Abstract
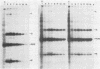
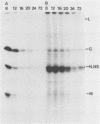
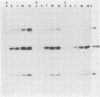
The time course of defective interfering (DI) particle and B particle release from vesicular stomatitis virus-infected BHK-21 cells was studied at different multiplicities of defective and infective particles. Particle release was progressively delayed in cells infected with an increasing DI-to-B particle ratio. The delayed particle release during interference was found to be connected with a reduced but prolonged synthesis of viral proteins, a slower accumulation of viral proteins, and a delayed shutoff of cellular protein synthesis. The relative synthesis of M and G proteins was reduced during interference, whereas the relative synthesis of N and NS proteins was increased. On the level of genomic RNA replication, we found that DI RNA was replicated more slowly during interference than the standard genomic RNA was during acute infection. The ratio of DI particles to B particles which were released increased throughout the infectious cycle. At a given time in the infectious cycle, this ratio was independent of the multiplicity of infecting DI and B particles. On the basis of the kinetic studies, we argue that cells infected with higher amounts of DI particles compared with B particles synthesize a higher DI-to-B particle ratio and release these progeny particles later than cells infected with a low DI-to-B particle ratio.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELLETT A. J., COOPER P. D. Some properties of the transmissible interfering component of vesicular stomatitis virus preparations. J Gen Microbiol. 1959 Dec;21:498–509. doi: 10.1099/00221287-21-3-498. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Garreis-Wabnitz C., Kruppa J. Intracellular appearance of a glycoprotein in VSV-infected BHK cells lacking the membrane-anchoring oligopeptide of the viral G-protein. EMBO J. 1984 Jul;3(7):1469–1476. doi: 10.1002/j.1460-2075.1984.tb01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeve L., Garreis-Wabnitz C., Zauke M., Breindl M., Kruppa J. The soluble glycoprotein of vesicular stomatitis virus is formed during or shortly after the translation process. J Virol. 1986 Mar;57(3):968–975. doi: 10.1128/jvi.57.3.968-975.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Breindl M. Factors involved in the generation and replication of rhabdovirus defective T particles. J Virol. 1976 Mar;17(3):805–815. doi: 10.1128/jvi.17.3.805-815.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Persistent noncytocidal vesicular stomatitis virus infections mediated by defective T particles that suppress virion transcriptase. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2956–2960. doi: 10.1073/pnas.71.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodyski F. M., Holland J. J. Viruses isolated from cells persistently infected with vesicular stomatitis virus show altered interactions with defective interfering particles. J Virol. 1980 Nov;36(2):627–631. doi: 10.1128/jvi.36.2.627-631.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodyski F. M., Nichol S. T., Spindler K. R., Holland J. J. Properties of DI particle resistant mutants of vesicular stomatitis virus isolated from persistent infections and from undiluted passages. Cell. 1983 Jul;33(3):801–810. doi: 10.1016/0092-8674(83)90022-3. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Manders E. K. Ribonucleic acid synthesis of vesicular stomatitis virus. IV. Transcription by standard virus in the presence of defective interfering particles. J Virol. 1972 Jun;9(6):909–916. doi: 10.1128/jvi.9.6.909-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Defective T particles of vesicular stomatitis virus. II. Biologic role in homologous interference. Virology. 1966 Oct;30(2):173–181. doi: 10.1016/0042-6822(66)90093-6. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Khan S. R., Lazzarini R. A. The relationship between autointerference and the replication of defective interfering particle. Virology. 1977 Mar;77(1):189–201. doi: 10.1016/0042-6822(77)90417-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Keene J. D., Schubert M. The origins of defective interfering particles of the negative-strand RNA viruses. Cell. 1981 Oct;26(2 Pt 2):145–154. doi: 10.1016/0092-8674(81)90298-1. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Translational control of protein synthesis after infection by vesicular stomatitis virus. J Virol. 1980 Dec;36(3):719–733. doi: 10.1128/jvi.36.3.719-733.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Cell killing by viruses. I. Comparison of cell-killing, plaque-forming, and defective-interfering particles of vesicular stomatitis virus. Virology. 1974 Feb;57(2):321–338. doi: 10.1016/0042-6822(74)90172-x. [DOI] [PubMed] [Google Scholar]
- Marvaldi J. L., Lucas-Lenard J., Sekellick M. J., Marcus P. I. Cell killing by viruses. IV. Cell killing and protein synthesis inhibition by vesicular stomatitis virus require the same gene functions. Virology. 1977 Jun 15;79(2):267–280. doi: 10.1016/0042-6822(77)90354-3. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Gatchell S. H. Intracellular events in the replication of defective interfering particles of vesicular stomatitis virus. Virology. 1979 Jan 15;92(1):168–179. doi: 10.1016/0042-6822(79)90222-8. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Davis N. L., Wertz G. W. N protein alone satisfies the requirement for protein synthesis during RNA replication of vesicular stomatitis virus. J Virol. 1984 Feb;49(2):303–309. doi: 10.1128/jvi.49.2.303-309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Holland J. J. Absence of transcriptase activity or transcription-inhibiting ability in defective interfering particles of vesicular stomatitis virus. Virology. 1972 Oct;50(1):150–170. [PubMed] [Google Scholar]
- Perrault J. Origin and replication of defective interfering particles. Curr Top Microbiol Immunol. 1981;93:151–207. doi: 10.1007/978-3-642-68123-3_7. [DOI] [PubMed] [Google Scholar]
- Popescu M., Schaefer H., Lehmann-Grube F. Homologous interference of lymphocytic choriomeningitis virus: detection and measurement of interference focus-forming units. J Virol. 1976 Oct;20(1):1–8. doi: 10.1128/jvi.20.1.1-8.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A., Kühne J., Zibirre R., Koch G. Poliovirus-induced alterations in HeLa cell membrane functions. J Virol. 1982 Nov;44(2):445–449. doi: 10.1128/jvi.44.2.445-449.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa M. Spectrophotometric quantitation of silver grains eluted from autoradiograms. Anal Biochem. 1983 Sep;133(2):511–514. doi: 10.1016/0003-2697(83)90117-3. [DOI] [PubMed] [Google Scholar]
- Wertz G. W. Replication of vesicular stomatitis virus defective interfering particle RNA in vitro: transition from synthesis of defective interfering leader RNA to synthesis of full-length defective interfering RNA. J Virol. 1983 May;46(2):513–522. doi: 10.1128/jvi.46.2.513-522.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]