Abstract
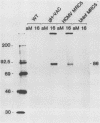
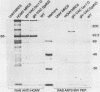
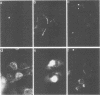
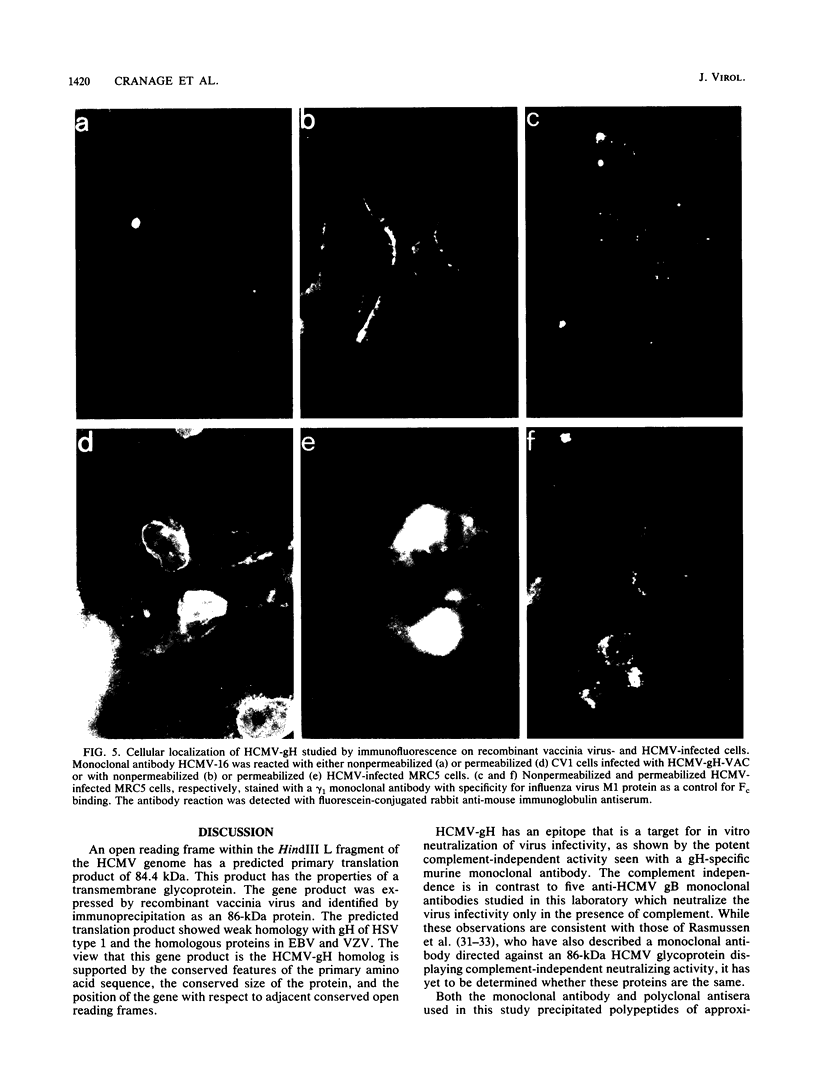
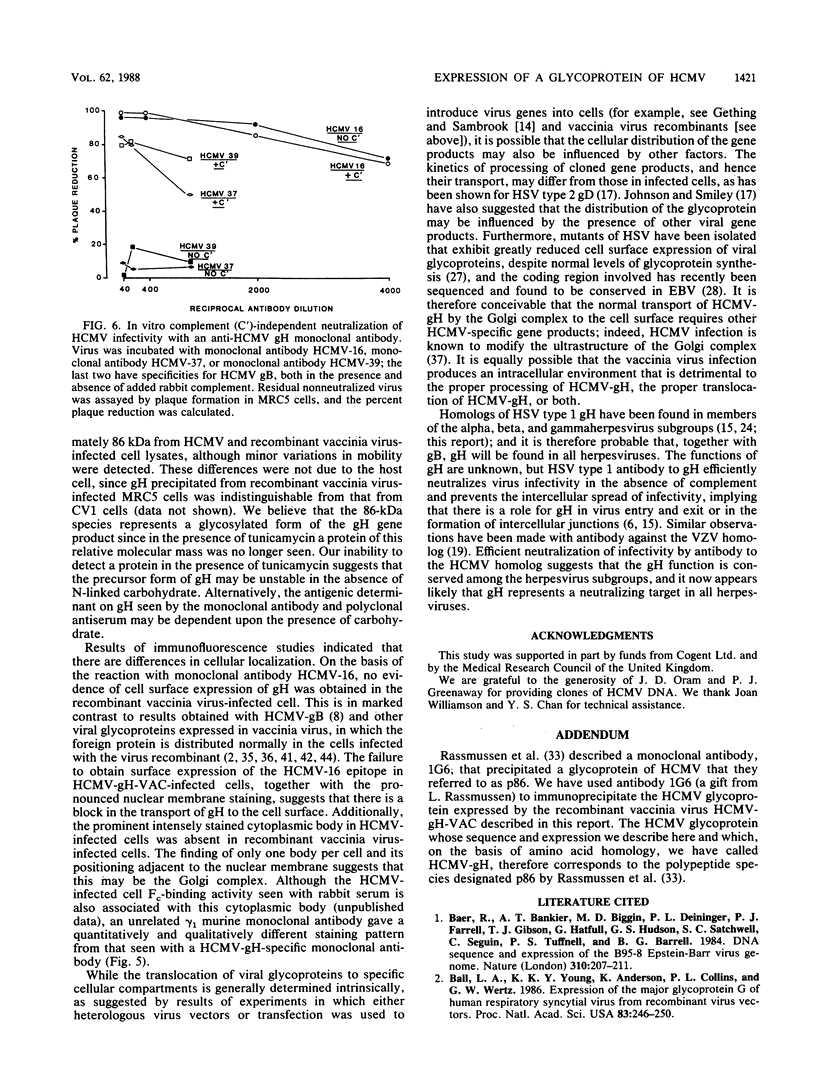
An open reading frame with the characteristics of a glycoprotein-coding sequence was identified by nucleotide sequencing of human cytomegalovirus (HCMV) genomic DNA. The predicted amino acid sequence was homologous with glycoprotein H of herpes simplex virus type 1 and the homologous protein of Epstein-Barr virus (BXLF2 gene product) and varicella-zoster virus (gpIII). Recombinant vaccinia viruses that expressed this gene were constructed. A glycoprotein of approximately 86 kilodaltons was immunoprecipitated from cells infected with the recombinant viruses and from HCMV-infected cells with a monoclonal antibody that efficiently neutralized HCMV infectivity. In HCMV-infected MRC5 cells, this glycoprotein was present on nuclear and cytoplasmic membranes, but in recombinant vaccinia virus-infected cells it accumulated predominantly on the nuclear membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Ball L. A., Young K. K., Anderson K., Collins P. L., Wertz G. W. Expression of the major glycoprotein G of human respiratory syncytial virus from recombinant vaccinia virus vectors. Proc Natl Acad Sci U S A. 1986 Jan;83(2):246–250. doi: 10.1073/pnas.83.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J., Auger D. Identification of a 65 000 dalton virion envelope protein of human cytomegalovirus. Virus Res. 1985 Dec;4(1):31–36. doi: 10.1016/0168-1702(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Britt W. J. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology. 1984 Jun;135(2):369–378. doi: 10.1016/0042-6822(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Buckmaster E. A., Gompels U., Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 X 10(3) molecular weight. Virology. 1984 Dec;139(2):408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Kouzarides T., Bankier A. T., Satchwell S., Weston K., Tomlinson P., Barrell B., Hart H., Bell S. E., Minson A. C. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986 Nov;5(11):3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Taylor P. Genetic relations between varicella-zoster virus and Epstein-Barr virus. J Gen Virol. 1987 Apr;68(Pt 4):1067–1079. doi: 10.1099/0022-1317-68-4-1067. [DOI] [PubMed] [Google Scholar]
- Durack D. T. Opportunistic infections and Kaposi's sarcoma in homosexual men. N Engl J Med. 1981 Dec 10;305(24):1465–1467. doi: 10.1056/NEJM198112103052408. [DOI] [PubMed] [Google Scholar]
- Farrar G. H., Greenaway P. J. Characterization of glycoprotein complexes present in human cytomegalovirus envelopes. J Gen Virol. 1986 Jul;67(Pt 7):1469–1473. doi: 10.1099/0022-1317-67-7-1469. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Hornberger E., Sakuma S., Plotkin S. A. Demonstration of immunoglobulin G receptors induced by human cytomegalovirus. J Clin Microbiol. 1975 Oct;2(4):332–336. doi: 10.1128/jcm.2.4.332-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Cell-surface expression of influenza haemagglutinin from a cloned DNA copy of the RNA gene. Nature. 1981 Oct 22;293(5834):620–625. doi: 10.1038/293620a0. [DOI] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Gönczöl E., Hudecz F., Ianacone J., Dietzschold B., Starr S., Plotkin S. A. Immune responses to isolated human cytomegalovirus envelope proteins. J Virol. 1986 May;58(2):661–664. doi: 10.1128/jvi.58.2.661-664.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Smiley J. R. Intracellular transport of herpes simplex virus gD occurs more rapidly in uninfected cells than in infected cells. J Virol. 1985 Jun;54(3):682–689. doi: 10.1128/jvi.54.3.682-689.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari B., Lussenhop N., Goertz R., Wabuke-Bunoti M., Radeke R., Gehrz R. Characterization of monoclonal antibodies reactive to several biochemically distinct human cytomegalovirus glycoprotein complexes. J Virol. 1986 Nov;60(2):345–352. doi: 10.1128/jvi.60.2.345-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P. M., Davison A. J., Lowe R. S., Riemen M. W., Ellis R. W. Identification and sequence of the gene encoding gpIII, a major glycoprotein of varicella-zoster virus. Virology. 1987 Apr;157(2):526–533. doi: 10.1016/0042-6822(87)90295-9. [DOI] [PubMed] [Google Scholar]
- Law K. M., Wilton-Smith P., Farrar G. H. A murine monoclonal antibody recognising a single glycoprotein within a human cytomegalovirus virion envelope glycoprotein complex. J Med Virol. 1985 Nov;17(3):255–266. doi: 10.1002/jmv.1890170307. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Davison A. J. DNA sequence of the herpes simplex virus type 1 gene encoding glycoprotein gH, and identification of homologues in the genomes of varicella-zoster virus and Epstein-Barr virus. Nucleic Acids Res. 1986 May 27;14(10):4281–4292. doi: 10.1093/nar/14.10.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancake B. A., Aschman D. P., Schaffer P. A. Genetic and phenotypic analysis of herpes simplex virus type 1 mutants conditionally resistant to immune cytolysis. J Virol. 1983 Sep;47(3):568–585. doi: 10.1128/jvi.47.3.568-585.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett P. E., Jenkins F. J., Ackermann M., Sarmiento M., Roizman B. Transcription initiation sites and nucleotide sequence of a herpes simplex virus 1 gene conserved in the Epstein-Barr virus genome and reported to affect the transport of viral glycoproteins. J Virol. 1986 Dec;60(3):1134–1140. doi: 10.1128/jvi.60.3.1134-1140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Hoffman M., Gallo D., Cremer N. Monoclonal antibodies to human cytomegalovirus: three surface membrane proteins with unique immunological and electrophoretic properties specify cross-reactive determinants. Infect Immun. 1982 Jun;36(3):924–932. doi: 10.1128/iai.36.3.924-932.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Hoffman M., Tatsuno M., Dondero D. Polymorphism of human cytomegalovirus glycoproteins characterized by monoclonal antibodies. Virology. 1984 Nov;139(1):73–86. doi: 10.1016/0042-6822(84)90331-3. [DOI] [PubMed] [Google Scholar]
- Rasmussen L. E., Nelson R. M., Kelsall D. C., Merigan T. C. Murine monoclonal antibody to a single protein neutralizes the infectivity of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Feb;81(3):876–880. doi: 10.1073/pnas.81.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L., Mullenax J., Nelson M., Merigan T. C. Human cytomegalovirus polypeptides stimulate neutralizing antibody in vivo. Virology. 1985 Aug;145(1):186–190. doi: 10.1016/0042-6822(85)90215-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen L., Mullenax J., Nelson R., Merigan T. C. Viral polypeptides detected by a complement-dependent neutralizing murine monoclonal antibody to human cytomegalovirus. J Virol. 1985 Aug;55(2):274–280. doi: 10.1128/jvi.55.2.274-280.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Levin J. Z., Palese P., Moss B. Synthesis and cellular location of the ten influenza polypeptides individually expressed by recombinant vaccinia viruses. Virology. 1987 Oct;160(2):336–345. doi: 10.1016/0042-6822(87)90004-3. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Mackett M., Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature. 1983 Apr 7;302(5908):490–495. doi: 10.1038/302490a0. [DOI] [PubMed] [Google Scholar]
- Smith J. D., De Harven E. Herpes simplex virus and human cytomegalovirus replication in WI-38 cells. I. Sequence of viral replication. J Virol. 1973 Oct;12(4):919–930. doi: 10.1128/jvi.12.4.919-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Graphic methods to determine the function of nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):521–538. doi: 10.1093/nar/12.1part2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagno S., Pass R. F., Dworsky M. E., Alford C. A. Congenital and perinatal cytomegalovirus infections. Semin Perinatol. 1983 Jan;7(1):31–42. [PubMed] [Google Scholar]
- Stephens E. B., Compans R. W., Earl P., Moss B. Surface expression of viral glycoproteins is polarized in epithelial cells infected with recombinant vaccinia viral vectors. EMBO J. 1986 Feb;5(2):237–245. doi: 10.1002/j.1460-2075.1986.tb04204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan V., Smith G. L. Expression and characterization of herpes simplex virus type 1 (HSV-1) glycoprotein G (gG) by recombinant vaccinia virus: neutralization of HSV-1 infectivity with anti-gG antibody. J Gen Virol. 1987 Oct;68(Pt 10):2587–2598. doi: 10.1099/0022-1317-68-10-2587. [DOI] [PubMed] [Google Scholar]
- Wentworth B. B., Alexander E. R. Seroepidemiology of infectious due to members of the herpesvirus group. Am J Epidemiol. 1971 Nov;94(5):496–507. doi: 10.1093/oxfordjournals.aje.a121347. [DOI] [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Reagan K. J., Dietzschold B., Curtis P. J., Wunner W. H., Kieny M. P., Lathe R., Lecocq J. P., Mackett M. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7194–7198. doi: 10.1073/pnas.81.22.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]