Abstract
Binding of dimeric immunoglobulin (Ig)A to the polymeric Ig receptor (pIgR) stimulates transcytosis of pIgR across epithelial cells. Through the generation of a series of pIgR chimeric constructs, we have tested the ability of ligand to promote receptor dimerization and the subsequent role of receptor dimerization on its intracellular trafficking. Using the cytoplasmic domain of the T cell receptor-ζ chain as a sensitive indicator of receptor oligomerization, we show that a pIgR:ζ chimeric receptor expressed in Jurkat cells initiates a ζ-specific signal transduction cascade when exposed to dimeric or tetrameric IgA, but not when exposed to monomeric IgA. In addition, we replaced the pIgR’s transmembrane domain with that of glycophorin A to force dimerization or with a mutant glycophorin transmembrane domain to prevent dimerization. Forcing dimerization stimulated transcytosis of the chimera, whereas preventing dimerization abolished ligand-stimulated transcytosis. We conclude that binding of dimeric IgA to the pIgR induces its dimerization and that this dimerization is necessary and sufficient to stimulate pIgR transcytosis.
INTRODUCTION
The polymeric immunoglobulin receptor (pIgR) is a type I membrane protein that is responsible for the transcytosis of dimeric IgA (dIgA) and pentameric IgM across the epithelial monolayer (Mostov 1994; Mostov and Cardone, 1995). Transcytosis of dIgA by pIgR is the first immunological defense against infections entering through mucosal surfaces, which constitute >95% of all infections. The pIgR itself is constitutively transcytosed across the cell; however, binding of dIgA stimulates its rate of transcytosis both in vitro (Hirt et al. 1993; Song et al., 1994a,b, 1995) and in vivo (Giffroy et al., 1998). Transcytosis therefore has two components, a constitutive baseline component and a stimulated component resulting from dIgA binding. Stimulation of transcytosis by ligand binding allows the pIgR system to respond to varying levels of dIgA presented to the epithelial cell.
The route of intracellular trafficking of the pIgR is well characterized. In brief, the pIgR is synthesized in the endoplasmic reticulum and transported through the biosynthetic pathway before being delivered directly to the basolateral plasma membrane domain (PM), where it interacts with its ligand. The pIgR, with or without bound ligand (dIgA) is rapidly endocytosed to a basolateral endosomal compartment and then transported in a microtubule-dependent manner to the apical recycling compartment (Apodaca et al., 1994a,b). From there it is transported to the apical cell surface, where the extracellular domain is proteolytically released as secretory component (SC).
We know that the intracellular processes of membrane trafficking and signal transduction are intimately related. For instance, proper signaling by many plasma membrane receptors, such as the EGF receptor, is dependent on their internalization (Vieira et al., 1996). Conversely, virtually all intracellular trafficking events are highly regulated. However, the signaling cascades involved in regulating membrane traffic and the molecular mechanisms by which they exert this control are poorly known. Recently we have found that, in addition to stimulating transcytosis, the binding of dIgA by the pIgR results in a rapid tyrosine phosphorylation of phosphatidyl inositol-specific phospholipase Cγ1, production of inositol triphosphate, activation of protein kinase C, and the elevation of intracellular Ca++ (Cardone et al., 1996) (Luton and Mostov, submitted). In addition, the ligand-stimulated component of transcytosis is inhibited in the presence of tyrosine kinase inhibitors, suggesting a direct link between a signal transduction cascade and ligand-stimulated transcytosis of the receptor (Luton and Mostov, submitted).
Monomeric IgA, which lacks the J chain, does not bind to or stimulate the transcytosis of the pIgR (Giffroy et al., 1998). Only J chain containing dimers or higher polymers of IgA can bind pIgR. One possibility is that one dIgA binds two pIgRs, thereby dimerizing the receptor. There are two pieces of indirect evidence to suggest that receptor dimerization may play a role in normal pIgR biology. First, Hirt et al. (1993) found that when MDCK cells expressing transfected rabbit pIgR were rapidly boiled in 3% SDS and then immunoprecipitated for the pIgR and analyzed by nonreducing SDS-PAGE, a high molecular weight species could be detected. Second, the pIgR contains within its transmembrane domain (TMD) a consensus sequence proposed to mediate ligand-dependent dimerization (Sternberg and Gullick, 1990). The presence of this motif in the pIgR suggests that the binding of ligand might alter the conformation of the pIgR TMD to facilitate receptor dimerization, leading to the modulation of pIgR transcytosis.
The stoichiometry of the interaction of free SC to dIgA has been under investigation for many years with conflicting results (Kuhn and Kraehenbuhl, 1982; Kraehenbuhl and Neutra, 1992). In fact, the results of two recent investigations that examined SC and dIgA are suggestive of a 1:1 ratio of SC to dIgA rather than a 2:1 ratio (Rindisbacher et al., 1995; Chintalacharuvu and Morrison, 1997). A 1:1 ratio of SC to dIgA would argue against the hypothesis that one dIgA binds two pIgRs, thereby causing pIgR dimerization. However, it must be kept in mind that binding of dIgA to the intact pIgR on the cell surface may differ substantially from dIgA binding to the soluble SC fragment. Even if one dIgA binds one pIgR, it is still possible that dIgA binding leads to pIgR dimerization (e.g., a complex of two dIgA and two pIgR), which in turn stimulates transcytosis.
Here we have investigated the hypothesis that ligand binding might promote or induce dimerization of the pIgR at the cell surface. This could facilitate pIgR transcytosis by several different processes, including alteration of the basolateral targeting signal, creation of a novel binding site for apical targeting machinery, changing the way pIgR aggregates during vesicular trafficking, and/or the initiation of the signal transduction cascade described above.
It can be quite difficult to directly ascertain the oligomeric state of a protein in the membrane and to determine the functional significance of dimerization (see DISCUSSION). Therefore, to determine both the existence and possible function of a putative pIgR dimer, we employed two complementary genetic approaches. First, to analyze whether the binding of dIgA results in the dimerization of the pIgR, we created a chimeric receptor using the extracellular and transmembrane domains from the pIgR with the cytoplasmic domain from the ζ chain of the T-cell receptor (TCR). The ζ chain of the TCR is responsible for signal transduction of the TCR, and it is highly responsive to its state of oligomerization. Although in vivo the ζ chain preexists as a covalently cross-linked dimer, both dimeric chimeras of CD8-ζ (Irving and Weiss, 1991) or monomeric and dimeric chimeras of Tac-ζ (Letourneur and Klausner, 1991; Eiseman and Bolen, 1992a) indicated that ζ cytoplasmic tail could be induced to signal upon antibody cross-linking. Even more importantly, their results indicated that signaling from ζ was induced by additional cross-linking. Therefore ζ in the cytoplasmic domain of a chimeric receptor could serve as a sensitive reporter of receptor oligomerization.
Second, we created chimeric receptors where dimerization is stabilized or prevented via the TMD. This has enabled us to explicitly test the functional role of dimerization on the intracellular trafficking of the pIgR, both in the presence and absence of the ligand. To study the effects of dimerization on the intracellular trafficking of the wild-type polymeric Ig receptor (pIgR-WT), we replaced its TMD with the TMD of human glycophorin A (pIgR-GpA). The glycophorin transmembrane domain contains the dimerization motif LIxxG79VxxG83VxxT, which has been demonstrated to be both necessary and sufficient for the oligomerization of glycophorin (Lemmon et al., 1992a,b). We therefore predict that the GpA TMD will artificially stabilize dimerization of the chimera (Bormann, 1989; Lemmon et al., 1992b; Treutlein et al., 1992; Challou et al., 1994; MacKenzie et al., 1997). Mutation of any one of the seven amino acids that are crucial to this motif abrogates dimerization (Lemmon et al. 1992a,b). GpA or a peptide corresponding to its TMD forms dimers that are stable even in the presence of SDS in a reducing environment. Previous studies found that the transfer of the GpA TMD onto the heterologous proteins v-neu, EGFR, and Staphylococcus aureus nuclease resulted in their detection as a SDS stable dimer (Lemmon et al., 1994). In addition, studies by (Lee and Nienhuis, 1992; Lee et al., 1992) found that when they transferred the TMD of GpA into colony-stimulating hormone receptor, the receptor was constitutively activated, suggesting that the dimerization of the receptor mimicked ligand binding.
Taken together, our constructs provide a useful tool to study the effects of dimerization on trafficking and are suggestive that for the pIgR dimerization is both necessary and sufficient for the ligand-induced stimulation of transcytosis.
MATERIALS AND METHODS
Reagents
General chemicals and supplies used in this study were from Fisher Scientific (Pittsburgh, PA). Trypsin, soybean trypsin inhibitor, and protease inhibitors were from Sigma Chemical (St. Louis, MO). Sulfo-NHS-biotin was obtained from Pierce Chemical (Rockford, IL). NP40 was from Calbiochem (San Diego, CA). The anti-mouse IgG-horseradish peroxidase (HRP) secondary antibody was purchased from Bio-Rad (Hercules, CA). The avidin-HRP and the enhanced chemiluminescence (ECL) system were obtained from Amersham (Arlington Heights, IL). Radiolabeled isotopes were obtained from New England Nuclear-Dupont (Boston, MA). Highly purified mIgA, dIgA, and tIgA was prepared and characterized as previously described (Song et al., 1995) and kindly provided by Prof. J.-P. Vaerman, Catholic University of Louvain (Brussels, Belgium).
Cells and Antibodies
MDCK cells were cultured in Eagle’s MEM with 5% FBS (Hyclone, Logan UT), penicillin, fungizone, and streptomycin (UCSF Cell Culture Facility, San Francisco, CA) at 37°C with 5% CO2. MDCK cells were transfected with pIgR-WT, pIgR-GpA, pIgR-GpA G83A, and pIgR-GpA G83A/G79L to generate stable cell lines by CaPO4 transfection (see PLASMID CONSTRUCTION). Four to five clones of each construct were tested for expression level of construct, delivery from the TGN to the cell surface, ability to transcytose dIgA, and the polarized secretion of gp80. Experiments on endocytosis and biotinylation of the receptor are from one of the chosen clones. Cells were routinely plated on plastic and passaged one time each week; they were discarded after eight passages. Cells plated on Transwell filters (Costar, Cambridge, MA) were harvested in 12 ml; 0.5 ml was plated on 12-mm filters and 1 ml was plated on 24-mm filters. Filters were fed everyday, after the first 48 h, and always used after 4 or 5 d of polarization.
Jurkat cells, kindly supplied by Art Weiss, were grown in suspension with RPMI supplemented with 25 mM HEPES, L-glutamine, penicillin, streptomycin, and 10% heat-inactivated FBS. A total of 107 cells were electroporated with 40 μg of pIgR, pIgR-ζ, or pIgRWT-ζ at 250 V and 960 μF in RPMI-20% FBS using a Bio-Rad Gene Pulser (Bio-Rad, Hercules, CA) and stably selected in G418 (2 mg/ml). Stable cells were than analyzed or sorted by FACScan/FACStar (Becton Dickinson, Franklin Lakes, NJ) based on their cell surface expression of pIgR, using an anti-SC antibody to detect the expression of pIgR-WT and the pIgR chimeras. A total of 1–2 × 106 cells in 150 μl were cooled to 4°C, stained with either sheep anti-SC or C305 (mouse monoclonal antibody directed against Jurkat Ti β-chain [Weiss and Stobo, 1984] to compare with the expression level of the TCR) in RPMI-10% FBS, washed 2× with RPMI, and stained with secondary antibody conjugated to FITC. Cells were exposed to 1 μg/ml propidium iodine to detect dead or dying cells. Background fluorescence determined for secondary antibody alone or Jurkat (untransfected cells) stained with sheep anti-SC. Cells were sorted twice in sequence based on a population of cells staining above the background fluorescence and gated consistent to the expression level for one another.
Plasmid Construction
Construction of the pIgR-ζ chimera was constructed using two polymerase chain reaction (PCR)-generated fragments each containing either pIgR or ζ with overlapping complimentary sequences. These were then annealed to each other and extended. This new fragment was shuttled into the pIgR sequences contained within the pCB6 expression vector (Brewer and Roth, 1991) and sequenced for confirmation. The ζ cytoplasmic domain sequences were taken from the CD8-ζ construct, kindly provided by Art Weiss. pCB6-pIgR and pCB6-pIgR-ζ were transfected into Jurkat cells as described by Irving and Weiss (1991), and stable cells expressing pIgR and pIgR-ζ were selected in G418 (2 mg/ml).
Construction of the pIgR-Glycophorin A (pIgR-GpA) chimera was a multistep process. First, a unique restriction site, AscI, was engineered into the 3′-end of the pIgR transmembrane domain by PCR. This was a silent mutation that did not change the amino acid sequence. The 5′-end of the transmembrane domain already contained a restriction site, ScaI. However, because it was not a unique site, a partial digest was performed to remove the transmembrane domain of pIgR. Two oligonucleotides encoding the transmembrane domain of glycophorin were synthesized, which, when joined, would create a 5′-blunt end for ligation into a ScaI site, and a 3′-AscI overhang:
5′-AACACTCATTATTTTTGGGGTGATGGCTGGTGTTATTGGAACGATCCTCTTAATTTCTTACGGTATTAGGG-3′
5′-TTGTGAGTAATAAAAACCCCACTACCGACCACAATAACCTTGCTAGGAGAATTAAAGAATGCCATAATCCCGCGC-3′
They were annealed, kinased, and ligated into place. The sequence of the construct was confirmed by DNA sequencing. The single- and double-point mutations were created by PCR mutagenesis and confirmed by DNA sequencing. pIgR-WT inserted into pCB7, a hygromycin resistance expression vector driven by the cytomegalovirus promoter and pCB6, a neomycin resistance expression vector driven by the cytomegalovirus promoter (Brewer and Roth, 1991) were used for the transfection into MDCK cells. All clones generated were screened for the polarized expression of the glycoprotein gp80, their ability to transcytose dIgA, and the delivery of the pIgR from the TGN. The clone MWTA4 used throughout this paper is designated pIgR-WT. The glycophorin constructs were created in pIgR-WT and inserted into pCB6. Four or five clones from each construct were again tested for the polarized expression of the glycoprotein gp80, their ability to transcytose dIgA, and the delivery of the pIgR from the TGN.
Luciferase Assay
The luciferase assay was performed as described by Chu et al. (1996). A total of 107 Jurkat cells stably expressing pIgR-WT, pIgR-ζ, and pIgRWT-ζ were transiently transfected with 20 μg of NFAT-luciferase reporter plasmid as described above. Twenty-four hours later, 105 cells in a total volume of 90 μl were stimulated with increasing concentrations of monomeric, dimeric, and tetrameric IgA. Cells were lysed 6.5 h later with 10 μl of 10× lysis buffer (final concentration of 100 mM KPO4, pH 7.8, 5 mM dithiothreitol, and 1% Triton X-100). The lysate was then mixed with 100 μl of assay buffer (200 mM KPO4, pH 7.8, 10 mM ATP, 20 mM MgCl2) followed by 100 μl of 1 mM luciferin. In general, stimulation of the TCR with C305 gave a two- to threefold stronger response (Singer, unpublished data). Luciferase activity, expressed in arbitrary units, was determined in triplicate for each experimental condition.
Jurkat Cell Stimulation
A total of 2 × 107 Jurkat cells in 200 μl were stimulated with C305 (anti-TCR at 1:500 dilution of ascites), tetrameric IgA (2 mg/ml), or left unstimulated for 2 min at 37°C, as described by Chu et al. (1996). Cells were rapidly lysed in buffer containing 1% NP40, 125 mM NaCl, 20 mM HEPES (pH 7.4), 10 mM NaF, 2 mM Na Vanadate, and a cocktail of protease inhibitors. After the nuclei had been spun out, lysates were cleared 2× with empty protein A Sepharose (Pharmacia, Uppsala, Sweden), immunoprecipitated with anti-ζ monoclonal 6810.2 (a kind gift from Art Weiss) bound to protein A, resolved by 15% SDS-PAGE, and transferred to Immobilon P (Millipore, Bedford, MA) for Western blot with mouse monoclonal 4G10 (anti-phosphotyrosine, Upstate Biotechnology, Lake Placid, NY). Blots were not stripped, but were directly reprobed with anti-ζ monoclonal 6810.2. Antibodies were detected by secondary antibody conjugated to HRP detected by enhanced chemiluminescence.
Receptor Dimerization
Cells transfected with the various constructs were examined for receptor dimerization by reducing SDS-PAGE. Cells were lysed from confluent 10-cm plastic tissue culture Petri dishes in ice-cold HEPES buffered saline (HBS, 50 mM HEPES, pH 8.0, 125 mM NaCl), plus 1% NP40 and protease inhibitors. The lysate was centrifuged at 4°C for 10 min at 14,000 rpm, to remove the nuclei and cellular debris. The supernatant was immunoprecipitated overnight with sheep anti-SC antibodies conjugated to protein G, followed by four washes with HBS + 1% NP40. Sample buffer contained 50 mM dithiothreitol (DTT). Cross-linking by boiling in SDS was performed as described by Hirt et al. (1993). Cells grown on a 10-cm Petri dish were washed in PBS (+ Ca++ and Mg++), scraped, pelleted, lysed with 75 μl of hot 3% SDS, and then boiled for 5 min. The lysate was diluted with dilution buffer (10 mM Tris, pH 7.5, 100 mM NaCl, 5 mM EDTA, and 1% Triton X-100) and immunoprecipitated as above and analyzed by 7% SDS-PAGE. Detection of protein was through Western blot, with monoclonal mouse antibody directed against the cytoplasmic domain of the pIgR (SC166), followed by detection with HRP.
Delivery from the TGN to the Apical and Basolateral Surface
A pulse-chase procedure combined with a protease sensitivity assay were applied to determine the vectorial transport of the pIgR from the TGN to the basolateral and apical surfaces of MDCK-derived clones. A detailed description of the assay is given by Aroeti et al. (Aroeti et al. 1993). In brief, duplicate 12-mm filter pairs containing a confluent monolayer of cells were used in each determination. Cells were pulsed on a 30 μl drop of [35S]cysteine (Du Pont-New England Nuclear, Boston, MA), 1 μCi/μl for 15 min at 37°C in a humidified chamber. Cells were quickly washed and chased plus or minus 25 μg/ml V8 protease (Boehringer-Mannheim Biochemicals, Indianapolis, IN) in the basal media, with MEM/BSA in the apical chamber for 60 min at 37°C. At the end of the chase period, receptor remaining in the cells and SC released into the apical and basal media were immunoprecipitated from the nonprotease-treated filters (total labeled pIgR), while for the protease-treated filters the receptor remaining in the cells and SC released into the apical media. Immunoprecipitations were performed with sheep anti-SC conjugated to protein G. Immunoprecipitates were analyzed by SDS-PAGE and quantified by PhosphorImager (Molecular Dynamics, Sunnyvale, CA). Basolateral delivery was derived from the reduction in the [35S]-labeled pIgR in the V8 protease-treated cells versus the nonprotease-treated (total pIgR). Basolateral delivery (%) = (1 − SC in apical medium and pIgR present in the cell after V8 treatment)/the total amount of labeled pIgR and SC in the apical medium in nonprotease-treated cells) × 100. Apical delivery (%) is derived from (SC in apical media and pIgR present in the cell after V8 treatment/the total amount of labeled pIgR and SC in the apical medium in nonprotease-treated cells) × 100. The data presented for the chimeras (wild-type and mutant) are combined from four to five clones, each assayed in at least two experiments in duplicate.
Ligand Transcytosis
The dIgA was iodinated by the ICl method (Goldstein et al., 1983) and stored at a concentration of 75 μg/ml, 4 × 105 to 1 × 106 cpm/μl. Cells cultured on 12-mm Transwell filters for 4–5 d were washed three times in MEM/BSA. The filter units were placed on a 10 μl drop of MEM/BSA containing 2 μl of [125I]-dIgA. The ligand was internalized for 10 min at 37°C. The filters were rapidly washed four to five times with MEM/BSA and transferred into a 12-well culture plate with fresh medium added to both apical (300 μl) and basolateral chambers (500 μl). The medium was collected and the filters transferred into new wells with fresh apical and basal MEM/BSA at 7.5, 15, 30, 60, and 120 min. At the end of the chase, the filters were cut out from the holders and all the fractions (cells, apical, and basolateral media) were counted in a Packard γ-counter. MDCK cells that do not express the pIgR were analyzed in parallel to control for nonspecific uptake of ligand, and the values obtained at each time point were subtracted as background.
Ligand Internalization
Radiolabeled dIgA was prepared as described above for the transcytosis assay. Cells were cultured on 12-mm Transwell filters for 4–5 d. Cells were cooled to 4°C in the cold room by placing the tray of cells on ice and washing 3× with ice-cold MEM/BSA. The basolateral cell surface was then exposed to 125I-dIgA to bind receptor for 1 h on ice. Cells were washed five to six times with ice-cold MEM/BSA to remove unbound ligand. The cells were then allowed to internalize ligand for various times at 37°C and then rapidly returned to 4°C. Remaining surface-bound ligand was stripped by incubation of the cells in 150 mM glycine, pH 2.5 (made in PBS plus 0.6% BSA) for 1 h at 4°C. Identical results were obtained when surface ligand was stripped with trypsin (Singer, unpublished data). The percentage of ligand endocytosed after each internalization period was calculated as the counts remaining in the cells after stripping divided by the total counts (the counts dissociated in the basolateral medium during the internalization period, those stripped from the cell surface, and those remaining intracellularly).
Biotinylation and Transcytosis of pIgR
MDCK cells were cultured on 24-mm Transwell filters for 4–5 d. Filters were washed three times with Hanks buffered saline solution plus 20 mM HEPES, pH 7.4 (HBSS+) at 18°C, then incubated with Sulfo-NHS-biotin (0.1 mg/ml) at their basolateral surface for 30 min at 18°C. HBSS+ was added to the apical surface. The filters were then washed three times over 5 min with MEM-BSA at 18°C. To determine the total amount of the biotinylated receptor, duplicate filters (+/- dIgA) were washed with PBS at 4°C, and cells were lysed in 0.5% SDS, 50 mM Tris, pH 8.0, boiled, and set aside for immunoprecipitation. To determine the effect of ligand on trafficking, one set of filters was then incubated on a 30-μl drop of MEM/BSA containing dIgA (300 μg/ml), on their basolateral surface for 10 min at 18°C. The second set of filters was incubated on a 30-μl drop of MEM/BSA lacking dIgA for 10 min at 18°C. Transcytosis of biotinylated receptor was determined for filters in triplicate or quadruplicate by placing the filters in a humidified 37°C chamber. Filters were placed on 100-μl drops of MEM/BSA, in the continued presence or absence of dIgA at 300 μg/ml. Fresh MEM/BSA (500 μl) was added apically. Apical medium was collected at the given time points and replaced with fresh medium. All samples were brought to 0.5% SDS and 1.25% Triton X-100 before immunoprecipitation with sheep anti-rabbit SC antibodies conjugated to protein G. Samples were analyzed by reducing SDS-PAGE, transferred to Immobilon P (Millipore) for Western blot with monoclonal antibody antiSC166 (which recognizes the cytoplasmic domain of the pIgR). Antibody was detected by secondary antibody conjugated to HRP, detected by enhanced chemiluminescence, and quantified by NIH image.
It should be noted that in this protocol, the cells are exposed to dIgA only after the pIgR at the basolateral surface has been biotinylated. We have previously found that inclusion of dIgA during the biotinylation step results in the inactivation of the dIgA, due to excessive biotinylation of the dIgA (Song et al., 1994a). Therefore, in this protocol many molecules of biotinylated pIgR may never actually have the opportunity to bind to the dIgA. This experiment therefore gives only a minimum estimate of the stimulation of pIgR transcytosis by dIgA.
RESULTS
We have addressed two fundamental questions about the pIgR. 1) Does the binding of dIgA to pIgR dimerize the pIgR? 2) Does dimerization of the pIgR have any consequences on its intracellular trafficking, particularly with regard to stimulation of transcytosis. To test whether dIgA binding dimerizes the receptor, we used the ζ chain from the TCR in a chimeric construct with the pIgR to serve as a reporter for cell surface oligomerization. To test for the effect of dimerization on the trafficking of the receptor, we have created chimeras of the pIgR with the wild-type TMD of GpA or mutant TMDs of GpA to stabilize or destabilize receptor dimerization, respectively.
Cell Surface Expression of the pIgR and the pIgR-ζ on Jurkat Cells
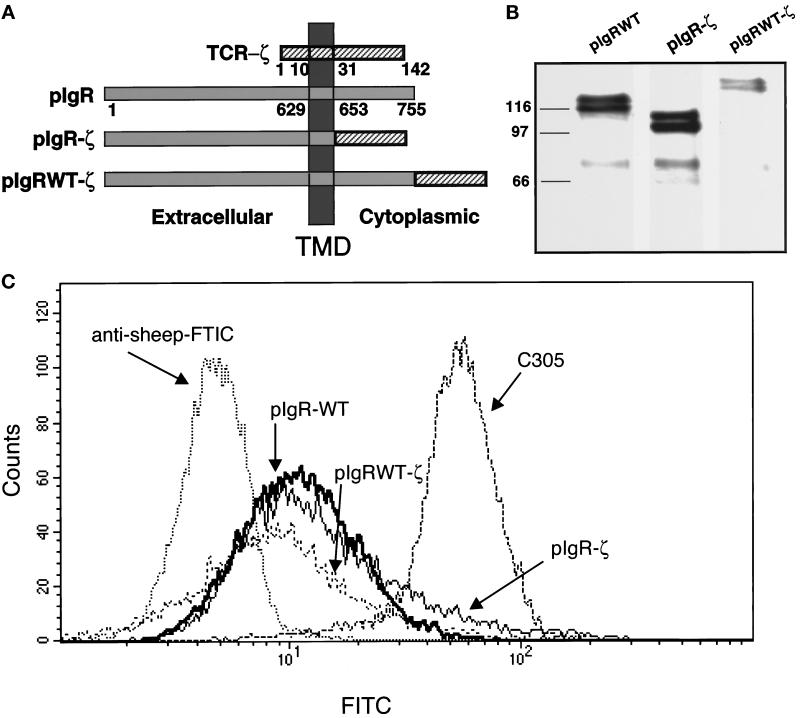
To test for receptor dimerization or oligomerization at the cell surface in response to ligand, we created two chimeric constructs with the pIgR and the ζ chain from the TCR (Figure 1a). In the first construct the cytoplasmic domain of the ζ chain was transferred onto the pIgR to precisely replace its cytoplasmic domain (pIgR-ζ). In the second construct, the cytoplasmic domain of the ζ chain was precisely transferred onto the C terminus of the full-length pIgR (pIgRWT-ζ). Both of these constructs and the wild-type pIgR (pIgR-WT) were stably transfected into the human T-cell line, Jurkat. Western blot analysis of the cells was used to demonstrate the expression of the transfected constructs in the cells. As shown in Figure 1b, the pIgR-ζ migrates with a faster mobility than the pIgR-WT, even though the size of their cytoplasmic domains is the same. This is consistent with the observation that the wild-type cytoplasmic domain of the pIgR migrates anomalously slowly, most likely due to its highly charged nature. When the ζ sequences are placed after the C terminus of the full- length pIgR (pIgRWT-ζ), the mobility is reduced commensurate with expected shift in size with respect to the pIgR-WT.
Figure 1.
Construction and stable expression of pIgR-ζ chimeras in Jurkat cells. (a) A schematic representation of the pIgR-WT, pIgR-ζ, and pIgRWT-ζ constructs. The extracellular, TMD, and cytoplasmic tails are indicated. Numbers correspond to amino acids after signal peptide cleavage. (b) Stable expression of pIgR-WT, pIgR-ζ, and pIgRWT-ζ in Jurkat cells selected with G418 (2 mg/ml). Cell lysates were immunoprecipitated for pIgR with guinea pig anti-SC conjugated to Protein A-Sepharose, separated by 10% SDS-PAGE, and transferred to immobilon P for detection by Western blot. The pIgR constructs were detected with sheep anti-SC, followed by HRP-labeled secondary antibody. The molecular weight markers in kilodaltons are indicated on the right. Note that each construct migrates as a dimer, due to heterogeneous glycosylation, as previously observed. The bands around 70 kDa are SC, which is cleaved from the pIgR due to the extremely protease-sensitive site where this cleavage normally occurs. (c) Flow cytometry was used to analyze cell surface expression of pIgR-WT, pIgR-ζ, and pIgRWT-ζ. Jurkat cells (1–2 × 106) were cooled to 4°C and stained with sheep anti-SC antibody followed by anti-sheep FITC-conjugated secondary antibody.
To ensure that these proteins are expressed at the cell surface at comparable levels, Jurkat cells expressing each of the constructs were subject to a fluorescence-activated cell sorter for both analysis of cell surface expression and subsequent selection of a population of equivalently expressing cells. As shown in Figure 1c, the mean expression of pIgR-WT, pIgR-ζ, and pIgRWT-ζ was 12.44, 19.63, and 11.4 FITC intensity (arbitrary units), respectively. The mean of the background was 4.92 FITC intensity for untransfected Jurkat cells stained with antibody against the pIgR or transfected Jurkat cells stained with FITC-labeled secondary antibody alone (Singer, unpublished). In comparison, the mean expression of the endogenous TCR, detected by antibody directed against Ti β-chain, was 55.62 FITC intensity. For reasons that are not clear, subsequent rounds of fluorescence-activated cell sorter selection did not stably increase the cell surface expression.
Dimerization of the pIgR-ζ by dIgA and tIgA
As described in the INTRODUCTION, activation of the ζ chain is sensitive to oligomerization. One of the downstream consequences of ζ activation is the phosphorylation of ζ and its associated protein, ZAP 70, leading to the induction of a signal transduction cascade that results in the transcriptional activation of the interleukin-2 (IL-2) gene. Studies initiated in Art Weiss’s laboratory developed a reporter plasmid that contained the IL-2 promoter (NFAT) driving the luciferase gene product (Chu et al., 1996). Using this reporter plasmid, they demonstrated that the activation of ζ could be quantified by analysis of luciferase activity.
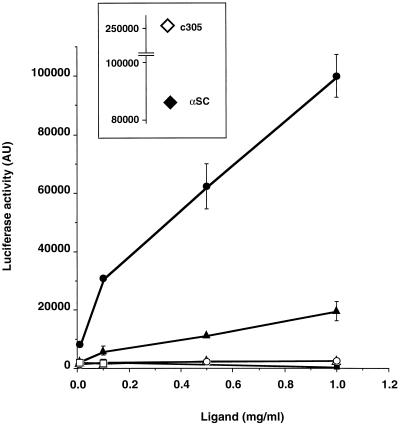
To test for the effect of dIgA on the pIgR-ζ, Jurkat cells expressing the pIgR, pIgR-ζ, and pIgRWT-ζ were transiently transfected with the NFAT-luc reporter plasmid. Twenty-four hours later they were stimulated with dIgA (as described in MATERIALS AND METHODS) for 6.5 h. As shown in Figure 2, the addition of dIgA (triangles) to pIgR-ζ (solid), but not to pIgR-WT (open), resulted in the initiation of luciferase production indicating the dimerization of the chimeric construct. It might have been possible that dIgA binding to the exogenously expressed pIgR-WT would result in a detectable transcription from the IL-2 promoter due to a nonspecific induction of signaling. However, this proved not to be the case and thus the pIgR-WT serves as a good negative control for the specificity of the response due to the chimeric construct. The addition of monomeric IgA (mIgA, squares) to pIgR-ζ (solid) or pIgR-WT (open) had no effect of the induction of luciferase production, in agreement with the known inability of monomeric IgA to bind pIgR. The binding of tetrameric IgA (tIgA, circles) to pIgR-ζ (solid) had a greatly enhanced induction of luciferase. This result is consistent with the earlier finding for the Tac-ζ chimera supporting the finding that ζ is responsive to the degree of protein oligomerization (Letourneur and Klausner, 1991; Eiseman and Bolen, 1992b). It also directly indicates that the binding of tIgA results in a higher order oligomerization of the pIgR. The binding of tIgA to pIgR-WT (open circle) had no effect on luciferase production. The addition of dIgA had no effect when ζ was placed at the end of the full-length pIgR, pIgRWT-ζ (Singer, unpublished). Most likely this is due to the unusual conformation of the wild- type pIgR cytoplasmic domain, as suggested by its anomalous migration by PAGE.
Figure 2.
Luciferase activity in Jurkat pIgR-ζ is stimulated by dIgA and tIgA in a dose-dependent manner. Jurkat pIgR-WT (open symbols) or pIgR-ζ (closed symbols) cells (1 × 107) were transiently transfected with a NFAT-luciferase plasmid. After 24 h, 1 × 105 cells were stimulated with increasing concentrations of monomeric IgA (squares), dIgA (triangles), or tIgA (circles) for 6.5 h. Cell lysates were analyzed for luciferase activity with 1 mM luciferin. The results are representative of three independent experiments. The inset provides the luciferase values for cells exposed to c305 antibody (anti-TCR, 1:500, open diamond) or anti-SC rabbit polyclonal antibody (1:500, closed diamond).
Activation of pIgR-ζ Is Independent of the Endogenous TCR-ζ
The Jurkat cells used for these experiments expressed their endogenous TCR. Therefore, we tested whether the activation of pIgR-ζ involved the endogenous TCR-ζ or whether the response was solely due to the pIgR-ζ chimera. Upon activation of the TCR, the ζ-chain becomes phosphorylated and can be detected by Western blot using the antiPO4-Tyr antibody, 4G10. Jurkat cells expressing both pIgR-ζ and pIgR-WT were stimulated for 2 min with either a TCR-activating antibody, C305, or the pIgR-ζ chimera-activating ligand, tIgA. The cells were immediately lysed and immunoprecipitated for ζ for subsequent analysis by PAGE and Western blot. The addition of tIgA to pIgR-ζ or pIgR-WT did not result in the phosphorylation/activation of the TCR-ζ. Phosphorylated TCR-ζ can only be detected when the cells are stimulated with C305 to activate the TCR specifically. We were unable to detect any increase in phosphorylation of the pIgR-ζ chimera itself in response to tIgA, as there was a background signal for the pIgR-ζ, even in the absence of ligand (Singer, unpublished).
The Role of Dimerization on the Intracellular Trafficking of the pIgR
To study the effects of dimerization on the intracellular trafficking of the wild-type polymeric Ig receptor (pIgR-WT), we replaced its 23-amino acid TMD with the 23-amino acid TMD of human glycophorin A (pIgR-GpA). Studies using resonance energy transfer, rotational resonance imaging, and nuclear magnetic resonance spectroscopy of the glycophorin transmembrane domain have indicated that the amino acids Gly79/Val80 and Gly83/Val84 are required for the physical interaction of the two helices (Adair and Engelman, 1994; Smith and Bormann, 1995; MacKenzie et al., 1997). The glycine residues are buried in the center of the dimer interface and contribute to the electrostatic interactions between the helix backbones (Lemmon et al. 1994; MacKenzie et al., 1997). Indeed, mutation of these residues has been shown to disrupt dimerization (Lemmon et al. 1992b). For this reason we choose to target these glycines for single- and double-point mutations to disrupt dimerization of the pIgR-GpA chimera. For the single-point mutation of the pIgR-GpA chimera, Ala was substituted for Gly83 (pIgR-GpA G83A). The double-point mutation combined G83A with Leu substituting for Gly79 (pIgR-GpA G83A/G79L) (Figure 3a). We analyzed four major trafficking steps for the pIgR-GpA dimeric chimera compared with the wild-type receptor. These included polarized delivery from the TGN to the apical and basolateral cell surfaces, transcytosis of the receptor in the presence or absence of ligand, transcytosis of ligand by the receptor, and internalization of ligand by the receptor. By comparing the trafficking pathways of the dimerizing chimera containing the wild-type GpA TMD (pIgR-GpA) with that of the nondimerizing point mutant chimeras (pIgR-GpA G83A, G83A/G79L), we can directly test the role of receptor dimerization and separate the effects of dimerization per se from those due to the presence of the heterologous TMD.
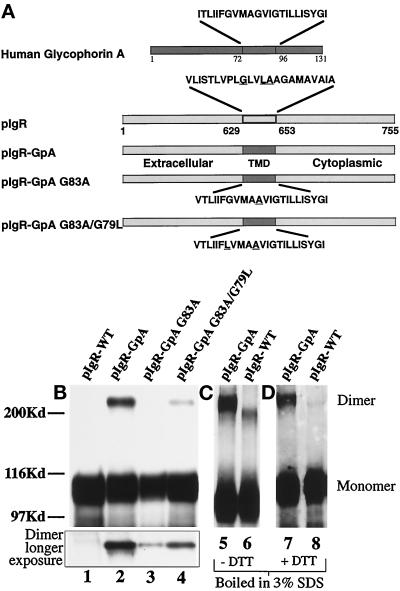
Figure 3.
Construction and expression of pIgR-GpA chimeras. (a) A schematic representation of the pIgR-WT, pIgR-GpA, pIgR-GpA-G83A, and pIgR-GpA-G83A/G79L. A unique restriction site was engineered into the pIgR at the 3′-end of the pIgR TMD. Oligonucleotides for the wild-type human glycophorin A TMD were generated with overhangs corresponding to the restriction sites ScaI and AscI. The mutations in the glycophorin TMD were created by PCR mutagenesis. Due to the 5′-restriction site, the first amino acid, Val, of the pIgR TMD was retained. The remaining amino acids correspond to the GpA TMD. Substitution of Val for Ile had no effect on the dimerization of GpA (Lemmon et al., 1992a). For the chimeric constructs, underlined amino acids represent those residues mutated. For the wild-type pIgR, the underlined residues are those corresponding to the dimerization motif described by Sternberg and Gullick (1990). (b) Detection of dimeric receptor by SDS-PAGE. MDCK cells transfected with pIgR-WT (lane 1), pIgR-GpA (lane 2), pIgR-GpA G83A (lane 3), and pIgR-GpA G83A/G79L (lane 4) constructs. Immunoprecipitates were analyzed by SDS-PAGE/Western blot (SC166 mouse monoclonal against pIgR cytoplasmic domain); 30 sec exposure by ECL. The inset represents the higher molecular weight dimer band at a longer exposure of the same gel. (c) Pellets of cells expressing pIgR-GpA (lane 5) or pIgR-WT (lane 6) were lysed with hot 3% SDS, boiled for 5 min, and then diluted 10-fold with 10 mM Tris, pH 7.5, 100 mM NaCl, 5 mM EDTA, and 1% Triton X-100, before immunoprecipitation with sheep anti-SC conjugated to protein G and analyzed by SDS-PAGE minus DTT. (d) Pellets of cells expressing pIgR-GpA (lane 7) or pIgR-WT (lane 8) were processed as in panel c, except that the samples were reduced with DTT before electrophoresis. In panels c and d, pIgR was detected by Western blot with sheep anti-SC followed by secondary anti-sheep-HRP and ECL.
Dimerization of pIgR-GpA Chimeras
Although dimerization of membrane receptors in situ is often difficult to detect, we were able to detect pIgR-GpA dimer after detergent solubilization by several techniques, including SDS-PAGE, cross-linking by diamide, and cross-linking by boiling in 3% SDS during lysis (Hirt et al., 1993). We detected approximately 13% of the pIgR-GpA chimera as an apparent stable dimer under reducing SDS-PAGE conditions (see Figure 3, b–d, lanes 2, 5, and 7; Table 1). This fraction of SDS-PAGE stable dimer was independent of the level of the pIgR-GpA chimera expressed in various clones. To control for nonspecific disulfide cross-linking during cell lysis, the cells were lysed in the presence of N-ethyl maleimide to alkylate free cysteines. This did not affect the detection of the pIgR-GpA dimerization. Dimerization of the point mutants, determined by their stability during lysis in SDS and SDS-PAGE, was decreased by 20-fold for pIgR-GpA G83A and 10-fold for pIgR-GpA G83A/G79L compared with the pIgR-GpA (Figure 3b, inset, lanes 3 and 4; Table 1). No dimerization of the pIgR-WT was detected under these conditions regardless of protein level expressed (see Figure 3b, lane 1). Treatment of the cells with diamide, which causes the oxidation of cytoplasmic cysteines (pIgR contains one cytoplasmic cysteine), or lysis of the samples by boiling in 3% SDS, resulted in detectable dimerization for both the pIgR-WT and pIgR-GpA. When boiled in 3%-SDS, the dimer of the pIgR-WT has a slightly faster mobility than the pIgR-GpA dimer on nonreducing SDS 8% PAGE (Figure 3c). As shown in Figure 3d, when these samples are treated with DTT to reduce the disulfide bond before electrophoresis, the pIgR-WT dimer is lost; however, the pIgR-GpA dimer remains. This is consistent with the fact that the interaction from the GpA TMD is not based on a covalent disulfide bond. In contrast, the pIgR-WT dimer seen on nonreducing SDS-PAGE after boiling in 3% SDS is dependent on a disulfide bond.
Table 1.
Quantitation of receptor monomers and dimers
| Cell line | Monomera | Dimera | % Dimer:Monomer |
|---|---|---|---|
| pIgR-WT | 7670 | 0 | 0 |
| pIgR-GpA | 7529 | 985 | 13 |
| pIgR-GpA G83A | 5122 | 31 | 0.6 |
| pIgR-GpA | 8505 | 114 | 1.3 |
| G83A/G79L |
Values are arbitrary densitometric units with a Molecular Dynamics scanner and Image Quant software, determined from Figure 3b.
Pulse chase analysis of the pIgR-WT and pIgR-GpA demonstrated that the rate of loss of the full-length protein from the cell was correlated to the release of SC in the apical media (Singer, unpublished data). The half-life of the pIgR-GpA chimera was 80 min versus 170 min for the pIgR-WT. This is consistent with the direct delivery of the pIgR-GpA chimera to the apical membrane (see below), whereas the pIgR-WT must be transported first to the basolateral PM and then transcytosed to the apical PM before being released as SC. Transport through the endoplasmic reticulum and Golgi, observed as a shift in mobility due to the addition of complex carbohydrates, was also normal for the pIgR-GpA chimera (Singer, unpublished data).
Dimerization Enhances Transcytosis of the pIgR
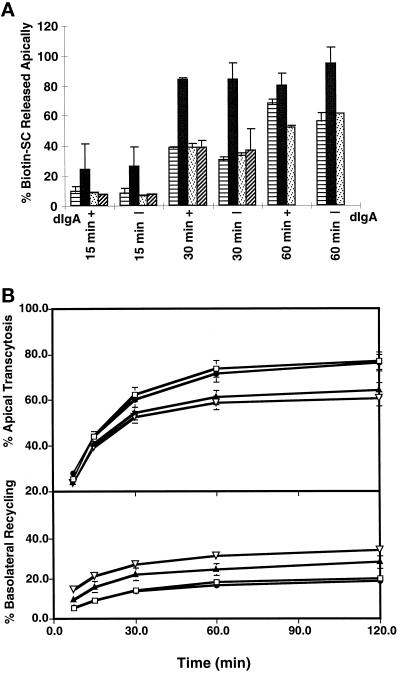
We would predict that if transcytosis of the WT receptor is stimulated by ligand-mediated dimerization, then stabilizing receptor dimerization through the GpA TMD would mimic ligand binding and result in an unoccupied receptor that transcytoses more rapidly. Conversely, when there is little dimerization, as for the unoccupied pIgR-WT or the mutant chimeras, then the constitutive rate of transcytosis would be decreased. Monitoring the transcytosis of the receptor by biotinylation enabled us to follow the kinetics of transcytosis of the receptor independent of ligand binding. For the chimeric receptors, both wild-type and mutant, measurements of transcytosis are normalized for the percentage of protein that is delivered to the basolateral cell surface (described later). To follow the basal to apical transcytosis of the receptor in MDCK cells, the receptor was labeled by biotinylation of the basolateral cell surface for 30 min at 18°C. This labels a cohort of receptors at the cell surface and those receptors recycling from the basal early endosomes. To compare the receptor transcytosis with and without ligand, the cells are incubated for an additional 10 min at 18°C with or without dIgA, washed, and then chased at 37°C for the given times. When the transcytotic receptor reaches the apical PM, it is cleaved and released into the apical media as secretory component. Biotinylated SC is quantified by immunoprecipitation of SC and then detected by a Western blot using HRP- conjugated streptavidin.
We found that the constitutive rate of transcytosis of the dimeric pIgR-GpA is dramatically accelerated (Figure 4a). Transcytosis of the pIgR-GpA reached a plateau of 84% by 30 min, whereas transcytosis of the pIgR-WT at this time was only 30%. In fact, within the first 15 min the transcytosis of the pIgR-GpA was already twofold higher than that of the pIgR-WT. Transcytosis of the pIgR-WT could be stimulated by the presence of ligand from 56% to 68% after 60 min of transcytosis, consistent with previous results (Song et al., 1994a). Those WT receptors that bound dIgA were clearly stimulated in transcytosis. However, in the presence of ligand, the rate of transcytosis of the dimeric pIgR-GpA was greater than that of the pIgR-WT. A likely explanation is that stimulation of transcytosis by the pIgR-WT is dependent on the binding of ligand, and that not all of the biotinylated receptor has the opportunity to bind ligand. Note that in this assay the cell surface pIgR is first biotinylated for 30 min, washed for 5 min, and then incubated with or without dIgA for 10 min, all at 18°C. The separation of the biotinylation from the dIgA incubation steps is necessary, as the dIgA is inactivated by reaction with the biotinylation reagent. As such, many of the biotinylated pIgR-WT do not have the opportunity to bind ligand, and as such the stimulation of transcytosis by dIgA is underestimated (Song et al., 1994b). In contrast, the enhanced rate of pIgR-GpA transcytosis is independent of ligand binding due to the stabilization of the dimer. We propose that dimerization of the receptor alone is sufficient to control the rate of receptor transcytosis.
Figure 4.
Rate of transcytosis of the receptor and [125I]dIgA. (a) Transcytosis of receptor in presence or absence of ligand. MDCK cells expressing pIgR-WT or the chimeras were grown on permeable supports for 4–5 d. The basolateral cell surface was biotinylated (0.1 mg/ml) for 30 min at 18°C, washed, incubated in the presence or absence of 300 μg/ml dIgA for 10 min at 18°C, washed briefly, and then placed at 37°C in the continued presence or absence of dIgA. Apical media were collected at 15, 30, and 60 min; 100% represents the total amount of pIgR biotinylated at time zero. Samples were immunoprecipitated for pIgR and detected by Western blot with avidin-HRP and ECL. The symbols are: ▤, pIgR-WT; ▪, for pIgR-GpA; ▨, pIgR-GpA G83A; , pIgA-GpA G83A/G79L. Results were scanned and quantified by NIH Image. Single clones were used for this experiment; for time points other than zero, n = 7. Error bars represent the SD. (b) Receptor-mediated transcytosis and recycling of [125I]dIgA. MDCK cells expressing pIgR-WT or the chimeras were grown on permeable supports for 4–5 d. Cells were pulsed with [125I]dIgA for 10 min at 37°C, washed, and then chased for the given times. Apical and basolateral media were collected at each time point and counted for [125I]dIgA. For each chimeric construct, three to four independent clones were tested in at least three separate experiments, and the results were combined; error bars represent the SD. The symbols are: □, pIgR-WT; •, pIgR-GpA; ▴, pIgR-GpA G83A; ▿, pIgA-GpA G83A/G79L. Transcytosis of [125I]dIgA is calculated as the percent cumulative apically released [125I]dIgA at each time point/total [125I]dIgA (apical + basal + intracellular). Recycling of [125I]dIgA is calculated as the percent basolaterally released [125I]dIgA at each time point/total [125I]dIgA.
Introduction of a single- or double-point mutation in the pIgR-GpA transmembrane domain, pIgR-GpA G83A and pIgR-GpA G83A/G79L, decreased the constitutive transcytosis at 30 min to 37% and 33%, respectively. In fact, the rate of transcytosis for the mutant chimeric receptors, with or without ligand, was comparable to the constitutive transcytosis of the pIgR-WT, suggesting that with the defect in dimerization the chimera was no longer stimulated in transcytosis by dIgA.
Transcytosis of [125I]dIgA Is Decreased for the Mutant pIgR-GpA Chimeric Constructs
We next determined the transcytosis of the ligand, [125I]dIgA, from the basal to the apical cell surface for all of the receptor constructs compared with the pIgR-WT. Transcytosis of ligand is a highly sensitive assay for transcytosis. A single cohort of [125I]dIgA was internalized for 10 min at 37°C followed by collection of both apical and basal media at 7.5, 15, 30, 60, and 120 min. Release of ligand into the apical media is the result of transcytosis, while release back into the basal media is indicative of receptor recycling.
Replacement of the pIgR TMD with the GpA TMD did not affect the ability of the chimera to bind and transcytose dIgA. Transcytosis of dIgA mediated by both the pIgR-WT and the pIgR-GpA reached 76% over a 2-h period (see Figure 4b upper panel). The nearly identical rates of transcytosis of [125I]dIgA by pIgR WT and pIgR-GpA strongly support our hypothesis that dimerization, induced either by dIgA binding or by the GpA TMD, is responsible for stimulation of transcytosis. Note that in this experiment only the transcytosis of pIgR carrying [125I]dIgA is relevant. This is in contrast to Figure 5a, which shows the transcytosis of biotinylated pIgR, where many of the biotinylated pIgR molecules are not bound to dIgA and are therefore not stimulated (Song et al., 1994b).
Figure 5.
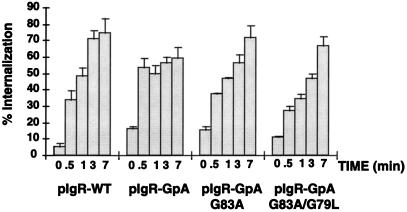
Receptor-mediated internalization of [125I]dIgA. MDCK cells expressing pIgR-WT or the chimeras were grown on permeable supports for 4 d. Cells were incubated at 4°C with [125I]dIgA at the basal surface for 1 h, washed, placed at 37°C for the given times, and then rapidly cooled back to 4°C. The cell surface was then stripped of remaining dIgA and the filters were counted. Calculations were based on the percent IgA internalized/total dIgA bound at time zero (stripped from the cell surface after internalization + dissociated into the basal media during warming at 37°C + internalized). Single clones were used for this experiment (n = 6), error bars represent the SD.
In contrast, the transcytosis of [125I]dIgA by the single- and double-point mutant receptors, pIgR-GpA G83A and pIgR-GpA G83A/G79L, was decreased to 64% and 60% over a 2-h time period, respectively. The reduction in the rate of transcytosis is consistent for a receptor that is unable to undergo stimulated transcytosis. In this experiment, the reduction in rate of transcytosis for pIgR-GpA G83A and pIgR-GpA G83A/G79L is 16% and 21% of pIgR-WT (or pIgR-GpA), respectively. Although this is not a large reduction, it is statistically significant and completely consistent with the magnitude of change for ligand-stimulated receptor transcytosis as determined by measurement of the biotinylated receptor (see Figure 4a). Twenty percent of the [125I]dIgA from both the pIgR-WT and the pIgR-GpA was recycled over the 2-h period (see Figure 4b, lower panel). In contrast, the recycling of [125I]dIgA by the single- and double-point mutant receptors, pIgR-GpA G83A and pIgR-GpA G83A/G79L, was increased to 28% and 34% over a 2-h time period, respectively. The decrease in transcytosis and the increase in recycling of the mutant chimeras compared with the pIgR-WT again suggest that dimerization of the receptor is a required event for the ligand-stimulated transcytosis of the pIgR.
Internalization of [125I]dIgA by the pIgR-Glycophorin A Chimeric Constructs Is Altered
The first step in transcytosis of ligand is internalization of the receptor from the cell surface. When assaying for the rate of transcytosis of ligand in the above experiment, the contribution of different rates of internalization is minimized. This is because after a 10 min-pulse at 37°C, the vast majority of the receptor is already internalized. Therefore, internalization of ligand by the pIgR-WT and the pIgR-GpA chimera constructs was directly measured by following a cohort of radiolabeled dIgA bound to the cell surface at 4°C. Cells grown on permeable supports were rapidly cooled to 4°C, followed by binding of ligand for 1 h. After extensive washing, filters were rapidly warmed to 37°C for 0–7 min to allow for internalization, and then rapidly cooled again to 4°C. The percent of internalized ligand was determined after stripping all remaining receptor from the cell surface. Although most of the chimeric receptors were delivered apically, sufficient receptor was present on the basolateral cell surface to permit analysis of the rate of ligand internalization. As shown in Figure 5, the pIgR-GpA chimera reached the plateau of internalization sixfold faster than pIgR-WT; i.e., approximately 30 sec for pIgR-GpA and by 3 min for pIgR-WT. In contrast, the plateau of internalization for the pIgR-GpA G83A and pIgR-GpA G83A/G79L chimeras was slower, suggesting a slower rate of internalization. The zero timepoint represents that population of receptor resistant to either stripping with low pH or trypsin without any warming from 4°C.
Polarized Trafficking in the Biosynthetic Pathway Is Independent of Dimerization
To determine the effect of dimerization on trafficking in the biosynthetic pathway, we compared the GpA chimeric constructs to the pIgR-WT in their polarized sorting from the TGN to the cell surface. For cells grown on permeable supports, the delivery from the TGN to the cell surface can be determined by the sensitivity of the receptor to protease added to the medium in contact with the basolateral surface. The cells are metabolically labeled and then chased for 1 h at 37°C in the continual presence of V8 protease in the basal media. If the receptor arrives at the basolateral PM, it is degraded and no longer detected by immunoprecipitation. The determination of basolateral delivery is based on the difference in the amount of receptor in the cell and released as SC at the apical surface from cells treated with or without V8 protease.
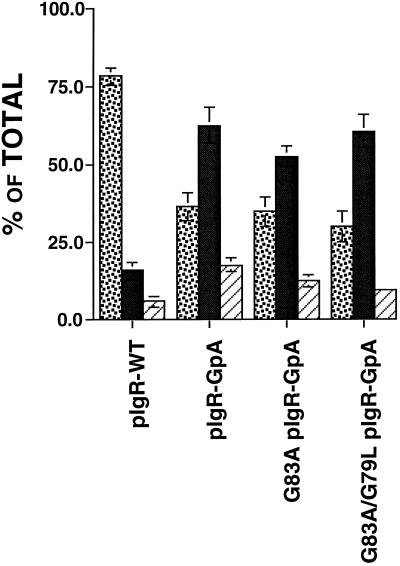
Consistent with previous reports, we find that 78% of the pIgR-WT is delivered directly to the basolateral membrane domain (Casanova et al., 1991; Aroeti et al., 1993) (see Figure 6). In contrast, as shown in Figure 6, delivery of pIgR-GpA from the TGN to the PM was altered, such that only 27% went directly to the basolateral PM domain, whereas 65% of the pIgR-GpA was delivered directly to the apical PM where it was released normally as SC. Importantly, the single- and double-point mutant chimeras, pIgR-GpA G83A and pIgR-GpA G83A/G79L, were also predominantly delivered from the TGN directly to the apical PM. As shown in Figure 6, for pIgR-GpA G83A and pIgR-GpA G83A/G79L, only 35% and 30% of the receptors were delivered directly to the basolateral domain, whereas 53% and 61% were delivered to the apical PM, respectively. Given the predominant apical delivery for both the pIgR-GpA and the dimerization mutant pIgR-GpA chimeras, it is most likely that the state of dimerization has no effect on the trafficking of the receptor in the biosynthetic pathway. Instead, these results suggest a role for the wild-type transmembrane domain of the pIgR in contributing to the targeting from the TGN to the basolateral domain.
Figure 6.
Delivery of the receptors from the TGN to the cell surface. MDCK cells were grown on permeable supports for 4–5 d to establish polarity. Cells were pulsed with [35S]Cysteine for 10 min, washed, and then chased for 1 h +/- V8 protease (1 μg/ml) in the basal medium. Apical, basal, and cell lysates were then collected, and subjected to immunoprecipitation with anti-SC antibody, analyzed by SDS-PAGE, and quantified by PhosphorImager. For each chimeric construct, three to four clones were tested in at least three separate experiments, and the results were combined. ░⃞, % basolateral; ▪, percent apical; ▨, percent intracellular. Error bars represent ± SEM.
DISCUSSION
We have used two disparate chimeric strategies to address different aspects of oligomerization of the pIgR. In the first approach a chimeric pIgR was created with the cytoplasmic domain of the TCR-ζ chain to determine whether ligand binding to the receptor induced receptor dimerization. The advantage to using the ζ chain is twofold. First, the activation of ζ is sensitive to the degree of oligomerization. This first became apparent through experiments with the Tac-ζ chimera. Antibody to Tac to cross-link either monomeric or dimeric chimera resulted in ζ signaling, indicating that it is not dimerization alone that is initiating activation, but rather the change in the state of ζ oligomerization (Letourneur and Klausner, 1991). Likewise, the addition of a secondary antibody or avidin to further cross-link the Tac-ζ chimera increased the activation of the ζ response (Letourneur and Klausner, 1991; Eiseman and Bolen, 1992b). We have also found a similar response at low concentrations of dIgA; the addition of anti-human IgA to cross-link the receptor increased the induction of ζ activation (Singer, unpublished). Second, the activation of ζ induces a catalytic response with a highly sensitive readout. This enabled us to detect interactions that may be transient and undetectable by other means. Our results clearly demonstrate that dIgA binding to the pIgR-ζ chimera induces its dimerization, and it is therefore quite likely that dIgA binding to pIgR-WT similarly causes dimerization.
In the second approach we used the GpA TMD and mutant forms of the GpA TMD as experimental tools to examine the effect of dimerization on four of the major intracellular trafficking pathways used by the pIgR: targeting from the TGN to the cell surface, transcytosis of the receptor, and the transcytosis and internalization of ligand. The mutant chimeric constructs represent receptors that are unable to form stable dimers and, as such, enable us to make predictions on the requirement for dimerization by the pIgR in these same assays. Comparing the trafficking of the pIgR-GpA with the nondimerizing point mutants also enabled us to separate the effects of dimerization per se from the effects of replacing the wild-type TMD of the pIgR with heterologous sequences. We find that transcytosis of the receptor and ligand and internalization are all stimulated by dimerization. In contrast, polarized delivery from the TGN to the basolateral or apical surface is affected by the TMD but is not dependent on dimerization, per se.
Our data are consistent with a model whereby the binding of dIgA to the pIgR induces the homotypic dimerization of the receptor, which then facilitates its transcytosis. The mechanism by which dimerization translates into stimulated transcytosis is not known; however, one likely mechanism is the induction of a signal transduction cascade. Signaling by the pIgR has been demonstrated to occur in response to dIgA and also to be required for stimulated transcytosis. As noted in the INTRODUCTION, the pIgR may signal through a novel mechanism, and our data on the role of dimerization in this are important in dissecting the first step in this pathway. It is also important to note that the primary function of pIgR is trafficking itself, and it is possible that pIgR dimerization may play a direct role in trafficking, independent of signal transduction.
We cannot absolutely exclude the possibility that the pIgR preexists as an inactive dimer in the absence of ligand, and that dIgA binding causes a conformational change or shift in equilibrium between monomer and dimer. However, our data on the activation of ζ activity in response to dIgA, which is highly sensitive to changes in oligomerization, are most consistent with a model whereby the binding of dIgA induces the receptor to dimerize, rather than binding to a preexisting dimer. Our data using the TMD of glycophorin to stabilize dimerization strongly suggests that dimerization in the absence of ligand is sufficient to drive transcytosis. We propose that stabilizing dimerization mimics the receptor with ligand bound. It should be noted that by replacing the TMD of the pIgR with one that is α helical we might have also simulated the conformation of the active ligand-bound receptor concomitant with a rotation in the cytoplasmic domain. However, as the mutant glycophorin TMDs contain only single or double amino acid residue mutations, these would not have been predicted to be so different in their secondary structure. Given this, we propose that in the case of the pIgR it is the dimerization per se that is important for the altered trafficking.
We also found that the binding of tIgA had a greatly enhanced effect on ζ activity, suggesting that the tIgA is binding to more than two receptors, which results in receptor oligomerization. Previous experiments on the transcytosis of tIgA found that it was transcytosed across MDCK cells at a slightly slower rate than dIgA. This may be due to slower movement of a larger complex in the plane of the membrane. Although it does not rule it out, our data suggest that stimulation of transcytosis is not via a mechanism dependent on a specific low-affinity interaction that is enhanced by multiple copies. Rather, it might suggest that both dimerization and further oligomerization have similar effects on a signal transduction cascade.
Our data also suggest that receptor dimerization is not only sufficient, but is also necessary for ligand-stimulated transcytosis. When we prevented dimerization through mutation of Gly83 or Gly83/Gly79 in the glycophorin transmembrane domain, we completely eliminated the stimulated rate of receptor transcytosis. Furthermore, we found that the rate of transcytosis of these nondimerizing receptors in the presence or absence of ligand was comparable to that of the unoccupied pIgR-WT. These finding were completely supported by the results from receptor-mediated transcytosis of [125I]dIgA. We found that the cells expressing either the wild-type pIgR or the pIgR-GpA gave almost identical rates of dIgA transcytosis. This is consistent with our prediction that the major effect of ligand binding is to facilitate dimerization, as such there should be no advantage in this assay given to the trafficking of dIgA by the pIgR-GpA chimera over the wild-type pIgR. In contrast, transcytosis of dIgA in cells expressing either of the nondimerizing pIgR-GpA chimeras was significantly slower. As these mutant receptors are unable to dimerize, or at least dimerize less well, we propose that they undergo little or no stimulation of transcytosis due to dIgA binding. Our results do not rule out that dIgA can bind and be transported by monomeric pIgR. In fact, the transcytosis of [125]I-dIgA by the pIgR-GpA G83A or pIgR-GpA G83A/G79L is indicative of this possibility. Importantly, our data are consistent with the hypothesis that in the absence of dimerization, receptor transcytosis is not stimulated and suggest that, under this condition, there is no alternative signal transduction from ligand binding.
Interestingly, the contribution of receptor dimerization to ligand- dependent signal transduction has recently become quite controversial. Although it is commonly believed that the binding of ligand induces receptor dimerization and that this dimerization is somehow necessary for signal transduction, there are increasing examples where this has been called into question. Studies involving the EGFR suggest that the high-affinity EGF receptor preexists as an inactive dimer at the cell surface (Gadella and Jovin, 1995). Subsequent binding of EGF is then required for the initiation of the signal transduction cascade. The authors suggest that it is not dimerization per se that mediates receptor activation, but rather a conformational change brought about through the binding of EGF. In contrast, Sorokin et al. (1994) found that cysteine residues in the receptor (added through mutational analysis) only became cross-linked subsequent to EGF binding, suggesting in this case that dimerization does not occur until after ligand has bound (Sorokin et al., 1994). However, it is not clear in their construct that self-association per se would have been enough to cause the cysteine disulfide bridge to form. Covalent cross-linking may be facilitated by the conformational change brought about by EGF binding. They also find that once cross-linked, the dimeric receptor was more active in phosphorylation of an exogenous substrate. Most recently, the role of receptor dimerization was challenged for the bacterial chemotaxis receptor for aspartate, Tar (Gardina and Manson, 1996; Stock, 1996; Tatsuno et al., 1996). In these studies Tar was genetically altered to form a dimer pair in which one of the partners had a truncated cytoplasmic domain. They found that with only a single cytoplasmic domain the dimeric receptor was still competent for signaling. These results called into question the necessity of dimerization of the cytoplasmic domain for signal transduction. These disparate results and models illustrate that the dimerization state of native receptors in the membrane, as well as the functional consequences of dimerization, can be very difficult to determine definitively. Additionally, they point out that the roles of dimerization of the receptor and of ligand binding to the receptor may be separate.
The endocytosis of the pIgR-WT occurs at a very rapid rate, half-time <1.5 min. Therefore it was surprising to find that the pIgR-GpA internalized even more rapidly, half-time< 30 sec. It is unclear why the plateau for GpA-pIgR is lower than pIgR-WT (60% vs. 75%); however, a higher affinity for the chimera might increase the efficiency of dIgA transcytosis. Apical media were not collected for analysis; therefore, any receptor/ligand that successfully transcytosed during the time of the assay would not be counted, thus lowering the plateau. Consistent with a role for dimerization in endocytosis, introduction of the point mutations in the GpA TMD decreased the rate of endocytosis to a half-time < 3 min. The changes in the rate of endocytosis of the various chimeras are unlikely to account for more than a small fraction of the changes in the rate of transcytosis. Previous mutations in the two tyrosine internalization signals of the pIgR led to great slowing of endocytosis, but very little effect on transcytosis (Okamoto et al. 1992).
In stark contrast to the effects of dimerization on endocytosis and transcytosis, we found that dimerization itself does not affect polarized sorting from the TGN to the apical or basolateral surface. Replacement of the transmembrane domain with the heterologous sequences of glycophorin A (dimerizing and nondimerizing mutants) disrupted the normal basolateral targeting of the pIgR. For all transcytotic trafficking events measured in this study, the mutant pIgR-GpA G83A and pIgR-GpA G83A/G79L chimeras were clearly distinct from the pIgR-GpA. Therefore, the finding that all of the chimeric constructs behave similarly in the biosynthetic pathway suggests that receptor dimerization is not the determinant for polarized sorting by the TGN, and as such indicates that the mechanism of apical delivery is distinct from transcytosis. These data suggest instead that either the wild-type pIgR TMD sequences contain targeting information necessary for basolateral targeting or that the presence of the heterologous GpA sequences disrupt the conformation of the basolateral targeting signal located within the cytoplasmic domain. Previous work led to the model that the membrane proximal 17 residues of the cytoplasmic domain of the pIgR contain a signal that is necessary for targeting the molecule from the TGN to the basolateral PM (Casanova et al., 1991; Aroeti et al., 1993). However, the ability of the basolateral targeting signal in the pIgR’s cytoplasmic domain to act independently of the TMD of pIgR was not investigated.
In conclusion, we have used a genetic approach to directly address the role of receptor dimerization in controlling receptor traffic. We provide clear evidence that dIgA binding to the pIgR causes receptor dimerization. Furthermore, using the superb tools of the structurally and genetically characterized GpA TMD, we can predictably force or abrogate dimerization and correspondingly stimulate or prevent ligand-stimulated transcytosis. This provides the clearest evidence presented to date that dimerization directly controls the trafficking of a membrane protein.
ACKNOWLEDGMENTS
We thank Professors J.-P. Vaerman for highly purified dIgA, mIgA, and tIgA and Art Weiss for kindly supplying all of the molecular reagents for the TCR-ζ chain and general assistance with the T-cells and luciferase assay received from his laboratory; Paul Dazin and the Howard Hughes Medical Institute for the expert assistance with flow cytometry; Kitty Tang for her excellent technical assistance; Gloria Pedro for laboratory support; Frédéric Luton and Steve Chapin for their thoughtful discussions throughout this project; and Frances Brodsky and Frédéric Luton for their critical reading of this manuscript. This work was supported by NIH grants R01 AI25144 and R01 AI36953. K.S. was supported by a grant from the Cystic Fibrosis Foundation (F770) and NIH Post Doctoral award (T32HL07731) from the Cardiovascular Research Institute.
Footnotes
Abbreviations used: dIgA, dimeric IgA; ECL, enhanced chemiluminescence; GpA, human glycophorin A; mIgA, monomeric IgA; pIgR, polymeric Ig receptor; PM, plasma membrane; SC, secretory component; tIgA, tetrameric IgA; TCR, T cell receptor TMD, transmembrane domain; WT, wild type.
REFERENCES
- Adair BD, Engelman DM. Glycophorin A helical transmembrane domains dimerize in phorpholipid bilayers: a resonance energy transfer study. Biochemistry. 1994;33:5539–5544. doi: 10.1021/bi00184a024. [DOI] [PubMed] [Google Scholar]
- Apodaca G, Enrich C, Mostov KE. The calmodulin antagonist, W-13, alters transcytosis, recycling, and the morphology of the endocytic pathway in MDCK cells. J Biol Chem. 1994;269:19005–19013. [PubMed] [Google Scholar]
- Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroeti B, Kosen PA, Kuntz ID, Cohen FE, Mostov KE. Mutational and secondary structural analysis of the basolateral sorting signal of the polymeric immunoglobulin receptor. J Cell Biol. 1993;123:1149–1160. doi: 10.1083/jcb.123.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann B-J, William JK, Marchesi WT. Synthetic peptides mimic the assembly of transmembrane glycoproteins. J Biol Chem. 1989;264:4033–4037. [PubMed] [Google Scholar]
- Brewer CB, Roth MG. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991;114:413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone MH, Smith BL, Mennitt PA, Mochly-Rosen D, Silver RB, Mostov KE. Signal transduction by the polymeric immunoglobulin receptor suggests a role in regulation of receptor transcytosis. J Cell Biol. 1996;133:997–1005. doi: 10.1083/jcb.133.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JE, Apodaca G, Mostov KE. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991;66:65–75. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- Challou N, Goormaghtigh E, Cabiaux V, Conrath K, Ruysschaert J. Sequence and structure of the membrane-associated peptide of glycophorin A. Biochemistry. 1994;33:6902–6910. doi: 10.1021/bi00188a020. [DOI] [PubMed] [Google Scholar]
- Chintalacharuvu KR, Morrison S. Production of secretory immunoglobulin A by single mammalian cell. Proc Natl Acad Sci USA. 1997;94:6364–6368. doi: 10.1073/pnas.94.12.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DH, Spits H, Peyron JF, Rowley RB, Bolen JB, Weiss A. The Syk protein tyrosine kinase can function independently of CD45 or Lck in T cell antigen receptor signaling. EMBO J. 1996;15:6251–6261. [PMC free article] [PubMed] [Google Scholar]
- Eiseman E, Bolen JB. Engagement of the high-affinity IgE receptor activates srcprotein-related tyrosine kinases. Nature. 1992a;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- Eiseman E, Bolen JB. Signal transduction by the cytoplasmic domains of Fc epsilon RI-gamma and TCR-zeta in rat basophilic leukemia cells. J Biol Chem. 1992b;267:21027–21032. [PubMed] [Google Scholar]
- Gadella Tw, Jr, Jovin TM. Oligomerization of epidermal growth factor receptors on A431 cells studied by time-resolved fluorescence imaging microscopy. A stereochemical model for tyrosine kinase receptor activation. J Cell Biol. 1995;129:1543–1558. doi: 10.1083/jcb.129.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardina PJ, Manson MD. Attractant signaling by an aspartate chemoreceptor dimer with a single cytoplasmic domain [see comments] Science. 1996;274:425–426. doi: 10.1126/science.274.5286.425. [DOI] [PubMed] [Google Scholar]
- Giffroy, D., Langendries, A., Maurice, M., Daniel, F., Lardeux, B., Courtoy, P.J., and Vaerman, J.-P. (1998). In vivo stimulation of polymeric Ig receptor-transcytosis by circulating polymeric IgA in rat liver. Int. Immunol. (in press). [DOI] [PubMed]
- Goldstein JL, Basu SK, Brown MS. Receptor-mediated endocytosis of low density lipoprotein in cultured cells. Methods Enzymol. 1983;96:241–259. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Hirt RP, Hughes GJ, Frutiger S, Michetti P, Perregaux C, Poulain-Godefroy O, Jeanguenat N, Neutra MR, J.-Kraehenbuhl P. Transcytosis of the polymeric Ig receptor requires phosphorylation of Serine 664 in the absence but not the presence of dimeric IgA. Cell. 1993;74:245–255. doi: 10.1016/0092-8674(93)90416-n. [DOI] [PubMed] [Google Scholar]
- Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Kraehenbuhl J-P, Neutra MR. Transepithelial transport and mucosal defence II: secretion of IgA. Trends Cell Biol. 1992;2:170–174. doi: 10.1016/0962-8924(92)90036-m. [DOI] [PubMed] [Google Scholar]
- Kuhn LC, Kraehenbuhl J-P. The sacrificial receptor-translocation of polymeric IgA across epithelia. Trends Biochem. 1982;7:299–302. [Google Scholar]
- Lee AW, Nienhuis AW. Functional dissection of structural domains in the receptor for colony-stimulating factor-1. J Biol Chem. 1992;267:16472–16483. [PubMed] [Google Scholar]
- Lee K, Hong K, Papahadjopoulos D. Recognition of liposomes by cells: in vitro binding and endocytosis mediated by specific lipid head groups and surface charge density. Biochim Biophys Acta, 1992;1103:185–197. doi: 10.1016/0005-2736(92)90086-2. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Flanagan JM, Hunt JF, Adair BD, Bormann BJ, Dempsey CE, Engelman DM. Glycophorin A dimerization is driven by specific interactions between transmembrane alpha-helices. J Biol Chem. 1992b;267:7683–7689. [PubMed] [Google Scholar]
- Lemmon MA, Glanagan JM, Treutlein R, Zhang J, Engelman DM. Sequence specificity in the dimerization of transmembrane α-helices. Biochemistry. 1992a;31:12719–12725. doi: 10.1021/bi00166a002. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Treutlein HR, Adams PD, Brunger AT, Engelman DM. A dimerization motif for transmembrane alpha-helices. Nat Struct Biol. 1994;1:157–163. doi: 10.1038/nsb0394-157. [DOI] [PubMed] [Google Scholar]
- Letourneur F, Klausner RD. T-cell and basophil activation through the cytopasmic tail of T-cell-receptor ζ family proteins. Proc Natl Acad Sci USA. 1991;88:8905–8909. doi: 10.1073/pnas.88.20.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie KR, Prestegard JH, Engelman DM. A transmembrane helix dimer: structure and implications. Science. 1997;276:131–133. doi: 10.1126/science.276.5309.131. [DOI] [PubMed] [Google Scholar]
- Mostov KE. Transepithelial transport of immunoglobulins. Annu Rev Immunol. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Cardone MH. Regulation of protein traffic in polarized epithelial cells. BioEssays. 1995;17:129–138. doi: 10.1002/bies.950170208. [DOI] [PubMed] [Google Scholar]
- Okamoto CT, S.-Shia P, Bird C, Mostov KE, Roth MG. The cytoplasmic domain of the polymeric immunoglobulin receptor contains two internalization signals that are distinct from its basolateral sorting signal. J Biol Chem. 1992;267:9925–9932. [PubMed] [Google Scholar]
- Rindisbacher L, Cottet S, Wittek R, Kraehenbuhl JP, Corthesy B. Production of human secretory component with dimeric IgA binding capacity using viral expression systems. J Biol Chem. 1995;270:14220–14228. doi: 10.1074/jbc.270.23.14220. [DOI] [PubMed] [Google Scholar]
- Smith SO, Bormann BJ. Determination of helix-helix interactions in membranes by rotational resonance NMR. Proc Natl Acad Sci USA. 1995;92:488–491. doi: 10.1073/pnas.92.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Apodaca G, Mostov K. Transcytosis of the polymeric immunoglobulin receptor is regulated in multiple intracellular compartments. J Biol Chem. 1994a;269:29474–29480. [PubMed] [Google Scholar]
- Song W, Bomsel M, Casanova J, Vaerman J-P, Mostov KE. Stimulation of transcytosis of the polymeric immunoglobulin receptor by dimeric IgA. Proc Natl Acad Sci USA. 1994b;91:163–166. doi: 10.1073/pnas.91.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Vaerman J-P, Mostov KE. Dimeric and tetrameric IgA are transcytosed equally by the polymeric immunoglobulin receptor. J Immunol. 1995;155:715–721. [PubMed] [Google Scholar]
- Sorokin A, Lemmon MA, Ullrich A, Schlessinger J. Stabilization of an active dimeric form of the epidermal growth factor receptor by introduction of an inter-receptor disulfide bond. J Biol Chem. 1994;269:9752–9759. [PubMed] [Google Scholar]
- Sternberg MJE, Gullick WJ. A sequence motif in the transmembrane region of growth factor receptors with tyrosine kinase activity mediates dimerization. Protein Eng. 1990;3:245–248. doi: 10.1093/protein/3.4.245. [DOI] [PubMed] [Google Scholar]
- Stock J. Receptor signaling: dimerization and beyond. Curr Biol. 1996;6:825–827. doi: 10.1016/s0960-9822(02)00605-x. [DOI] [PubMed] [Google Scholar]
- Tatsuno I, Homma M, Oosawa K, Kawagishi I. Signaling by the Escherichia coliaspartate chemoreceptor Tar with a single cytoplasmic domain per dimer [see comments] Science. 1996;274:423–425. doi: 10.1126/science.274.5286.423. [DOI] [PubMed] [Google Scholar]
- Treutlein HR, Lemmon MA, Engelman DM, Brunger AT. The glycophorin A transmembrane domain dimer: sequence-specific propensity. Biochemistry. 1992;31:12726–12733. doi: 10.1021/bi00166a003. [DOI] [PubMed] [Google Scholar]
- Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- Weiss A, Stobo JD. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984;160:1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]