Abstract
The ssp1 gene encodes a protein kinase involved in alteration of cell polarity in Schizosaccharomyces pombe. ssp1 deletion causes stress sensitivity, reminiscent of defects in the stress-activated MAP kinase, Spc1; however, the two protein kinases do not act through the same pathway. Ssp1 is localized mainly in the cytoplasm, but after a rise in external osmolarity it is rapidly recruited to the plasma membrane, preferentially to active growth zones and septa. Loss of Ssp1 function inhibits actin relocalization during osmotic stress, in cdc3 and cdc8 mutant backgrounds, and in the presence of latrunculin A, implicating Ssp1 in promotion of actin depolymerization. We propose a model in which Ssp1 can be activated independently of Spc1 and can partially compensate for its loss. The ssp1 deletion mutant exhibited monopolar actin distribution, but new end take-off (NETO) could be induced in these cells by exposure to KCl or to latrunculin A pulse treatment. This treatment induced NETO in cdc10 cells arrested in G1 but not in tea1 cells. This suggests that cells that contain intact cell end markers are competent to undergo NETO throughout interphase, and Ssp1 is involved in generating the NETO stimulus by enlarging the actin monomer pool.
INTRODUCTION
In the fission yeast, Schizosaccharomyces pombe, the stress-activated MAP kinase is Spc1 (synonymous with Sty1) (Millar et al., 1995; Shiozaki and Russell, 1995). Spc1 kinase is activated by a variety of different environmental insults, including high osmolarity, heat shock, oxidative and nutritional stress, UV light, some DNA-damaging drugs, and protein synthesis inhibitors. Regulation of Spc1 follows the generic MAP kinase cascade pattern, i.e., Spc1 is activated by a MAP kinase kinase called Wis1, which itself is activated by two MAP kinase kinase kinases, Win1 and Wik1 (the latter also isolated as Wak1 and Wis4; reviewed in Banuett, 1998). The two pathways converging on Wis1 appear to have different ranges of sensitivities to different forms of stress. In addition, activation of Spc1 independent of either of these two pathways has been suggested. Wik1 and possibly Win1 receive signals from Mcs4, a response regulator of the histidine kinase-containing two-component system, which transduces environment-related signals from the plasma membrane (Banuett, 1998). Activities of at least two transcription factors, Atf1 and Pap1, are dependent on Spc1. These proteins control expression of a number of stress-related genes as well as genes involved in meiosis and nutritional regulation (reviewed in Wilkinson and Millar, 1998). Stimulation of glycerol synthesis by Atf1-dependent induction of glycerol-3-phosphate dehydrogenase is the crucial event controlled by the Spc1 pathway in response to osmotic stress (Aiba et al., 1995).
Two negative regulators of the Spc1 protein kinase have been characterized, namely protein tyrosine phosphatases Pyp1 and Pyp2, that remove the phosphate group from Spc1 Tyr173 (Millar et al., 1995; Shiozaki and Russell, 1995). The tyrosine phosphatase activity is necessary to down-regulate the Spc1 pathway, and the pyp1 pyp2 double deletion is lethal (Ottilie et al., 1992). Pyp2 is regulated by Atf1 at the gene expression level and contributes to down-regulation of Spc1 via a negative feedback loop (reviewed in Banuett, 1998).
In many cell types, adaptive remodeling of the actin cytoskeleton is a vital component of the osmotic stress response. Mammalian cells, facing mostly mild fluctuations in osmolarity of the media, developed mechanisms allowing an increase or decrease in cell volume, leading to a decrease or increase in intracellular osmolarity, respectively. The early response is based on regulation of ion channels residing in the plasma membrane. An important component of this regulation is reversible reorganization of the cortical actin (Cantiello, 1997). Organisms exposed to wide swings in osmolarity in their natural environments have developed potent mechanisms for protection from osmotic damage. Cells of Dictyostelium discoideum reduce cell size and stiffen their cortex in response to hyperosmotic shock. During this process myosin II becomes phosphorylated in a cGMP-dependent manner and relocalizes to the cell cortex (Kuwayama et al., 1996). Also required is at least one of the two major actin cross-linking proteins, α-actinin and ABP120 (Rivero et al., 1996).
Yeast morphology is dominated by a rigid cell wall. Yeast cells maintain a substantial osmotic gradient across the plasma membrane that allows them to expand the cell wall by internal turgor pressure while new cell wall components are being incorporated (Mulholland et al., 1994). During interphase, filamentous actin in both budding and fission yeast appears in two morphologically distinct forms: discrete patches and continuous cables (reviewed in Robinow and Hyams, 1989; Botstein et al., 1997). Actin patches accumulate at the sites of cell wall growth and have been shown to associate directly with the plasma membrane (Mulholland et al., 1994). When the osmolarity gradient is reversed by hyperosmotic shock, actin is thought to support the cell surface, at least during the period before cells can produce enough glycerol to reestablish a sufficient osmotic gradient and resume growth. This view is supported by three types of arguments. First, during exposure to hyperosmotic medium, actin patches in Saccharomyces cerevisiae undergo profound but reversible redistribution from growing buds (Chowdhury et al., 1992). Second, many conditional actin mutants and mutants in proteins involved in actin organization in S. cerevisiae are osmosensitive (reviewed in Botstein et al., 1997). Third, mutants in three genes, RAH1–3, have been isolated that specifically rescue osmosensitivity in actin mutants (Chowdhury et al., 1992).
Little is known about the signals responsible for actin reorganization in yeasts following osmotic shock. The S. pombe gene ssp1 encodes a serine/threonine protein kinase previously shown to be required for alteration of growth polarity and actin localization at high temperature (Matsusaka et al., 1995). The ssp1 gene was obtained as a suppressor of the ppe1 and sts5–7 mutant strains. The ppe1 gene encodes a type 6 protein phosphatase (Shimanuki et al., 1993). The exact function of the Sts5 protein is unknown, but its mutation confers supersensitivity to the protein kinase C inhibitor staurosporin (Toda et al., 1996). Both ppe1 and sts5 cells are characterized by spherical cell shape and loss of actin polarity, at least under certain conditions. ssp1 mutants are unable to undergo transition from monopolar to bipolar growth (new end take-off [NETO]) and delay cell cycle progression into mitosis (Matsusaka et al., 1995). Two conditions seem necessary for commitment of cells to NETO: completion of DNA synthesis and critical cell length (Mitchison and Nurse, 1985). Thus, two opposing mechanisms, one stabilizing and the other destabilizing cortical actin architecture, have been postulated to regulate actin dynamics in a cell cycle-dependent manner (Matsusaka et al., 1995).
Here we show that the ssp1 mutant exhibits stress response phenotypes reminiscent of mutants in the Spc1 stress-activated MAP kinase pathway but that Ssp1 can act independently of Spc1. After a rise in external osmolarity, Ssp1 is recruited to the proximity of the plasma membrane and is involved in promoting actin reorganization. In addition, Ssp1 is involved in controlling the release of free actin monomers, and we propose a model in which Ssp1 can partially compensate for the loss of the Spc1 MAP kinase. Last, we demonstrate that release of free actin monomers is a sufficient stimulus to promote NETO in S. pombe cells regardless of their DNA content.
MATERIALS AND METHODS
Strains and Media
All S. pombe strains used in this work (Table 1) were derived from wild-type strains 972 h−S or 975 h+N (Leupold, 1970). Strains were grown in yeast extract medium containing adenine (YEA complex medium) or Edinburgh minimal medium (EMM) containing nutritional supplements when necessary (Alfa et al., 1993). Low pH media were prepared as described in Saleki et al. (1997).
Table 1.
S. pombe strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| Q868 | h−leu1-32 | Lab collection |
| Q431 | h+leu1-32 | Lab collection |
| Q1528 | h+ssp1∷sup3-5 leu1-32 ade6-704 | This study |
| KS1366 | h−spc1∷ura4 leu1-32 ura4-D18 | Shiozaki and Russell (1995) |
| Q1540 | h−ssp1∷sup3-5 spc1∷ura4 leu1-32 ade6-704 ura4-D18 | This study |
| Q1521 | h−cdc25-22 leu1-32 | This study |
| Q353 | h+wee1-50 cdc25-22 ade6-M210 | Lab collection |
| Q1541 | h−ssp1∷sup3-5 wee1-50 cdc25-22 ade6-704 leu1-32 | This study |
| Q1518 | h−cdc10-129 leu1-32 | This study |
| Q1532 | h−leu1-32/pRO6-1 (nmt∶ssp1 LEU2) | This study |
| Q1590 | h+leu1-32/pZA69 (nmt∶GFP LEU2) | This study |
| Q1591 | h+leu1-32/pIR2-22 (nmt∶GFP-ssp1 LEU2) | This study |
| Q1592 | h−leu1-32/pRUP5 (nmt∶GFP LEU2) | This study |
| Q1593 | h−leu1-32 ura4-D18/pIR8 (nmt∶ssp1-GFP LEU2) | This study |
| Q1576 | h−cdc10-129 leu1-32/pIR2-22 (nmt∶GFP-ssp1 LEU2) | This study |
| Q1577 | h−cdc25-22 leu1-32/pIR2-22 (nmt∶GFP-ssp1 LEU2) | This study |
| Q207 | h+cdc3-6 | Nurse et al. (1976) |
| Q1535 | h+ssp1∷sup3-5 cdc3-6 | This study |
| Q209 | h+cdc8-27 | Nurse et al. (1976) |
| Q1536 | h+ssp1∷sup3-5 cdc8-27 | This study |
| Q1580 | h−spc1∷ura4 leu1-32 ura4-D18/pIR2-22 (nmt∶GFP-ssp1 LEU2) | This study |
| Q910 | h−pyp1∷ura4 leu1-32 ura4-D18 | Sabine Ottilie |
| Q1592 | h+pyp1∷ura4 leu1-32 ura4-D18/pIR2-22 (nmt∶GFP-ssp1 LEU2) | This study |
| JM101 | h+tea1∷ura4 ura4-D18 ade6-M210 | Mata and Nurse (1997) |
| Q1589 | h−tea1∷ura4 ura4-D18 ade6-M210/pIR2-22 (nmt∶GFP-ssp1 LEU2) | This study |
| TP235-2C | h−ssp1-11 leu1-32 | Matsusaka et al. (1995) |
Cloning of the ssp1 Gene
Standard molecular biological and genetic techniques were used (Moreno et al., 1991; Alfa et al., 1993). To clone the ssp1 gene, the strain carrying the mutant ssp1 allele was transformed with a wild-type genomic library in the pWH5 vector (P.G. Young and D. Beach, unpublished observations), and transformants were selected on EMM plates, pH 3.5, at 35°C. The locus was confirmed by integration mapping and a complementation test against ssp1–11. Sequence data showed the gene to be identical with ssp1 (Matsusaka et al., 1995).
Construction of the ssp1Δ Allele
A 1.2-kb XhoI–NheI region in the ssp1 gene carrying plasmid was replaced with the 1.0-kb sup3–5 fragment. The resulting disruption plasmid contained only 15% of the ssp1 ORF and was not capable of rescuing the ssp1 point mutation. Stable integrants of this plasmid were selected and sporulated from an ade6–704 diploid strain and 2:2 cosegregation of the low pH sensitivity phenotype, and ade6–704 allele suppression was confirmed by tetrad analysis of the progeny. The existence of an ssp1 deletion in two haploid progeny was confirmed by Southern blot hybridization.
Overproduction of Ssp1 and Green Fluorescent Protein-Ssp1 Fusion Proteins
A DNA fragment containing the ssp1 ORF flanked by the NdeI and XmaI restriction sites, which were added by PCR, was inserted between the corresponding sites of the pREP1 expression vector. The pREP1 vector carries the thiamine-repressible nmt1 promoter and the LEU2 selectable marker (Maundrell, 1993). The construct, designated pRO6–1, was transformed into S. pombe strains carrying the leu1–32 auxotrophic marker, and positive transformants were selected on EMM plates lacking leucine and containing 4 μM thiamine. The Ssp1 overproduction phenotype was assessed in cells growing overnight in EMM lacking thiamine.
To construct the N-terminal Green Fluorescent Protein (GFP)-Ssp1 fusion, a DNA fragment containing the ssp1 gene flanked with SalI and XmaI restriction sites in the proper reading frame was inserted into the pZA69 vector containing the nmt1 promoter-controlled gene encoding the GFP S65T protein (Helm et al., 1995), followed by the multiple cloning site and the LEU2 selection marker. The resulting construct was designated pIR2–22. The assessment of the resulting phenotype in S. pombe cells was performed as above.
To verify that the location of the GFP tag does not affect Ssp1 function, the Ssp1-GFP C-terminal fusion was constructed as follows. First, the NdeI restriction site residing in the GFP ORF was removed by a silent mutation created by overlap extension PCR (Horton et al., 1993). The resulting fragment carrying the BamHI site at the 5′-terminus and the XmaI site at the 3′-terminus in the proper reading frame, which were added by PCR, was then inserted between the corresponding sites of the pREP1 vector. The resulting construct, pRUP5, was used to create the Ssp1-GFP fusion. The ssp1 ORF flanked by the NdeI and SalI sites generated by PCR was inserted between the corresponding sites of the pRUP5 vector. The final construct, designated pIR8, was transformed into S. pombe, and the overproduction phenotype was assessed as above. It was found that the phenotypes and GFP fluorescence of both pIR2–22 and pIR8 harboring wild-type and ssp1Δ cells were identical, and therefore only the pIR2–22 construct was used for detailed analysis.
Treatment with Latrunculin A
The whole procedure was performed at a constant temperature. Cells were first grown in 20 ml YEA precultures. To minimize the impact of the transfer into fresh media, part of the preculture was collected by centrifugation, and the supernatant was saved, maintained at the same temperature, and used later as the preconditioned medium for the release from the drug. Latrunculin A (Molecular Probes, Eugene, OR) was added to 0.2–1 ml cultures, which were taken from the same precultures, to a final concentration of 10 or 20 μM from a 10 mM stock solution in DMSO. Cells were incubated with the drug for up to 2 h. To release cells from the drug, cells were washed once with 1 ml of the preconditioned medium and resuspended in the initial volume of the same medium. Aliquots (25 μl) were collected at desired timepoints, and cells were fixed and stained for actin as described below.
Calcofluor and Actin Staining
Cells were stained essentially as described (Rupes̆et al., 1997). Cells from 1 ml of liquid culture were collected into 50 μl 0.6 M sorbitol and stained with an equal volume of 5 mg/ml Calcofluor White M2R (Polysciences, Warrington, PA) for 5 min, washed with 0.6 M sorbitol, and observed under the microscope. During experiments using latrunculin A, cells were fixed with 7.4% formaldehyde in PEM (Rupes̆et al., 1997) for 7 min, washed once with PEM, permeabilized with Triton X-100, washed two times with PEM, and stained with TRITC–phalloidin as above. A Leica DMRB (Leica Microsystems, Nussloch, Germany) fluorescence microscope and a Meridian laser scanning confocal microscope (Genomic Solutions, Ann Arbor, MI) equipped with the MCID (Imaging Research, St. Catharines, Canada) image analysis software were used for observations.
Fluorescence-activated Cell Sorter Analysis
Fluorescence-activated cell sorter (FACS) analysis was performed according to the protocol of Alfa et al. (1993) using a Coulter (Hialeah, FL) Epics Elite flow cytometer.
RESULTS
Isolation and Identification of ssp1
S. pombe strains carrying ura4-D18 or leu1–32 auxotrophic markers are nonviable at high external pH (Saleki et al., 1997; Karagiannis et al., 1999). In a search aimed primarily at identifying new elements involved in transport across the plasma membrane and in pH homeostasis, mutants rescuing this phenotype were obtained. One of these mutants displayed a distinct temperature-sensitive cell division cycle defect when grown at low pH (Saleki et al., 1997). After outcrossing to the wild-type background, mutant cells proliferated on minimal media at pH 3.5–6.5 at 25°C. On shifting to 35°C at pH 3.5, the cells were unable to form colonies and arrested as single elongated cells. To test whether the low pH sensitivity of this strain was due to intracellular acidification, cells were acid-loaded by the addition of acetic acid to medium with a final pH of 5.5. This medium caused cell cycle arrest in the mutant strain but not in the wild type, suggesting that the mutant was indeed sensitive to intracellular acidification. The gene was cloned by complementation of the low pH sensitivity phenotype, and subsequent sequencing revealed its identity as ssp1, a gene encoding a protein kinase previously shown to be involved in alteration of growth polarity and actin localization (Matsusaka et al. 1995).
ssp1Δ Has an Overlapping Phenotype with spc1Δ
In accordance with previously published results (Matsusaka et al. 1995), we found that the ssp1 gene is nonessential under normal growth conditions. The deletion (see MATERIALS AND METHODS) caused cells to arrest at low pH and high temperature (Figure 1A). The presence of leu1− or ade6− did not alter the phenotype. In the presence of the ura4-D18 mutation, ssp1Δ cells became less viable when exposed to suboptimal conditions, and therefore ura4− strains were excluded from further analysis. To determine whether the low pH sensitivity of the ssp1Δ cells may be a part of a broader stress response deficiency, we tested the ssp1Δ cells in parallel to mutants carrying the spc1 gene deletion (spc1Δ). Both ssp1Δ and spc1Δ cells proliferated well in complex media at 30°C. On temperature shift from 30 to 35°C, the doubling period of the ssp1Δ cells was only 15% longer than that of the wild type. Both ssp1Δ and spc1Δ cells, however, delayed entry into mitosis relative to wild type, as can be monitored by increased cell length at septation (Figure 1B). In complex media, a temperature shift-up caused a larger delay in ssp1Δ than in spc1Δ. Minimal media prolonged the delay in both strains, suggesting that ssp1Δ as well as spc1Δ (Shiozaki and Russell, 1995) may be sensitive to nutritional conditions. The effect of nutrient limitation was more obvious after the cells were shifted to medium lacking a nitrogen source. On depletion for nitrogen, wild-type cells transiently accelerate their cell division rate and enter a stationary phase with reduced cell size (Fantes, 1984; Young and Fantes, 1987). In contrast, under the same conditions both ssp1Δ and spc1Δ cells remained distinctly elongated (our unpublished results).
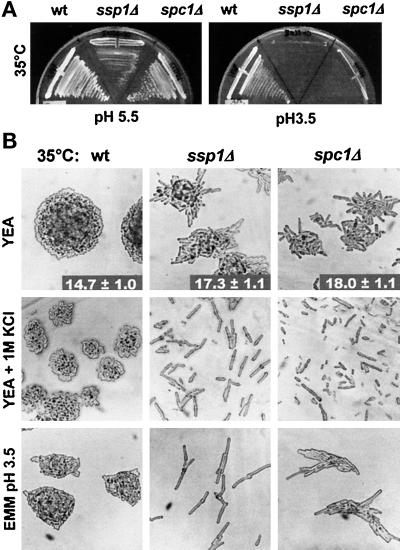
Figure 1.
ssp1Δ and spc1Δ cells are hypersensitive to KCl and low pH at 35°C. (A) ssp1Δ and spc1Δ cells are unable to form colonies at low pH. Strains Q868 (wt), Q1528 (ssp1Δ), and KS1366 (spc1Δ) were grown on EMM at 30°C overnight and then streaked onto fresh EMM plates, pH 5.5, or EMM plates, pH 3.5, and incubated overnight at 35°C. (B) High osmolarity and low pH induce mitotic delay or arrest in both ssp1Δ and spc1Δ cells. The same strains as in A were grown on YEA complex medium at 30°C overnight and then streaked onto fresh YEA and YEA + 1 M KCl plates and incubated overnight at 35°C. To assess the effect of low pH, cells were treated as in A. The numbers represent cell sizes at septation in μm ± SD (n > 100).
We tested whether spc1Δ was also sensitive to low external pH. When the pH of minimal media was lowered, spc1Δ cells became more elongated but were still able to proliferate; however, when plated on EMM at pH 3.5 and incubated at 35°C, their growth became severely impaired (Figure 1A), although they were still able to form microcolonies of highly elongated cells (Figure 1B).
Mutants defective in the Spc1 protein kinase pathway are highly sensitive to elevated concentrations of KCl (Millar et al., 1995; Shiozaki and Russell, 1995). ssp1Δ cells were unable to proliferate on plates containing 1 M KCl at 35°C (Figure 1B), and they did not adapt even if the exposure to KCl was preceded by prolonged incubation at 35°C. When tested over a range of KCl concentrations, ssp1Δ had a less severe KCl sensitivity than spc1Δ. The inhibitory effect of KCl on ssp1Δ cells was temperature dependent and resulted in complete failure to enter mitosis at 35°C; at lower temperatures ssp1Δ cells were still distinctly elongated. In contrast, the response of spc1Δ cells to increasing KCl concentrations did not markedly change with temperature. We conclude that although the degree of sensitivity varies under different conditions, both ssp1- and spc1-deficient cells respond to environmental challenges in a closely related manner by delaying entry into mitosis or with complete G2/M arrest.
ssp1 and spc1 Respond to Stress through Different Pathways
To find out whether SspI may act through the Spc1 kinase pathway, we constructed a double-deletion mutant, ssp1Δ spc1Δ. When plated on YEA complex medium containing 0.6 M KCl and incubated at 30°C, i.e., conditions permissive for both single mutants, the ssp1Δ and spc1Δ mutations exhibited profound negative interaction. The double mutant was unable to proliferate and arrested as single elongated cells (Figure 2A). Similar arrest was caused by incubation on minimal media at pH 4.5 at 30°C, again conditions permissive for both single mutants (our unpublished results).
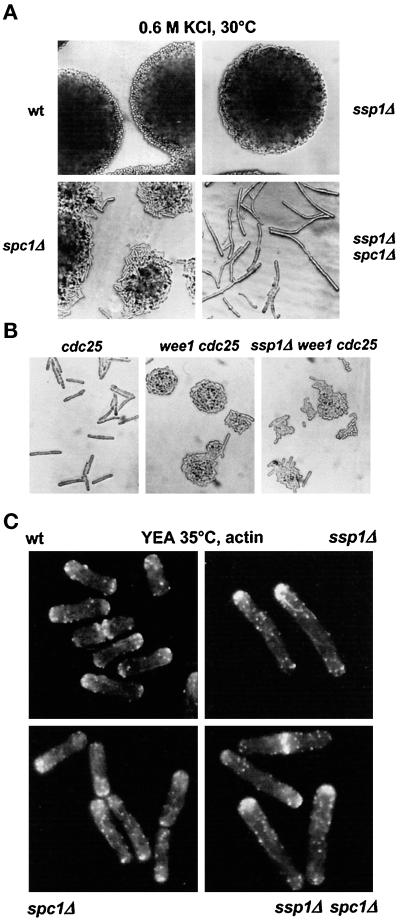
Figure 2.
SspI and Spc1 act through independent pathways. (A) Strains Q868 (wt), Q1528 (ssp1Δ), KS1366 (spc1Δ), and Q1540 (ssp1Δ spc1Δ) were grown on YEA complex medium at 30°C overnight and then streaked onto fresh YEA + 0.6 M KCl plates and incubated for 24 h at 30°C. (B) Strains Q1521 (cdc25–22ts), Q353 (wee1–50ts cdc25–22ts), and Q1541 (ssp1Δ wee1–50ts cdc25–22ts) were picked from overnight 25°C YEA plates, streaked onto fresh YEA plates, and incubated overnight at 35°C. (C) The same strains as in A were grown in liquid YEA cultures for 6 h and then stained with TRITC–phalloidin to visualize filamentous actin.
Wee1 and Cdc25 are the key negative and positive mitotic regulators, respectively, that affect the level of Tyr15 phosphorylation on Cdc2 protein kinase (MacNeill and Nurse, 1997). spc1− reverts the suppression of the conditional thermosensitive (ts) cdc25–22 phenotype by wee1–50ts (Shiozaki and Russell, 1995). In contrast, the presence of ssp1Δ in the wee1–50ts cdc25–22ts genetic background did not cause substantial mitotic delay, although some cells were longer than the wee1–50ts cdc25–22ts double mutant (Figure 2B). Consistent with this result, wee1–50ts rescues the cell cycle delay in the ssp1Δ mutant but not its stress sensitivity (our unpublished results). These data strongly suggest that Ssp1 and Spc1 protein kinases do not impinge on mitotic control through the same pathway.
Actin patches in the ssp1Δ cells grown in complex media at 35°C appeared consistently brighter and more concentrated at the growing tips relative to wild type (Figure 2C). By contrast, actin patches were more dispersed outside the polar zones in the spc1Δ cells compared with wild type. In the double mutant, cells were largely monopolar with actin patches highly concentrated at the poles as in the ssp1Δ single mutant, although visually the distribution appeared to be slightly more relaxed (Figure 2C). On the basis of these data, we propose that Ssp1 and Spc1 can respond to stress through different pathways.
Localization of Ssp1 in Cells
The native level of ssp1 expression is too low to allow its localization to be detected by fluorescence microscopy (Matsusaka et al., 1995). Wild-type cells were therefore transformed with an expression vector carrying the ssp1 gene placed under the control of the thiamine-repressible nmt1 promoter (see MATERIALS AND METHODS). In the absence of thiamine, cells grown on plates became shorter and more rounded and had actin patches noticeably less polarized to the tips than control cells harboring the vector alone (Matsusaka et al., 1995; our unpublished results). To monitor the localization of Ssp1 in cells, we constructed a GFP-Ssp1 fusion under the control of the nmt1 promoter. When expressed, the resulting phenotype matched that of cells overproducing the untagged version of Ssp1. Also, the presence of the GFP-Ssp1 fusion protein was able to rescue the NETO defect, cell elongation phenotype, and the KCl sensitivity of the ssp1Δ mutant at 35°C (our unpublished results). These results indicate that the GFP-tagged version of Ssp1 remained functional.
GFP-Ssp1 overproduction generated a heterogenous population of cells. A small fraction of cells grown in liquid culture (∼5%) had an extremely bright fluorescent signal localized to the cytoplasm and a rim of fluorescence along the cell surface, suggesting that a fraction of the Ssp1 protein localizes to or near the plasma membrane during strong GFP-Ssp1 overexpression. These cells were oval or spherical and contained depolarized actin patches. The majority of cells, however, fluoresced only moderately and retained their normal or near-normal rod-like shape. Confocal microscopy showed that GFP-Ssp1 was distributed throughout the cytoplasm, excluded from the nucleus and vacuolar compartments, and with a faint signal at the plasma membrane, mostly and in some cells exclusively at polar zones (Figure 3A). During cell division, a strong GFP-Ssp1 signal was associated with both forming and completed septa.
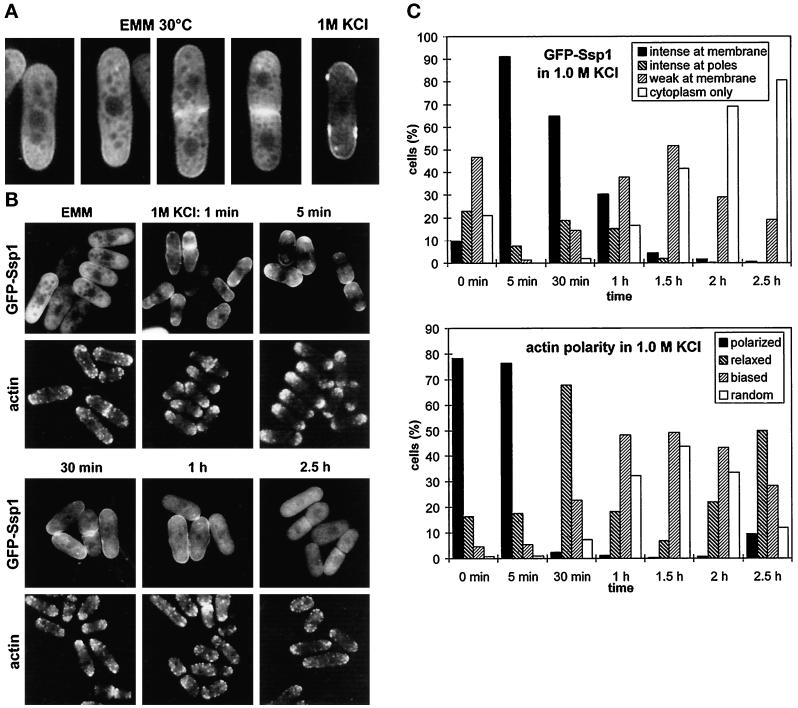
Figure 3.
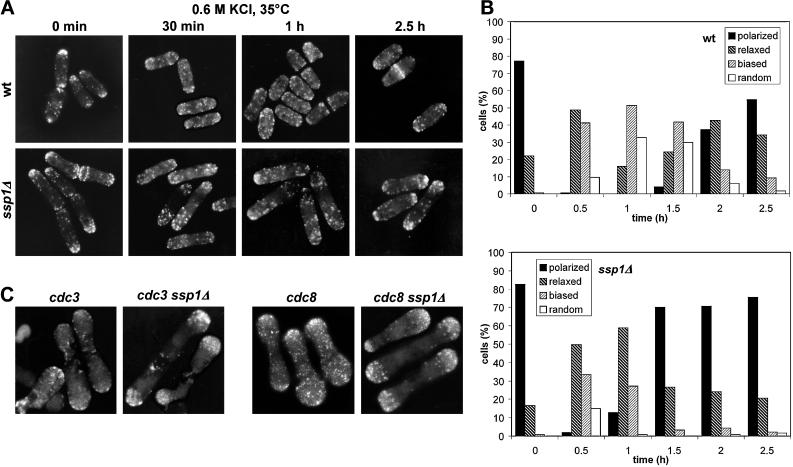
Cellular localization of GFP-SspI. (A) Strain Q1591 (wild type harboring the pIR2–22 plasmid) was grown in EMM lacking thiamine (EMM, 30°C) and then transferred to EMM + 1 M KCl. A sample was collected from this culture after 10 min of incubation at 30°C. Cells were analyzed by confocal microscopy. (B) The same strain was grown in EMM as in A and then shifted to EMM + 1 M KCl and incubated at 30°C. Cells were taken at the indicated times, observed to localize GFP-Ssp1, and also fixed and stained with TRITC–phalloidin to visualize filamentous actin. Images were taken using the fluorescence microscope. (C) Quantitative scoring of the cell population in experiment B. The presence of GFP-Ssp1 at the plasma membrane (top graph) was scored in living cells; the polarity of actin patches (bottom graph) was blind-scored in cells fixed and stained for actin (n ≥ 200). Intense at membrane: distinct rim of fluorescence along the surface. intense at poles: distinct only at poles, none or threshold of detection in the middle; weak at membrane: weak rim all over the surface or at poles only; polarized: wild-type aggregations of actin patches at cell ends; relaxed: relaxed aggregations, more patches outside poles; biased: patches all over the cell surface but bias toward the poles maintained; random: completely random distribution.
When cells were exposed to 1 M KCl, GFP-Ssp1 protein became largely associated with the plasma membrane, primarily at the polar zones (Figure 3A, right panel). In addition, many cells exhibited multiple foci of increased fluorescence under the cell surface near the poles. The GFP-Ssp1 localization at the plasma membrane was apparent as early as 1 min after the shift to KCl and persisted for at least 1 h with slowly diminishing intensity. After 1 h of exposure to KCl the plasma membrane localization of GFP-Ssp1 lost its preference for the poles, and after 2.5 h it completely relocalized back to the cytoplasm (Figure 3, B and C). A similar transient relocalization of the GFP-Ssp1 protein was triggered by the shift of cells into media containing 1.2 M sorbitol, confirming that the change was caused by the increase in external osmolarity (our unpublished results). A striking temporal correlation was revealed by comparing changes in GFP-Ssp1 localization to reorganization of the actin cytoskeleton. After exposure to KCl, actin patches started to disappear gradually from cell poles and septal zones until after 1 h they became virtually randomly distributed along the cell surface. At the time when the GFP-Ssp1 signal disappeared from the plasma membrane (2.5 h), actin patches accumulated back at the poles, although now they were not as tightly packed as in untreated cells (Figure 3, B and C). This distribution remained unchanged for at least another 2.5 h of incubation. This experiment was reproduced at 30°C as well as 35°C with similar results. The correlation between the changes in GFP-Ssp1 and actin relocalization was also confirmed by double fluorescence. We observed, however, that the addition of formaldehyde immediately triggered the stress-related GFP-Ssp1 shift to the membrane, and therefore only the GFP-Ssp1 signal in living cells is presented.
The cdc10–129ts mutant arrests at START before DNA synthesis but continues extension growth in a monopolar manner. In contrast, the cdc25–22ts mutant arrests in G2 and grows at both ends (Marks et al., 1986). If the sites of Ssp1 stress-induced localization represent the sites of extension growth, the prediction would be that Ssp1 will localize preferentially at one end in cdc10–129ts and at both ends in cdc25–22ts cells. Overproduction of the GFP-Ssp1 protein in these cells, however, had an effect on the normal progression through NETO. In most of the cdc25–22ts cells arrested in G2, only an abnormally small contribution to the total cell length was made by extension at the new end, suggesting that NETO was delayed in these cells (Figure 4A). On the other hand, the majority of the cdc10–129ts cells did progress through NETO, despite their cell cycle arrest. In both strains Ssp1 was preferentially localized to both cell ends. Another mutant normally unable to initiate growth at the new end is tea1Δ (Mata and Nurse, 1997). Overexpression of GFP-Ssp1 protein did not promote NETO in these cells, and the fluorescence signal at the plasma membrane was associated mainly, although not exclusively, with the single growing end (Figure 4A).
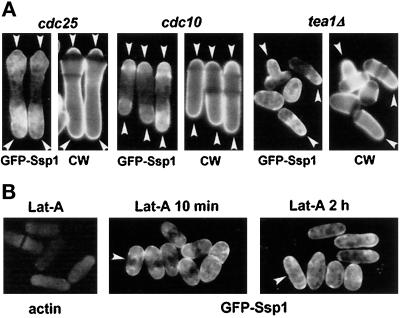
Figure 4.
Polarity of the GFP-Ssp1 localization to the plasma membrane. (A) Strains Q1576 (cdc10–129ts) and Q1577 (cdc25–22ts), both harboring the pIR2–22 plasmid, were grown in EMM lacking thiamine at 25°C overnight, then shifted to 36°C for 4 h. Cells were then collected, stained with Calcofluor for 2 min to visualize cell wall (CW), transferred to EMM + 1 M KCl, and incubated for another 10 min at 36°C. Strain Q1589 (tea1Δ, harboring the pIR2–22 plasmid), was maintained at 30°C and treated as above. Arrowheads mark sites of extension growth. (B) The presence of actin at growth zones is not required for Ssp1 localization. Cells (the Q1591 strain) were grown at 30°C overnight in EMM lacking thiamine and then incubated in the presence of 20 μM latrunculin A for 10 min. A sample was stained for actin as a control that actin patches had disappeared (Lat-A, actin); the rest was exposed to 1 M KCl in EMM containing 20 μM latrunculin A, and a sample was observed (Lat-A 10 min). Another sample was taken after another 2 h of incubation in the presence of latrunculin A (Lat-A 2 h). After 2 h Ssp1 lost its preference for cell poles (arrowheads).
Next, we wondered whether the presence of cortical actin patches is required for Ssp1 localization in stressed cells. Latrunculin A is a potent inhibitor of actin polymerization in yeast (Ayscough et al., 1997). Cells were first treated with latrunculin A for 10 min to allow all actin patches to disappear (Figure 4B, left panel). When these cells were subsequently stressed with KCl, still in the presence of latrunculin A, Ssp1 localized to the cell surface proximal to the poles (Figure 4B, middle panel). This suggests that the presence of actin at growth sites is not absolutely required for normal localization of Ssp1; however, when cells were treated with latrunculin A for 2 h and then exposed to KCl, Ssp1 localized almost evenly along the cell surface (Figure 4B, right panel). This indicates that an actin-dependent process or structure is important for Ssp1 localization. The simplest explanation of these data is that the Ssp1 protein kinase responds to osmotic stress by relocalization to the plasma membrane, preferentially to or near active growth zones, where it takes part in the reorganization of actin cytoskeleton.
Ssp1 Is Involved in Actin Depolymerization
To obtain further evidence supporting a causal relationship between Ssp1 function and actin reorganization, we examined the effect of ssp1 deletion on actin reorganization during osmotic stress. We induced stress-related actin movements in cells that were grown in YEA complex medium at 35°C for 4 h and subsequently stressed by addition of KCl to a final concentration of 0.6 M (Figure 5, A and B). In the ssp1Δ strain, the initial incubation at 35°C generated a monopolar population of cells. After the addition of KCl, actin patches were partially released from the poles, but after 30 min they started gradually to relocalize at the poles again. Throughout the duration of the experiment, the distribution of actin patches maintained a noticeable bias toward the poles in the majority of the ssp1Δ cells. Wild-type cells, on the other hand, reached more or less random distribution of actin patches at 1 h in the presence of KCl and only then started to repolarize. When the experiment was repeated with the concentration of KCl raised to 1 M, depolarized actin patches appeared in ssp1Δ cells within 5 h after the shift, along with a number of dead or dying cells, whereas wild-type cells recovered similarly as at the lower KCl concentration. These data suggest that Ssp1 is required to allow relocalization of actin at high temperature.
Figure 5.
Ssp1 is involved in actin depolarization. (A) ssp1Δ cells exhibit delayed actin redistribution after osmotic stress. Strains Q868 (wt) and Q1528 (ssp1Δ) were grown in YEA complex medium at 35°C for 4 h and then exposed to 0.6 M KCl in the same medium and incubated further at 35°C. Samples were stained with TRITC–phalloidin to visualize filamentous actin. (B) Blind scoring of actin polarity in cells from experiment A. For explanation of the categories see Figure 3C (n ≥ 200). (C) The presence of the ssp1Δ allele inhibits actin redistribution in the cdc3 and cdc8 mutants. Strains Q207 (cdc3–6ts), Q1535 (cdc3–6ts ssp1Δ), Q209 (cdc8–27ts), and Q1536 (cdc8–27ts ssp1Δ) were grown in YEA complex medium at 25°C overnight and then shifted to 35°C for 4 h. Samples were stained for filamentous actin.
If SspI acts on actin, directly or indirectly, loss of SspI function should also inhibit actin relocalization caused by factors other than stress. cdc3–6ts is an allele of a gene encoding S. pombe profilin, a protein believed to be primarily involved in stimulating actin polymerization (Balasubramanian et al., 1994; Ayscough, 1998). At the restrictive temperature, cdc3–6ts cells become swollen at the poles and arrest at cytokinesis, unable to form the actin contractile ring and having actin patches more or less evenly scattered along the cell surface. Deletion of the ssp1 gene did not restore viability of the cdc3–6ts cells at 35°C, but actin patches remained largely bound to the polar zones even after 5 h of incubation (Figure 5C, two left panels). cdc8–27ts is an allele of a gene coding for S. pombe tropomyosin, a protein implicated in stabilization of actin filaments (Balasubramanian et al. 1992; Ayscough, 1998). At the restrictive temperature, cdc8–27ts causes a lethal defect similar to that caused by cdc3–6ts. Again, ssp1Δ did not rescue lethality caused by the cdc8–27ts mutation but confined actin patches to the polar zones in these cells (Figure 5C, two right panels). In both cases actin patches were large and bright in the ssp1Δ background, whereas the cdc3 and cdc8 single mutants contained mostly faint dots lining the cell surface.
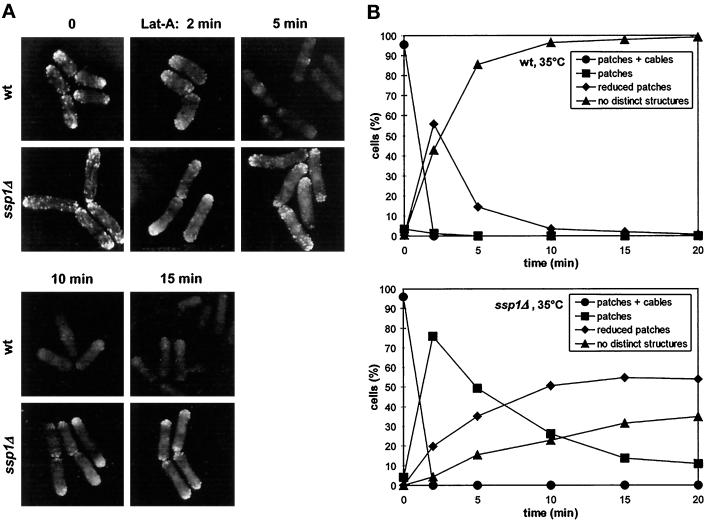
Finally, we asked whether the ssp1 gene deletion can specifically prevent complete actin depolymerization in S. pombe cells. The inhibitory effect of latrunculin A is mediated by its efficient binding to actin monomers, thus preventing their reassembly while allowing disassembly (Couéet al., 1987). Because of its actin sequestering properties, the rate of disappearance of actin filaments in cells in the presence of latrunculin A provides a reliable measure of net actin depolymerization in vivo (Ayscough et al., 1997). Wild-type and ssp1Δ cells were first grown at 35°C for 4 h to allow monopolar extension growth to be established in ssp1Δ cells. When the wild-type cells were treated with 10 μM latrunculin A, they lost actin cables in <2 min and the majority of actin patches within 5 min of the treatment. In striking contrast, some ssp1Δ cells displayed nearly normal accumulation of actin patches at the poles and septa even after 15 min in the presence of the drug, although actin cables were lost as rapidly as in wild type (Figure 6, A and B). We also repeated this experiment with cells cultured at 25°C. We found that the actin depolymerization rate was again significantly lower in ssp1Δ cells compared with wild type, although the defect does not cause completely penetrant cell morphology and stress sensitivity phenotype at this temperature. Taken together these data strongly suggest that the Ssp1 protein kinase is involved in stimulation of actin depolymerization and thus promotion of actin turnover in S. pombe.
Figure 6.
ssp1Δ cells exhibit delayed actin disassembly in the presence of latrunculin A. (A) Strains Q868 (wt) and Q1528 (ssp1Δ) were grown in YEA complex medium at 35°C for 4 h and then exposed to 20 μM latrunculin A in the same medium and incubated further at 35°C. Samples were stained for filamentous actin. Pictures of wild type and ssp1Δ were taken under the same imaging settings. (B) Blind scoring of actin distribution in cells from experiment A (n ≥ 200).
Relationship between Ssp1 and Spc1 during Adaptation to Stress
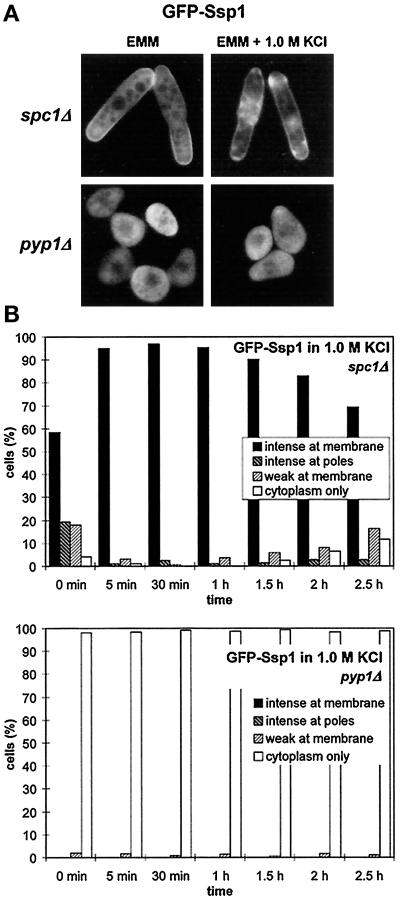
Actin reorganization is required for adaptation to osmotic stress in budding yeast (Chowdhury et al., 1992; Botstein et al., 1997). Therefore it is conceivable that the Ssp1-mediated stimulation of actin turnover may be required to allow rapid reinforcement of the cell surface exposed to the changed outer environment. The Spc1 pathway and its stimulation of the synthesis of osmolyte molecules such as glycerol (Degols et al., 1996) could then be viewed as the second line of defense against unfavorable osmotic conditions. If this view is correct then one would expect Ssp1 to be constitutively hyperactivated in cells lacking a functional Spc1 MAP kinase pathway, as the means by which a cell can partially compensate for the loss. The GFP-Ssp1 construct was expressed in spc1Δ cells, and its localization was monitored under the fluorescence microscope. Indeed, distinct localization of GFP-Ssp1 to the plasma membrane in nearly all cells was noted even when cells were grown in EMM either at 30 or 35°C. The presence of GFP-Ssp1 at the plasma membrane in these cells was dramatically enhanced by the shift to 1 M KCl, and no relocalization back to the cytoplasm occurred within 2.5 h after the shift (Figure 7, A and B). Identical results were obtained in the spc1Δ pyp1Δ background, ruling out the possibility that Pyp1 independent of the Spc1 pathway is required for the Ssp1 localization.
Figure 7.
Ssp1 localization depends on the activity of the Spc1 MAP kinase. (A) Ssp1 shows increased localization at the plasma membrane in the spc1Δ background and reduced localization in the pyp1Δ background. Strains Q1580 (spc1Δ) and Q1592 (pyp1Δ), both harboring the pIR2–22 plasmid, were grown overnight at 30°C in EMM lacking thiamine. Half of each culture was then transferred to the presence of 1 M KCl in the same medium and incubated for another 10 min. All four samples were then taken, and the GFP-Ssp1 fluorescence was observed. (B) A time course of the GFP-Ssp1 redistribution after transfer to 1 M KCl in experiment A. For explanation of categories and wild-type control, see Figure 3C (n ≥ 200).
Conversely, hyperactivation of the Spc1 pathway should lessen the burden laid on the actin-dependent maintenance of the cell surface and that should result in a lesser requirement for Ssp1 function. In pyp1 deletion strains, Spc1 remains hyperactivated even under nonstress conditions (Shiozaki and Russell, 1995). pyp1Δ cells overproducing GFP-Ssp1 were oval, pear-shaped, or spherical and exhibited markedly retarded growth (Figure 7A). No localization of GFP-Ssp1 to the plasma membrane was seen in these cells when they were cultured in minimal media either at 30 or 35°C, and the relocalization was not promoted even in the presence of 1 M KCl (Figure 7, A and B). Thus, these data support the model that Ssp1 can partially compensate for the loss of the Spc1 MAP kinase, giving a basis for understanding the profound synthetic interaction seen after exposure of the ssp1Δ spc1Δ double mutant to moderate stress conditions.
The Role of Ssp1 in NETO
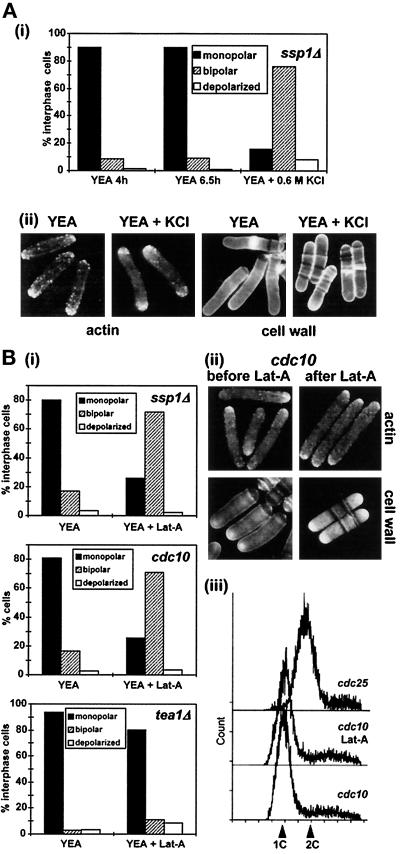
If Ssp1 is involved in promoting actin depolymerization, then ssp1Δ cells should still retain some potential for the bipolar switch. If so, then it should be possible to promote NETO in these cells after a sufficient pool of actin monomers becomes available. The first clue relevant to this prediction originated from the experiments that demonstrated the transient redistribution of actin patches after KCl stress. When ssp1Δ cells were maintained at 35°C and stressed with 0.6 M KCl, they reconstituted normal polarized distribution of actin patches within ∼2.5 h (Figure 5A); however, we noticed that many of these cells now had actin patches localized symmetrically at both poles. Quantitation of actin polarity in interphase ssp1Δ cells during undisturbed growth and in cells that have reconstituted actin polarity in the presence of KCl is shown in Figure 8A. It is clear that the ratio of monopolar to bipolar cells is fully reversed in KCl-stressed cells, suggesting that these cells have passed through NETO.
Figure 8.
The effect of release of actin monomers on NETO in different strains. (A) Exposure to KCl alters cell growth polarity in ssp1Δ cells. Strain Q1528 (ssp1Δ) was grown in YEA complex medium at 35°C for 4 h. The culture was then split into two: half was left untreated and incubated for another 2.5 h, KCl was added to the other half to a final concentration of 0.6 M, and cells were incubated for 2.5 h at 35°C. Samples were stained with TRITC–phalloidin to visualize actin and polarity of actin distribution in interphase cells recorded [(i), n > 200], and photographs of representative cells were taken (ii). (B) Pulse treatment with latrunculin A alters growth polarity in the ssp1Δ and cdc10–129ts cells but not in the tea1Δ cells. Strains Q1528 (ssp1Δ) and Q1518 (cdc10–129ts) were incubated in YEA complex media at 35° and 36°C, respectively, for 4 h, then exposed to 10 μM latrunculin A at the same respective temperatures for 2 min, washed, and incubated for another 1 h at the respectivetemperatures. Strain JM101 (tea1Δ) was grown in the same medium at 30°C, exposed to 20 μM latrunculin A for 10 min, washed, and incubated in the preconditioned medium for another 1 h at 30°C. Samples from untreated and treated cultures were stained with Calcofluor to visualize cell wall (cdc10–129ts) and with TRITC–phalloidin to visualize actin (all strains). Actin polarity in interphase cells was recorded [(i), n > 200], and representative photographs were taken (ii). The experiment was repeated with the cdc10–129ts and cdc25–22ts cells, and samples were taken for FACS analysis to determine the DNA content (iii).
To verify that NETO in ssp1Δ cells specifically follows the increase of the free actin monomer pool, we repeated this experiment using latrunculin A. In the presence of the drug, actin monomers are sequestered by the drug, but after the drug is removed they become available for reassembly. Cells were grown for 4 h and then treated with 10 μM latrunculin A for 2 min. As shown in Figure 6A, this treatment does not cause complete disappearance of actin patches but visibly interferes with the integrity of actin cables. After wash-out the cells were left to recover from the treatment for 1 h, and the polarity of actin distribution was then monitored. The whole experiment was performed at 35°C to ensure that the cells would not leak through the NETO block because of a drop in temperature. The quantitation [Figure 8B(i)] confirms the results of the previous experiment. Calcofluor staining of the cell wall revealed that an increased portion of these cells effectively passed through NETO (45% of latrunculin A pulse-treated cells versus 25% of untreated ssp1Δ cells had visible cell wall extension at the new end; the value for untreated wild type was 35%). This demonstrates that increasing the concentration of free actin monomers is sufficient to bypass the NETO block in ssp1Δ cells.
On the basis of these observations, we asked whether the release of free actin monomers may be generally a sufficient stimulus for cells to undergo NETO. We have seen earlier that the population of temperature-arrested cdc10–129ts cells overexpressing Ssp1 contained many bipolar cells. To investigate this phenomenon further, cdc10–129ts cells were arrested in G1 by incubation at 36°C and subsequently treated with latrunculin A, released from the drug, and left to recover for an additional hour at 36°C. After this treatment, bipolar distribution of actin patches was seen in a great majority of cells [Figure 8B(i, ii)]. These cells initiated cell wall growth at the new end, as could be visualized by Calcofluor staining, but they did not enter mitosis [Figure 8B(ii)]. Continuing arrest in G1 was also confirmed by FACS analysis [Figure 8B(iii)]. These data suggest that these cells passed through NETO even without previous initiation of DNA synthesis. Taken together, these results demonstrate that the release of a sufficient number of actin monomers, which is prevented in cells lacking Ssp1 function, can serve as a stimulus for cells to undergo NETO regardless of the DNA content.
The above data suggest that the positional signal required to mark the new end as a potential site of extension growth must be in place far ahead of the actual NETO event. In this case cells lacking such a signal should be unable to undergo NETO even with an excess of free actin monomers. The Tea1 protein is known to mark the cell ends throughout the cell cycle, regardless of whether the ends are actively growing or not (Mata and Nurse, 1997). As predicted, the latrunculin A pulse treatment did not promote bipolarity in these cells [Figure 8B(i)]. Thus, the latrunculin A pulse treatment distinguishes between two major events in NETO regulation: the first is establishment and maintenance of the polarity signal, which appears to be independent of cell cycle phase, and the second is the timely signal that involves release of actin monomers.
DISCUSSION
The Ssp1 protein kinase plays an important role in stimulating reorganization of the actin cytoskeleton after osmotic stress as well as during the normal cell division cycle. Our major conclusions can be summarized as follows: 1) the Ssp1 protein kinase stimulates actin depolymerization and thus actin turnover in S. pombe cells; 2) during hyperosmotic stress, Ssp1 rapidly translocates to the sites of active growth and promotes actin redistribution; 3) Ssp1 function is important for osmotic stress adaptation and can be regulated independently of the Spc1 MAP kinase pathway; and 4) induced actin depolymerization facilitates NETO regardless of the cell cycle phase.
Role of Ssp1 in Actin Depolymerization
The Ssp1 protein kinase has been implicated in destabilizing the actin cytoskeleton (Matsusaka et al., 1995). The actin cytoskeleton can be destabilized by several mechanisms. Destabilization factors may interfere with cross-linking or bundling of actin filaments, induce their fragmentation, inhibit their polymerization, or promote their depolymerization. Our data show that Ssp1 promotes actin depolymerization in vivo and thus represents a novel element involved in the control of actin cytoskeleton. By the structure of its catalytic domain, Ssp1 is related to the ELM family of protein kinases in S. cerevisiae (Matsusaka et al., 1995; Hunter and Plowman, 1997). At present, the functional relationship between Ssp1 and most of the members of this group remains unclear; however, mutants in the prototype representative of this group, Elm1, are unable to switch from polar to the isotropic phase of bud growth and constitutively execute the pseudohyphal growth mode (Blacketer et al., 1993). This suggests that Elm1 may represent a functional homologue of Ssp1 in S. cerevisiae, although there is little sequence homology outside the kinase domain.
One major question that arises from our findings is by what mechanism Ssp1 promotes actin depolymerization. Organization of the actin cytoskeleton is regulated at multiple levels, and indeed, conserved members of the major families of actin-binding proteins have been identified in S. cerevisiae and some of them also in S. pombe (reviewed in Ayscough, 1998). Thus, profilin is believed to promote actin filament formation by its interaction with the formin homology domain-containing proteins such as the S. cerevisiae Bni1 and Bnr1, the targets of the Rho family of small GTPases (Evangelista et al., 1997; Imamura et al., 1997), which also have their homologues in S. pombe (Chang et al., 1997; Petersen et al., 1998). Our data show that ssp1 gene deletion prevents actin depolarization in the S. pombe profilin mutant cdc3ts, although it does not restore cell viability. This indicates that the rates of actin polymerization and depolymerization tend to be balanced in the ssp1 cdc3ts double mutant, but the fine local orchestration of these events is lost, possibly as a consequence of the regulatory pathways converging on these elements having been destroyed. Stabilization of actin filaments by tropomyosin is as important for S. pombe cell viability as the presence of functional profilin. Again, our data show that the apparent actin stabilization that occurs in the ssp1Δ mutant cannot compensate for tropomyosin loss, but it does inhibit actin redistribution along the cell cortex in tropomysin-deficient cells.
The sole factor identified in yeast that is directly responsible for stimulation of actin depolymerization in vivo as well as in vitro is cofilin, encoded by COF1 (Lappalainen and Drubin, 1997). Phenotypically, ssp1Δ cells are reminiscent of the conditional thermosensitive cof1 mutants of S. cerevisiae in that ssp1Δ also delays actin disassembly in the presence of latrunculin A (this study). Cofilin homologues are ubiquitous proteins. In many vertebrate cells cofilin is regulated by inhibitory phosphorylation on the serine residue near the N-terminus, but the regulation of its yeast homologue remains unclear (Lappalainen et al., 1997). A putative cofilin homolog (60% identity to Cof1) is present in the S. pombe genome (Sanger Center genome database). Thus, cofilin becomes an attractive, if indirect, candidate downstream effector of the Ssp1-mediated signaling.
Ssp1 function is required only at high temperature. At present we do not understand the reason for the thermosensitivity of the ssp1 deletion. Presumably, Ssp1 is not the only factor regulating actin depolymerization in S. pombe. High temperature may be sensed by cells as a mild stress, and indeed heat shock is one of the stimuli that activates the Spc1 pathway (Degols et al., 1996). This might suggest that thermosensitivity is merely another manifestation of the mutant’s stress response deficiency. The defect, however, persists throughout the exposure to high temperature and does not tend to be alleviated by later physiological adaptations. Therefore it is also likely that the thermosensitivity reflects some intrinsic temperature-dependent property of the actin system kinetics. Deletion of the Ppe1 protein phosphatase, an activity thought to be opposing that of Ssp1 (Matsusaka et al., 1995), causes a cold-sensitive phenotype characterized by short, rounded cells (Shimanuki et al., 1993), which supports this argument.
Relationship between Ssp1 Localization and Its Function
One striking feature of the Ssp1 protein kinase is its translocation to the plasma membrane, promoted primarily by osmotic stress. It is important to remember that our data are based on detection of the overproduced version of the Ssp1 protein, and therefore they may not reflect precisely the native Ssp1 localization. Also, we do not know at this time whether the translocation is directly associated with activation of the kinase. Despite this, however, our results strongly support the notion that Ssp1 translocation is linked to cortical actin redistribution. First, a timing correlation exists between transient localization of Ssp1 to the growth zones and gradual disappearance of actin patches from these sites after an increase in osmolarity. Second, stress-induced actin redistribution is incomplete in cells lacking the ssp1 gene. Third, a small fraction of Ssp1 associated with sites of active growth is detectable even in nonstressed cells. The most prominent example is the association with the septum, arguing for the role of Ssp1 in stimulation of actin turnover during septation. The ssp1Δ mutant is not visibly affected in septation even at high temperature, and therefore it is likely that another activity stimulating actin depolymerization can compensate for the loss of ssp1 during septation. The association of Ssp1 with the plasma membrane, whether direct or indirect at the molecular level, provides an explanation for the seeming contradiction that appeared in the earlier report (Matsusaka et al., 1995). In accordance with our results, the authors reported Ssp1 as being localized throughout the cytoplasm during normal growth. Cell fractionation, however, revealed that ∼50% of the protein was associated with the insoluble fraction. Given the capability of the Ssp1 protein to rapidly translocate to the plasma membrane, it is possible that the preparation of cells for extraction may have been a cause of stress severe enough to trigger translocation of the protein to the membrane. Consistent with this, our experiments showed that centrifugation alone is sufficient to increase the Ssp1 presence at the plasma membrane (Rupes̆, unpublished results). Others have shown that centrifugation stress causes activation of the Spc1 kinase (Shiozaki et al., 1998).
Role of Ssp1 in Stress Response
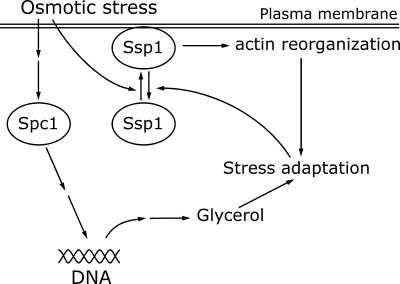
The role of Ssp1 in stimulation of actin depolymerization, along with the fact that the ssp1 gene is nonessential under normal growth conditions, provides an intriguing opportunity for insight into the control of actin organization in response to environmental stress. An important finding in this context is that Ssp1 can act independently of Spc1. The significance of an independent pathway stands out clearly if one considers the timely relationship between the responses of these two elements. In this regard, response to a sudden jump in osmolarity turns out to be particularly enlightening because it represents an immediate challenge for the integrity of the cell surface structures, especially the plasma membrane (Chowdhury et al., 1992; Mulholland et al., 1994). Translocation of Ssp1 to the plasma membrane occurs within <1 min and is thus, along with the production of phosphatidylinositol-3,5-bisphosphate (Dove et al., 1997), among the earliest cell responses to high osmolarity. Visible redistribution of actin patches occurs only minutes later, but one must be aware that we are not able to observe acute changes in the architecture of the actin cytoskeleton at the local level. If the view is correct that cell wall extension occurs at invaginations of the plasma membrane and actin is required to support weakened bonds between cell wall and the plasma membrane at these sites (Mulholland et al., 1994), then these sites may be especially vulnerable to osmotic challenge and will become primary targets for reinforcement by the network of cortical actin filaments. In contrast, full activation of the Spc1 protein kinase occurs only after 5–10 min of exposure to KCl (Gaits et al., 1998), and the accumulation of the gpd1 message, whose expression is controlled by Spc1 and whose product is involved in glycerol synthesis, does not peak until ∼30–60 min later (Degols et al., 1996; Shieh et al., 1997). Therefore S. pombe cells can spend a considerable period of time in the hyperosmotic environment without sufficient osmolyte protection. Thus it would make physiological sense if an early response of cells to hyperosmotic shock included reorganization of actin and reinforcing the cell cortex in order to prevent immediate effects caused by the reversal of the osmotic gradient and initial water efflux. The time profile of the levels of gpd1 expression (Degols et al., 1996; Shieh et al., 1997) provides another notable parallel, because the expression peaks at the time after which, according to our data, actin begins to relocalize back to the cell poles. Thus it seems that the transient dispersal of actin patches spans the period that cells spend with no protection by the osmolyte and is ended when cells generate sufficient osmotic potential to be able to resume polar growth. The model of parallel involvement of the Ssp1 and Spc1 protein kinases in response to osmotic stress is summarized in Figure 9.
Figure 9.
A model of cooperation between the Ssp1 and Spc1 pathways during osmotic stress. Osmotic stress promotes recruitment of the Ssp1 kinase at the plasma membrane, which in turn stimulates actin reorganization. At the same time the stress signal activates the Spc1 MAP kinase pathway, resulting in increased expression of stress-response genes. One of the consequences is stimulation of the glycerol biosynthesis pathway. Both Spc1 and Ssp1 pathways contribute to the osmotic stress adaptation process. Once the balance is reestablished, Ssp1 is released from the plasma membrane.
One prediction of this model is that Ssp1 should remain hyperactivated in the absence of functional Spc1 kinase. Indeed, our data suggest that in the spc1Δ strain, Ssp1 is present at the plasma membrane throughout incubation even under nonstress conditions. Conversely, if Spc1 is constitutively hyperactivated because of the pyp1 deletion, the Ssp1 localization to the membrane is barely detectable even in the hyperosmotic environment. Thus, although we cannot rule out partial involvement of Spc1 in Ssp1 regulation, our data strongly suggest that the major component of Ssp1 activation is not directly dependent on the Spc1 activity. Ssp1 overproduction in the pyp1Δ background results in the spherical cell morphology, indicating increased interference with actin polarity. It seems, therefore, that actin can be affected by changes in the Spc1 activity, although the precise mechanism remains to be seen. Consistent with this, the spherical cell shape of the sts5–7 mutant can be suppressed by both ssp1− and wis1Δ mutations, the latter resulting in constitutive inactivation of the Spc1 kinase (Matsusaka et al., 1995; Toda et al., 1996). Thus SspI and the Spc1 pathway, at least under certain conditions, may both act synergistically to influence actin organization.
Role of Ssp1 in NETO
The transition from monopolar to bipolar growth is presumably controlled at multiple levels. The phenotypes of representative mutants defective in NETO control other than ssp1, such as tea1, ban2, orb2/pak1, or pom1 (Verde et al., 1995, 1998; Bähler and Pringle, 1998), include relaxed control of the growth direction, depolarized growth resulting in spherical cell shape, and random initiation of growth at the old or new end. So far, there is no simple model of the relationship among these components. Theoretically, NETO might occur because of a positive signal at the new end or a loss of a negative signal preventing growth at the new end or both occurring in a timely orchestrated manner. The role of Ssp1 provides some insight into one essential component of NETO regulation. Our data show for the first time that the availability of free actin monomers is an important factor in this process. Surprisingly, artificial increase in the actin monomer concentration by latrunculin A pulse treatment is sufficient to trigger NETO even in cdc10 cells arrested in G1. This finding has an important implication. It shows that the potential for NETO is an intrinsic property of interphase cells and does not absolutely depend on the completion of DNA synthesis, as previously thought (Mitchison and Nurse, 1985). So, is the positive localization signal required for NETO? When the actin monomer pool in the tea1Δ cells is raised by latrunculin A pulse treatment, the cells continue to grow in the monopolar mode (this report). Thus the absence of the cell end marker (Mata and Nurse, 1997) cannot be overriden by making more free actin available. Tea1 can therefore be viewed as a part of a system maintaining competence for bipolarity that is kept in place throughout interphase. The Pom1 protein kinase is required, among other things, to determine that the old end of a newborn cell will become the primary growth pole and appears to be partially involved in mediating the positional signal from Tea1 to the growth machinery (Bähler and Pringle, 1998). It is possible that after polar growth has been established at one end, actin becomes bound at this end and requires additional stimulus to be released and newly organized at the opposite end. Our data show that the promotion of actin disassembly by Ssp1 plays an important part in delivering such a stimulus, at least at high temperature.
ACKNOWLEDGMENTS
We thank Paul Russell, Takashi Toda, Sabine Ottilie, and Juan Mata for providing strains and Mike Moser for the pZA69 plasmid. We thank Nancy Russell for technical support and the members of the lab for useful discussions. This work was supported by research grants from the Natural Science and Engineering Research Council of Canada and The National Cancer Institute of Canada to P.G.Y., and a group infrastructure support grant for FACS and confocal microscopy from the Medical Research Council of Canada.
REFERENCES
- Aiba H, Yamada H, Ohmiya R, Mizuno T. The osmo-inducible gpd1+ gene is a target of the signaling pathway involving Wis1 MAP-kinase kinase in fission yeast. FEBS Lett. 1995;376:199–201. doi: 10.1016/0014-5793(95)01277-4. [DOI] [PubMed] [Google Scholar]
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with fission yeast. A laboratory course manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1993. [Google Scholar]
- Ayscough KR. In vivo functions of actin-binding proteins. Curr Opin Cell Biol. 1998;10:102–111. doi: 10.1016/s0955-0674(98)80092-6. [DOI] [PubMed] [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler J, Pringle JR. Pom1p, a fission yeast protein kinase that provides positional information for both polarized growth and cytokinesis. Genes Dev. 1998;12:1356–1370. doi: 10.1101/gad.12.9.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian MK, Helfman DM, Hemmingsen SM. A new tropomyosin essential for cytokinesis in the fission yeast S. pombe. Nature. 1992;360:84–87. doi: 10.1038/360084a0. [DOI] [PubMed] [Google Scholar]
- Balasubramanian MK, Hirani BR, Burke JD, Gould KL. The Schizosaccharomyces pombe cdc3+ gene encodes a profilin essential for cytokinesis. J Cell Biol. 1994;125:105–114. doi: 10.1083/jcb.125.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F. Signaling in the yeasts: an informational cascade with links to the filamentous fungi. Microbiol Mol Biol Rev. 1998;62:249–274. doi: 10.1128/mmbr.62.2.249-274.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacketer MJ, Koehler CM, Coats SG, Myers AM, Madaule P. Regulation of dimorphism in Saccharomyces cerevisiae: involvement of the novel protein kinase homolog Elm1p and protein phosphatase 2A. Mol Cell Biol. 1993;13:5567–5581. doi: 10.1128/mcb.13.9.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D, Amberg D, Mulholland J, Huffaker T, Adams A, Drubin D, Stearns T. The yeast cytoskeleton. In: Pringle JR, Broach JR, Jones EW, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces. Vol. 3. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1997. pp. 1–90. [Google Scholar]
- Cantiello HF. Role of actin filament reorganization in cell volume and ion channel regulation. J Exp Zool. 1997;279:425–435. doi: 10.1002/(sici)1097-010x(19971201)279:5<425::aid-jez4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Chang F, Drubin D, Nurse P. Cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J Cell Biol. 1997;137:169–182. doi: 10.1083/jcb.137.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Smith KW, Gustin MC. Osmotic stress and the yeast cytoskeleton: phenotype-specific suppression of an actin mutation. J Cell Biol. 1992;118:561–571. doi: 10.1083/jcb.118.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coué M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin-A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- Degols G, Shiozaki K, Russell P. Activation and regulation of the spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:2870–2877. doi: 10.1128/mcb.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, Michell RH. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature. 1997;390:187–192. doi: 10.1038/36613. [DOI] [PubMed] [Google Scholar]
- Evangelista M, Blundell K, Longtine MS, Chow CJ, Adames N, Pringle JR, Peter M, Boone C. Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- Fantes P. Cell Cycle Clocks. L.N. Edmunds, New York: Dekker; 1984. Temporal control of the Schizosaccharomyces pombe cell cycle; pp. 233–252. [Google Scholar]
- Gaits F, Degols G, Shiozaki K, Russell P. Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast. Genes Dev. 1998;12:1464–1473. doi: 10.1101/gad.12.10.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm R, Cubitt AB, Tsien RY. Improved green fluorescence. Nature. 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- Horton RM, Ho SN, Pullen JK, Hunt HD, Cai Z, Pease LR. Gene splicing by overlap extension. Methods Enzymol. 1993;217:270–279. doi: 10.1016/0076-6879(93)17067-f. [DOI] [PubMed] [Google Scholar]
- Hunter T, Plowman GD. The protein kinases of budding yeast: six score and more. Trends Biochem Sci. 1997;22:18–22. doi: 10.1016/s0968-0004(96)10068-2. [DOI] [PubMed] [Google Scholar]
- Imamura H, Tanaka K, Hihara T, Umikawa M, Kamei T, Takahashi K, Sasaki T, Takai Y. Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 1997;16:2745–2755. doi: 10.1093/emboj/16.10.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis, J., Saleki, R., and Young, P.G. (1999). The pub1 E3 ubiquitin ligase negatively regulates leucine uptake in response to NH4 + in fission yeast. Curr. Genet. (in press). [DOI] [PubMed]
- Kuwayama H, Ecke M, Gerisch G, Van Haastert PJM. Protection against osmotic stress by cGMP-mediated myosin phosphorylation. Science. 1996;271:207–209. doi: 10.1126/science.271.5246.207. [DOI] [PubMed] [Google Scholar]
- Lappalainen P, Drubin DG. Cofilin promotes rapid actin filament turnover in vivo. Nature. 1997;388:78–82. doi: 10.1038/40418. [DOI] [PubMed] [Google Scholar]
- Lappalainen P, Fedorov EV, Fedorov AA, Almo SC, Drubin DG. Essential functions and actin-binding surfaces of yeast cofilin revealed by systematic mutagenesis. EMBO J. 1997;16:5520–5530. doi: 10.1093/emboj/16.18.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leupold U. Genetical methods for Schizosaccharomyces pombe. Methods Cell Physiol. 1970;4:169–177. [Google Scholar]
- MacNeill SA, Nurse P. Cell cycle control in fission yeast. In: Pringle JR, Broach JR, Jones EW, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces. Vol. 3. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1997. pp. 697–763. [Google Scholar]
- Marks J, Hagan IM, Hyams JS. Growth polarity and cytokinesis in fission yeast: the role of the cytoskeleton. J Cell Sci Suppl. 1986;5:229–241. doi: 10.1242/jcs.1986.supplement_5.15. [DOI] [PubMed] [Google Scholar]
- Mata J, Nurse P. tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell. 1997;89:939–949. doi: 10.1016/s0092-8674(00)80279-2. [DOI] [PubMed] [Google Scholar]
- Matsusaka T, Hirata D, Yanagida M, Toda T. A novel protein kinase gene ssp1+ is required for alteration of growth polarity and actin localization in fission yeast. EMBO J. 1995;14:3325–3338. doi: 10.1002/j.1460-2075.1995.tb07339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- Millar JB, Buck V, Wilkinson MG. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Mitchison JM, Nurse P. Growth in cell length in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1985;75:357–376. doi: 10.1242/jcs.75.1.357. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Mulholland J, Preuss D, Moon A, Wong A, Drubin D, Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol. 1994;125:381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P, Thuriaux P, Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976;146:167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Ottilie S, Chernoff J, Hanning G, Hoffman CS, Erikson RL. The fission yeast genes pyp1+ and pyp2+ encode protein tyrosine phosphatases that negatively regulate mitosis. Mol Cell Biol. 1992;12:5571–5580. doi: 10.1128/mcb.12.12.5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J, Nielsen O, Egel R, Hagan IM. FH3, a domain found in formins, targets the fission yeast formin Fus1 to the projection tip during conjugation. J Cell Biol. 1998;141:1217–1228. doi: 10.1083/jcb.141.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero F, Köppel B, Peracino B, Bozzaro S, Siegert F, Weijer CJ, Schleicher M, Albrecht R, Noegel A. The role of the cortical cytoskeleton: F-actin cross-linking proteins protect against osmotic stress, ensure cell size, cell shape and motility, and contribute to phagocytosis and development. J Cell Sci. 1996;109:2679–2691. doi: 10.1242/jcs.109.11.2679. [DOI] [PubMed] [Google Scholar]
- Robinow CF, Hyams JS. General cytology of fission yeasts. In: Nasim A, Young P, Johnson BF, editors. Molecular Biology of the Fission Yeast. San Diego: Academic; 1989. pp. 273–330. [Google Scholar]
- Rupes̆ I, Jochová J, Young PG. Markers of cell polarity during and after nitrogen starvation in Schizosaccharomyces pombe. Biochem Cell Biol. 1997;75:697–708. doi: 10.1139/o97-084. [DOI] [PubMed] [Google Scholar]
- Saleki R, Jia Z, Karagiannis J, Young PG. Tolerance of low pH in Schizosaccharomyces pombe requires a functioning pub1 ubiquitin ligase. Mol Gen Genet. 1997;254:520–528. doi: 10.1007/s004380050447. [DOI] [PubMed] [Google Scholar]
- Shieh J-C, Wilkinson MG, Buck V, Morgan BA, Makino K, Millar JBA. The Mcs4 response regulator coordinately controls the stress-activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev. 1997;11:1008–1022. doi: 10.1101/gad.11.8.1008. [DOI] [PubMed] [Google Scholar]
- Shimanuki M, Kinoshita N, Ohkura H, Yoshida T, Toda T, Yanagida M. Isolation and characterization of the fission yeast protein phosphatase gene ppe1+ involved in cell shape control and mitosis. Mol Biol Cell. 1993;4:303–313. doi: 10.1091/mbc.4.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P. Heat stress activates fission yeast Spc1/Sty1 MAPK by a MEKK-independent mechanism. Mol Biol Cell. 1998;9:1339–1349. doi: 10.1091/mbc.9.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Niwa H, Nemoto T, Dhut S, Eddison M, Matsusaka T, Yanagida M, Hirata D. The fission yeast sts5+ gene is required for maintenance of growth polarity and functionally interacts with protein kinase C and an osmosensing MAP-kinase pathway. J Cell Sci. 1996;109:2331–2342. doi: 10.1242/jcs.109.9.2331. [DOI] [PubMed] [Google Scholar]
- Verde F, Mata J, Nurse P. Fission yeast cell morphogenesis: identification of new genes and analysis of their role during cell cycle. J Cell Biol. 1995;131:1529–1538. doi: 10.1083/jcb.131.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F, Wiley DJ, Nurse P. Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle. Proc Natl Acad Sci USA. 1998;95:7526–7531. doi: 10.1073/pnas.95.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MG, Millar JBA. SAPKs and transcription factors do the nucleocytoplasmic tango. Genes Dev. 1998;12:1391–1397. doi: 10.1101/gad.12.10.1391. [DOI] [PubMed] [Google Scholar]
- Young PG, Fantes PA. Schizosaccharomyces pombe mutants affected in their division response to starvation. J Cell Sci. 1987;88:295–304. doi: 10.1242/jcs.88.3.295. [DOI] [PubMed] [Google Scholar]