Abstract
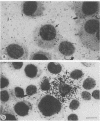
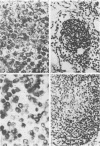
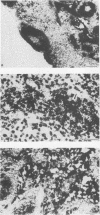
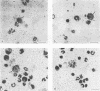
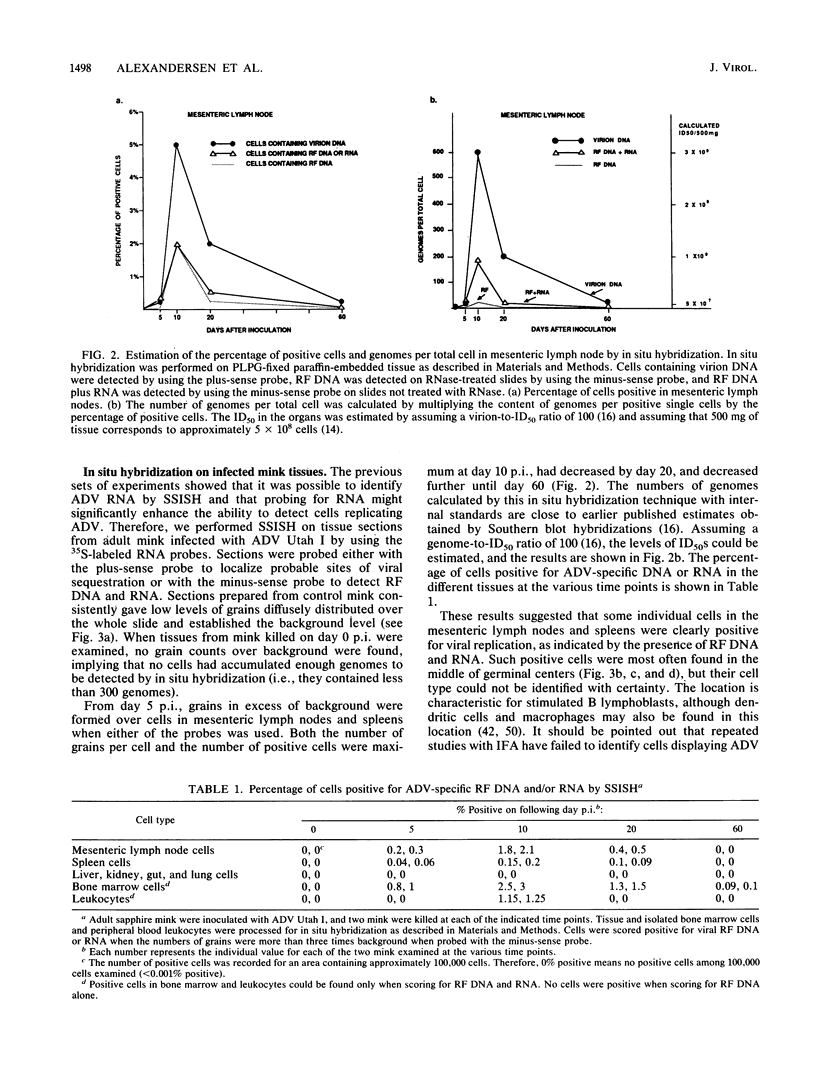
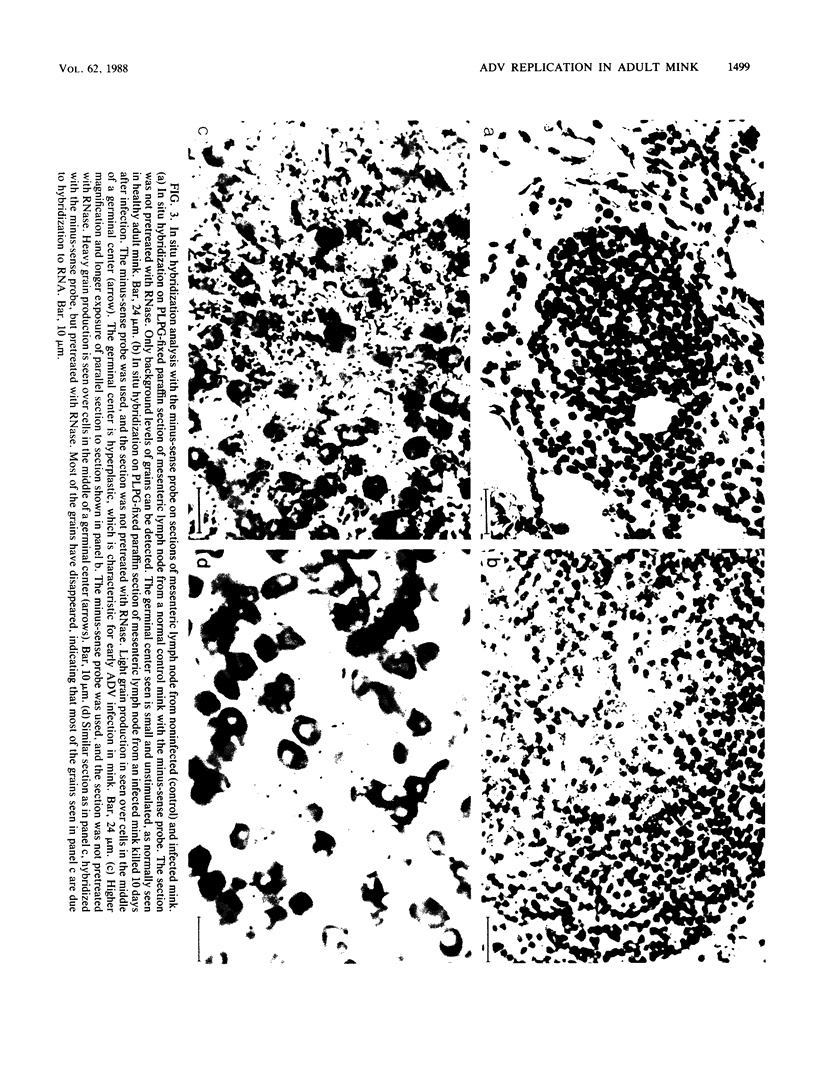
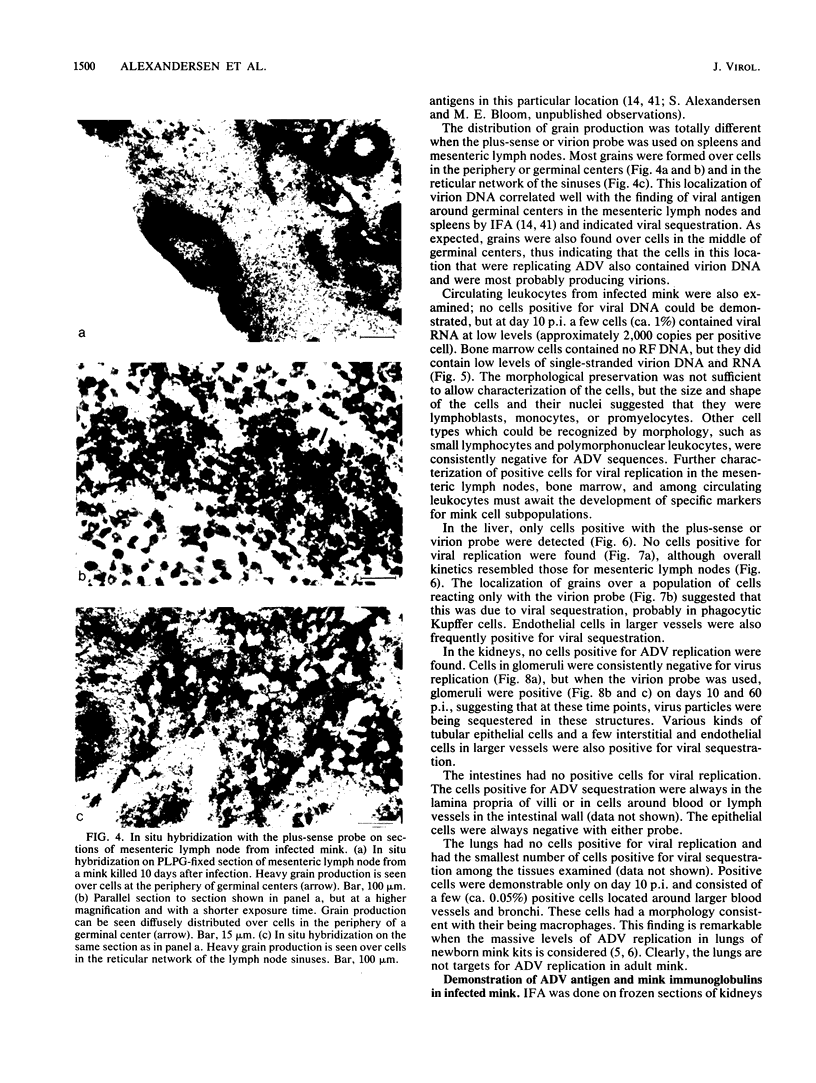
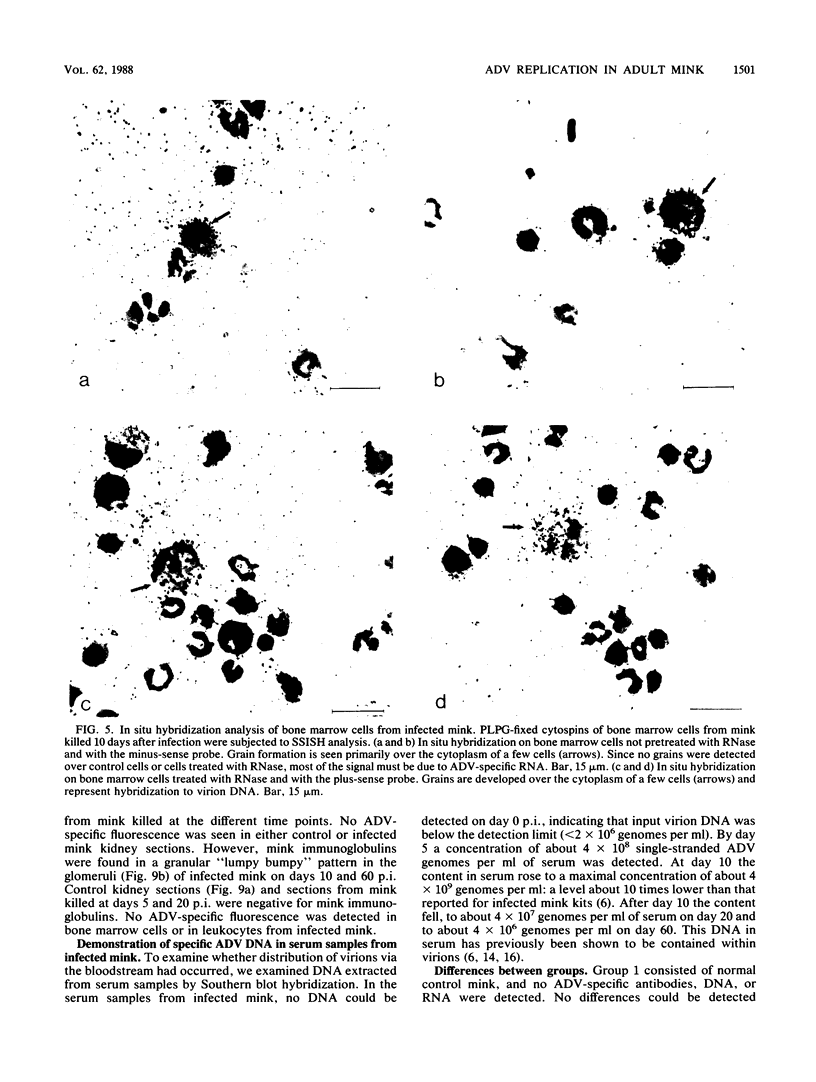
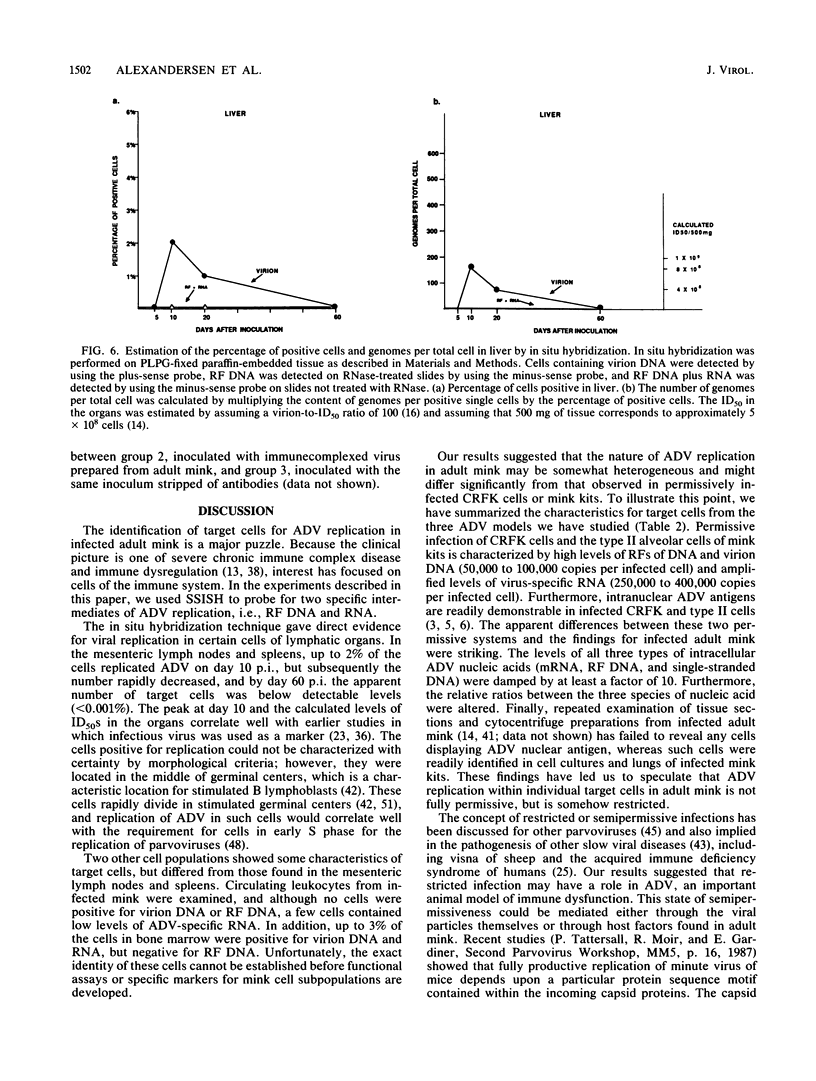
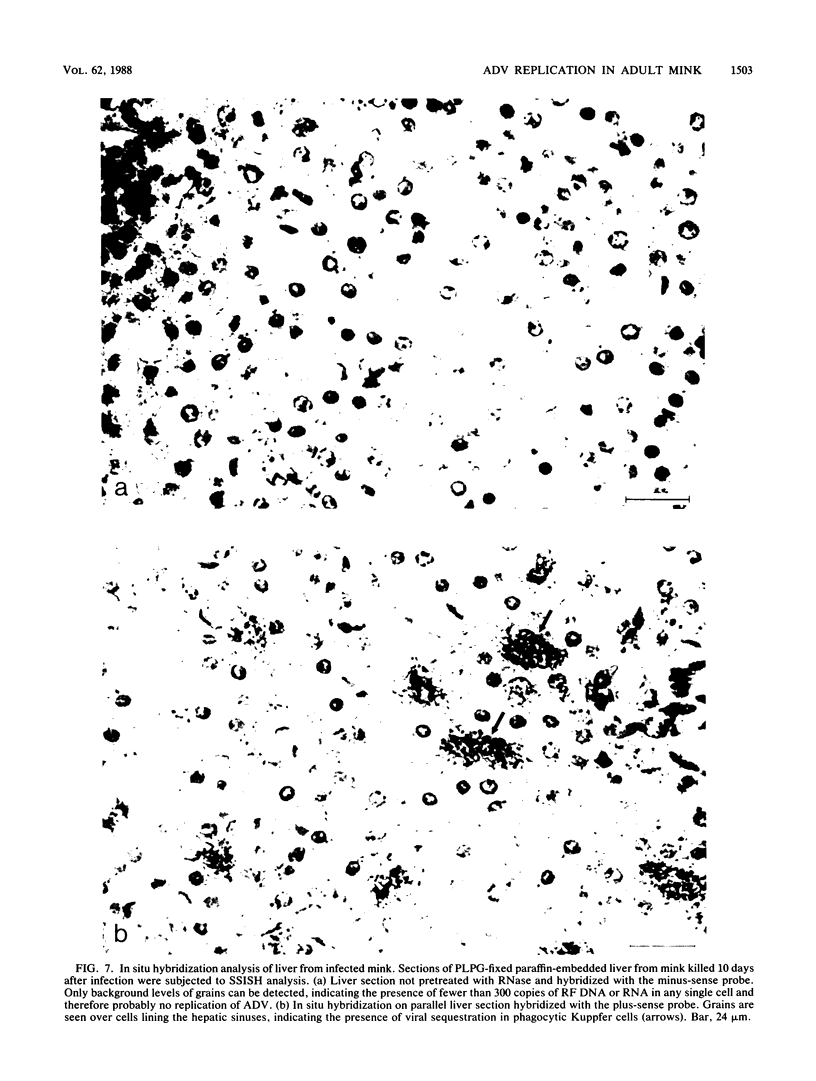
Strand-specific hybridization probes were used in in situ molecular hybridization specifically to localize cells containing replicative intermediates of Aleutian disease of mink parvovirus (ADV). When adult mink of Aleutian genotype were infected with ADV Utah I, the largest number of cells positive for viral replication (i.e., containing replicative-form DNA and RNA) were found in the mesenteric lymph nodes and spleens at 10 days after infection. The localization of positive cells in the middle of germinal centers suggested that they were B lymphoblasts. Circulating leukocytes and bone marrow cells also contained viral RNA, but the levels of replicative-form DNA were below detectability. The levels of viral DNA and RNA in adult mink cells replicating ADV were decreased compared with those in permissively infected cell cultures or neonatal mink, suggesting that the replication of ADV in adult mink might be semipermissive or restricted at some early stage of viral gene expression. The low level of viral replication and transcription in lymphoid cells might provide a mechanism for the development of immune disorders and for the maintenance of persistent infection. Single-stranded virion DNA was found in other organs, but the strand-specific probes made it possible to show that this DNA represented virus sequestration. In addition, glomerular immune complexes containing virion DNA were detected, suggesting that ADV virions, or perhaps free DNA, may have a role in the development of ADV-induced glomerulonephritis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasted B., Avery B., Cohn A. Serological analyses of different mink Aleutian disease virus strains. Arch Virol. 1984;80(1):11–22. doi: 10.1007/BF01315290. [DOI] [PubMed] [Google Scholar]
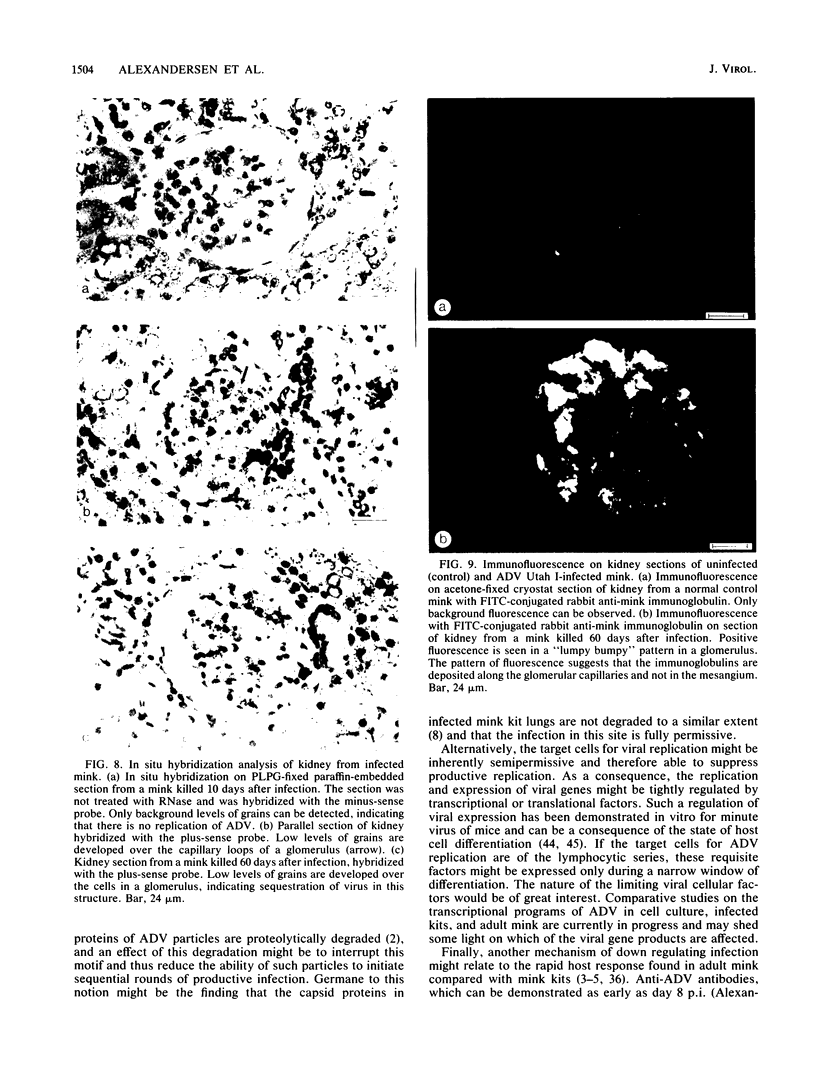
- Aasted B., Race R. E., Bloom M. E. Aleutian disease virus, a parvovirus, is proteolytically degraded during in vivo infection in mink. J Virol. 1984 Jul;51(1):7–13. doi: 10.1128/jvi.51.1.7-13.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Aasted B. Restricted heterogeneity of the early antibody response to Aleutian disease virus in mink kits. Acta Pathol Microbiol Immunol Scand C. 1986 Aug;94(4):137–143. doi: 10.1111/j.1699-0463.1986.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Alexandersen S. Acute interstitial pneumonia in mink kits: experimental reproduction of the disease. Vet Pathol. 1986 Sep;23(5):579–588. doi: 10.1177/030098588602300506. [DOI] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E. Studies on the sequential development of acute interstitial pneumonia caused by Aleutian disease virus in mink kits. J Virol. 1987 Jan;61(1):81–86. doi: 10.1128/jvi.61.1.81-86.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Wolfinbarger J., Race R. E. In situ molecular hybridization for detection of Aleutian mink disease parvovirus DNA by using strand-specific probes: identification of target cells for viral replication in cell cultures and in mink kits with virus-induced interstitial pneumonia. J Virol. 1987 Aug;61(8):2407–2419. doi: 10.1128/jvi.61.8.2407-2419.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Hau J. Rocket line immunoelectrophoresis: an improved assay for simultaneous quantification of a mink parvovirus (Aleutian disease virus) antigen and antibody. J Virol Methods. 1985 Feb;10(2):145–151. doi: 10.1016/0166-0934(85)90100-4. [DOI] [PubMed] [Google Scholar]
- Alexandersen S., Uttenthal-Jensen A., Aasted B. Demonstration of non-degraded Aleutian disease virus (ADV) proteins in lung tissue from experimentally infected mink kits. Brief report. Arch Virol. 1986;87(1-2):127–133. doi: 10.1007/BF01310549. [DOI] [PubMed] [Google Scholar]
- Astry C. L., Jakab G. J. Influenza virus-induced immune complexes suppress alveolar macrophage phagocytosis. J Virol. 1984 May;50(2):287–292. doi: 10.1128/jvi.50.2.287-292.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett E. V., Williams R. C., Jr, Kenyon A. J., Henson J. E. 'Nuclear' antigens and antinuclear antibodies in mink sera. Immunology. 1969 Feb;16(2):241–253. [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Labow M. A. Parvovirus gene regulation. J Gen Virol. 1987 Mar;68(Pt 3):601–614. doi: 10.1099/0022-1317-68-3-601. [DOI] [PubMed] [Google Scholar]
- Bloom M. E. Parvovirus infections: features reminiscent of AIDS. Ann N Y Acad Sci. 1984;437:110–120. doi: 10.1111/j.1749-6632.1984.tb37128.x. [DOI] [PubMed] [Google Scholar]
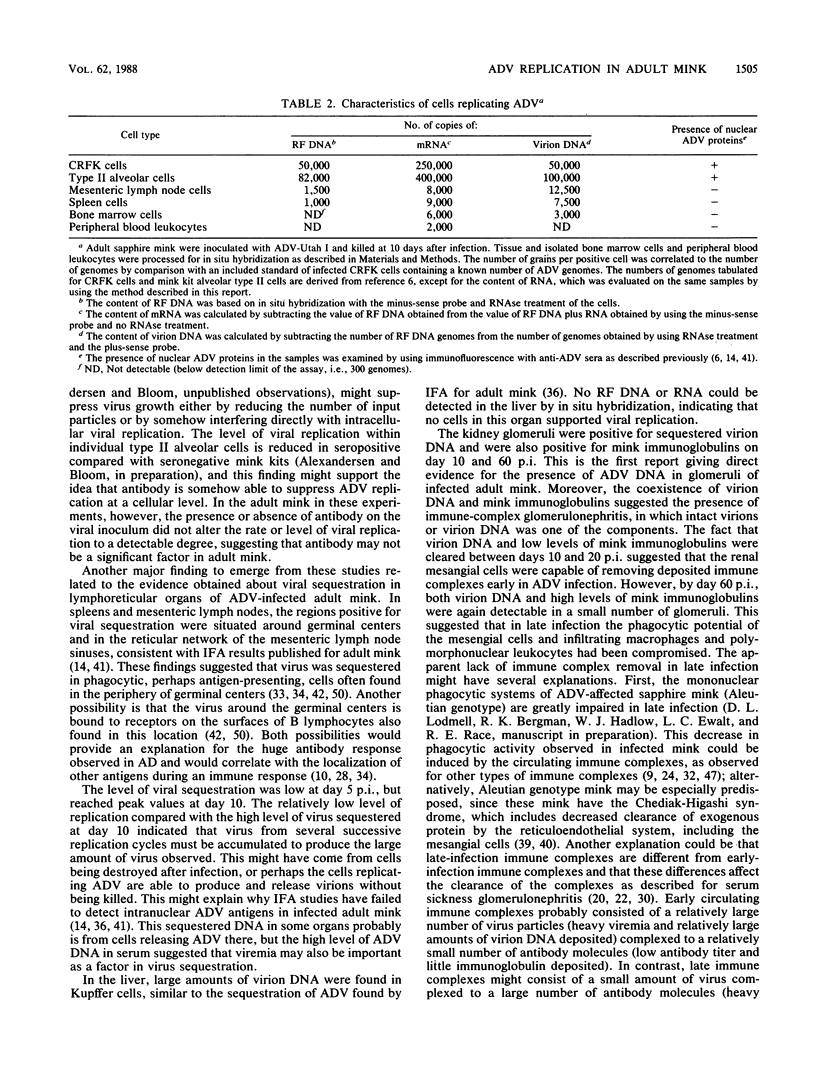
- Bloom M. E., Race R. E., Aasted B., Wolfinbarger J. B. Analysis of Aleutian disease virus infection in vitro and in vivo: demonstration of Aleutian disease virus DNA in tissues of infected mink. J Virol. 1985 Sep;55(3):696–703. doi: 10.1128/jvi.55.3.696-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Analysis of Aleutian disease of mink parvovirus infection using strand-specific hybridization probes. Intervirology. 1987;27(2):102–111. doi: 10.1159/000149727. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Characterization of Aleutian disease virus as a parvovirus. J Virol. 1980 Sep;35(3):836–843. doi: 10.1128/jvi.35.3.836-843.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Bloom M., Hadlow W., Race R. Purification and ultrastructure of Aleutian disease virus of mink. Nature. 1975 Apr 3;254(5499):456–457. doi: 10.1038/254456a0. [DOI] [PubMed] [Google Scholar]
- Cho H. J., Ingram D. G. Antigen and antibody in Aleutian disease in mink. I. Precipitation reaction by agar-gel electrophoresis. J Immunol. 1972 Feb;108(2):555–557. [PubMed] [Google Scholar]
- Cochrane C. G., Koffler D. Immune complex disease in experimental animals and man. Adv Immunol. 1973;16(0):185–264. doi: 10.1016/s0065-2776(08)60298-9. [DOI] [PubMed] [Google Scholar]
- Crandell R. A., Fabricant C. G., Nelson-Rees W. A. Development, characterization, and viral susceptibility of a feline (Felis catus) renal cell line (CRFK). In Vitro. 1973 Nov-Dec;9(3):176–185. doi: 10.1007/BF02618435. [DOI] [PubMed] [Google Scholar]
- Devey M. E., Bleasdale K., Collins M., Steward M. W. Experimental antigen-antibody complex disease in mice. The role of antibody levels, antibody affinity and circulating antigen-antibody complexes. Int Arch Allergy Appl Immunol. 1982;68(1):47–53. doi: 10.1159/000233066. [DOI] [PubMed] [Google Scholar]
- Eklund C. M., Hadlow W. J., Kennedy R. C., Boyle C. C., Jackson T. A. Aleutian disease of mink: properties of the etiologic agent and the host responses. J Infect Dis. 1968 Dec;118(5):510–526. doi: 10.1093/infdis/118.5.510. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr Effects of soluble immune complexes on Fc receptor- and C3b receptor-mediated phagocytosis by macrophages. J Exp Med. 1980 Oct 1;152(4):905–919. doi: 10.1084/jem.152.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T. The pathogenesis of slow virus infections: molecular analyses. J Infect Dis. 1986 Mar;153(3):441–447. doi: 10.1093/infdis/153.3.441. [DOI] [PubMed] [Google Scholar]
- Hadlow W. J., Race R. E., Kennedy R. C. Comparative pathogenicity of four strains of Aleutian disease virus for pastel and sapphire mink. Infect Immun. 1983 Sep;41(3):1016–1023. doi: 10.1128/iai.41.3.1016-1023.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn E. C., Hahn P. S. Autoimmunity in Aleutian disease: contribution of antiviral and anti-DNA antibody to hypergammaglobulinemia. Infect Immun. 1983 Aug;41(2):494–500. doi: 10.1128/iai.41.2.494-500.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A., Niwa Y., Shigematsu H., Taniguchi M., Tada T. Studies on passive serum sickness. II. Factors determining the localization of antigen-antibody complexes in the murine renal glomerulus. Lab Invest. 1978 Mar;38(3):253–262. [PubMed] [Google Scholar]
- Larsen S., Alexandersen S., Lund E., Have P., Hansen M. Acute interstitial pneumonitis caused by Aleutian disease virus in mink kits. Acta Pathol Microbiol Immunol Scand A. 1984 Sep;92(5):391–393. doi: 10.1111/j.1699-0463.1984.tb04419.x. [DOI] [PubMed] [Google Scholar]
- MILLER J. J., 3rd, NOSSAL G. J. ANTIGENS IN IMMUNITY. VI. THE PHAGOCYTIC RETICULUM OF LYMPH NODE FOLLICLES. J Exp Med. 1964 Dec 1;120:1075–1086. doi: 10.1084/jem.120.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Unkeless J. C., Pieczonka M. M., Silverstein S. C. Modulation of Fc receptors of mononuclear phagocytes by immobilized antigen-antibody complexes. Quantitative analysis of the relationship between ligand number and Fc receptor response. J Exp Med. 1983 Jun 1;157(6):1746–1757. doi: 10.1084/jem.157.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Ada G. L., Austin C. M., Pye J. Antigens in immunity. 8. Localization of 125-I-labelled antigens in the secondary response. Immunology. 1965 Oct;9(4):349–357. [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. Aleutian disease of mink. Adv Immunol. 1980;29:261–286. doi: 10.1016/s0065-2776(08)60046-2. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. 3. Immune complex arteritis. Am J Pathol. 1973 May;71(2):331–344. [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J Exp Med. 1969 Sep 1;130(3):575–593. doi: 10.1084/jem.130.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieur D. J., Collier L. L. Animal model of human disease: Chédiak-Higashi syndrome. Am J Pathol. 1978 Feb;90(2):533–536. [PMC free article] [PubMed] [Google Scholar]
- Prieur D. J., Davis W. C., Padgett G. A. Defective function of renal lysosomes in mice with the Chediak-Higashi syndrome. Am J Pathol. 1972 May;67(2):227–236. [PMC free article] [PubMed] [Google Scholar]
- Race R. E., Chesebro B., Bloom M. E., Aasted B., Wolfinbarger J. Monoclonal antibodies against Aleutian disease virus distinguish virus strains and differentiate sites of virus replication from sites of viral antigen sequestration. J Virol. 1986 Jan;57(1):285–293. doi: 10.1128/jvi.57.1.285-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz B. A., Tattersall P. Interaction of minute virus of mice with differentiated cells: strain-dependent target cell specificity is mediated by intracellular factors. J Virol. 1983 Jun;46(3):937–943. doi: 10.1128/jvi.46.3.937-943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Bratton J. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J Virol. 1983 Jun;46(3):944–955. doi: 10.1128/jvi.46.3.944-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., Tal J., Carter B. J. Negative and positive regulation in trans of gene expression from adeno-associated virus vectors in mammalian cells by a viral rep gene product. Mol Cell Biol. 1986 Aug;6(8):2884–2894. doi: 10.1128/mcb.6.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Unanue E. R. Suppression of the immune response to Listeria monocytogenes. I. Immune complexes inhibit resistance. J Immunol. 1984 Jul;133(1):104–109. [PubMed] [Google Scholar]
- Witmer M. D., Steinman R. M. The anatomy of peripheral lymphoid organs with emphasis on accessory cells: light-microscopic immunocytochemical studies of mouse spleen, lymph node, and Peyer's patch. Am J Anat. 1984 Jul;170(3):465–481. doi: 10.1002/aja.1001700318. [DOI] [PubMed] [Google Scholar]