Abstract
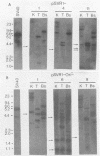
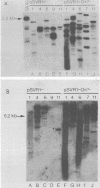
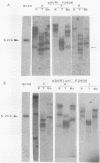
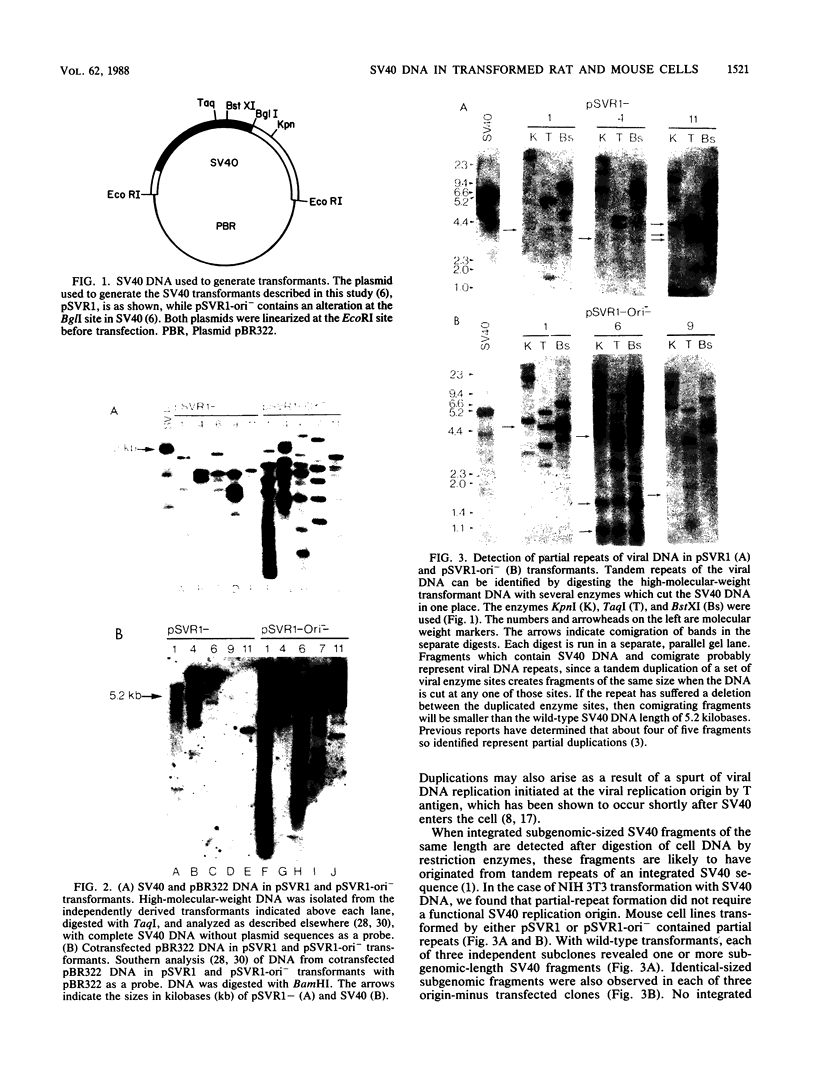
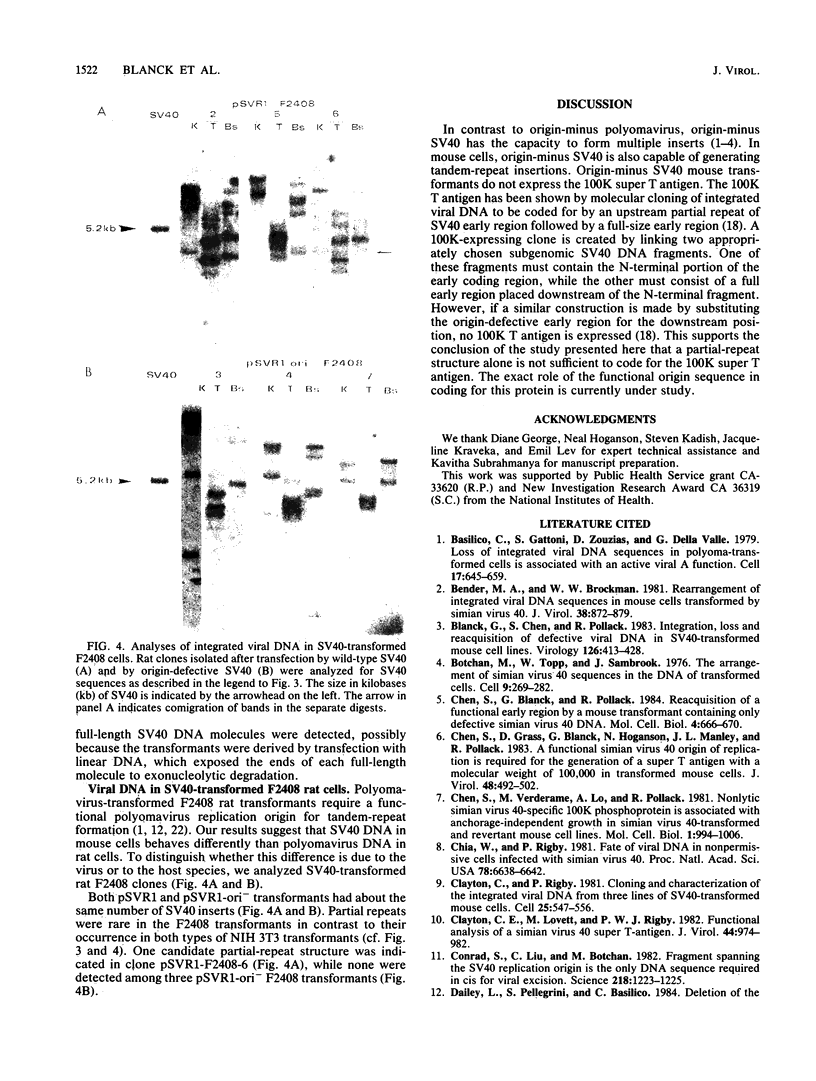
Stable simian virus 40 (SV40) transformation requires integration and expression of the early region of the SV40 genome. We have examined the amount and state of integrated viral DNA of SV40-transformed NIH 3T3 mouse and F2408 rat fibroblast lines generated by transfection with either wild-type or origin-defective SV40 DNA. A functional SV40 replication origin was not required for multiple inserts and partial-repeat structures to form in NIH 3T3 mouse transformants. In contrast, partial repeats in F2408 rat transformants were rare when the SV40 replication origin was intact and not detected at all when it was defective.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Gattoni S., Zouzias D., Valle G. D. Loss of integrated viral DNA sequences in polyomatransformed cells is associated with an active viral A function. Cell. 1979 Jul;17(3):645–659. doi: 10.1016/0092-8674(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Bender M. A., Brockman W. W. Rearrangement of integrated viral DNA sequences in mouse cells transformed by simian virus 40. J Virol. 1981 Jun;38(3):872–879. doi: 10.1128/jvi.38.3.872-879.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanck G., Chen S., Pollack R. Integration, loss, and reacquisition of defective viral DNA in SV40-transformed mouse cell lines. Virology. 1983 Apr 30;126(2):413–428. doi: 10.1016/s0042-6822(83)80001-4. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Chen S., Blanck G., Pollack R. Reacquisition of a functional early region by a mouse transformant containing only defective simian virus 40 DNA. Mol Cell Biol. 1984 Apr;4(4):666–670. doi: 10.1128/mcb.4.4.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Grass D. S., Blanck G., Hoganson N., Manley J. L., Pollack R. E. A functional simian virus 40 origin of replication is required for the generation of a super T antigen with a molecular weight of 100,000 in transformed mouse cells. J Virol. 1983 Nov;48(2):492–502. doi: 10.1128/jvi.48.2.492-502.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Verderame M., Lo A., Pollack R. Nonlytic simian virus 40-specific 100K phosphoprotein is associated with anchorage-independent growth in simian virus 40-transformed and revertant mouse cell lines. Mol Cell Biol. 1981 Nov;1(11):994–1006. doi: 10.1128/mcb.1.11.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia W., Rigby P. W. Fate of viral DNA in nonpermissive cells infected with simian virus 40. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6638–6642. doi: 10.1073/pnas.78.11.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E., Lovett M., Rigby P. W. Functional analysis of a simian virus 40 super T-antigen. J Virol. 1982 Dec;44(3):974–982. doi: 10.1128/jvi.44.3.974-982.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E., Rigby P. W. Cloning and characterization of the integrated viral DNA from three lines of SV40-transformed mouse cells. Cell. 1981 Aug;25(2):547–559. doi: 10.1016/0092-8674(81)90073-8. [DOI] [PubMed] [Google Scholar]
- Conrad S. E., Liu C. P., Botchan M. R. Fragment spanning the SV40 replication origin is the only DNA sequence required in cis for viral excision. Science. 1982 Dec 17;218(4578):1223–1225. doi: 10.1126/science.6293055. [DOI] [PubMed] [Google Scholar]
- Dailey L., Pellegrini S., Basilico C. Deletion of the origin of replication impairs the ability of polyomavirus DNA to transform cells and to form tandem insertions. J Virol. 1984 Mar;49(3):984–987. doi: 10.1128/jvi.49.3.984-987.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y., Frisque R. J., Sambrook J. Origin-defective mutants of SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):293–300. doi: 10.1101/sqb.1980.044.01.033. [DOI] [PubMed] [Google Scholar]
- Hiscott J. B., Murphy D., Defendi V. Instability of integrated viral DNA in mouse cells transformed by simian virus 40. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1736–1740. doi: 10.1073/pnas.78.3.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J., Murphy D., Defendi V. Amplification and rearrangement of integrated SV40 DNA sequences accompany the selection of anchorage-independent transformed mouse cells. Cell. 1980 Nov;22(2 Pt 2):535–543. doi: 10.1016/0092-8674(80)90363-3. [DOI] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Jensen F. C., Steplewski Z. Activation of production of infectious tumor virus SV40 in heterokaryon cultures. Proc Natl Acad Sci U S A. 1967 Jul;58(1):127–133. doi: 10.1073/pnas.58.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt A., Chen S., Blanck G., George D., Pollack R. E. Two integrated partial repeats of simian virus 40 together code for a super-T antigen. Mol Cell Biol. 1985 Apr;5(4):742–750. doi: 10.1128/mcb.5.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litzkas P., Jha K. K., Ozer H. L. Efficient transfer of cloned DNA into human diploid cells: protoplast fusion in suspension. Mol Cell Biol. 1984 Nov;4(11):2549–2552. doi: 10.1128/mcb.4.11.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni M. A., Roufa D. J. Replication of viral DNA sequences integrated within the chromatin of SV40-transformed Chinese hamster lung cells. Cell. 1981 Oct;26(2 Pt 2):245–258. doi: 10.1016/0092-8674(81)90307-x. [DOI] [PubMed] [Google Scholar]
- May E., Kress M., Daya-Grosjean L., Monier R., May P. Mapping of the viral mRNA encoding a super-T antigen of 115,000 daltons expressed in simian virus 40-transformed rat cell lines. J Virol. 1981 Jan;37(1):24–35. doi: 10.1128/jvi.37.1.24-35.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini S., Dailey L., Basilico C. Amplification and excision of integrated polyoma DNA sequences require a functional origin of replication. Cell. 1984 Apr;36(4):943–949. doi: 10.1016/0092-8674(84)90044-8. [DOI] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robins D. M., Ripley S., Henderson A. S., Axel R. Transforming DNA integrates into the host chromosome. Cell. 1981 Jan;23(1):29–39. doi: 10.1016/0092-8674(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Sager R., Anisowicz A., Howell N. Genomic rearrangements in a mouse cell line containing integrated SV40 DNA. Cell. 1981 Jan;23(1):41–50. doi: 10.1016/0092-8674(81)90268-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]