Abstract
In wild-type yeast mitochondrial inheritance occurs early in the cell cycle concomitant with bud emergence. Cells lacking the PTC1 gene initially produce buds without a mitochondrial compartment; however, these buds later receive part of the mitochondrial network from the mother cell. Thus, the loss of PTC1 causes a delay, but not a complete block, in mitochondrial transport. PTC1 encodes a serine/threonine phosphatase in the high-osmolarity glycerol response (HOG) pathway. The mitochondrial inheritance delay in the ptc1 mutant is not attributable to changes in intracellular glycerol concentrations or defects in the organization of the actin cytoskeleton. Moreover, epistasis experiments with ptc1Δ and mutations in HOG pathway kinases reveal that PTC1 is not acting through the HOG pathway to control the timing of mitochondrial inheritance. Instead, PTC1 may be acting either directly or through a different signaling pathway to affect the mitochondrial transport machinery in the cell. These studies indicate that the timing of mitochondrial transport in wild-type cells is genetically controlled and provide new evidence that mitochondrial inheritance does not depend on a physical link between the mitochondrial network and the incipient bud site.
INTRODUCTION
Mitochondria are essential organelles that produce the majority of the cellular ATP required for the growth and proliferation of eukaryotic cells. Cytological studies indicate that mitochondrial morphology and distribution can vary in different cell types, ranging from small, spherical organelles to a complex reticulum or network (Bereiter-Hahn, 1990; Bereiter-Hahn and Vöth, 1994). Although it is clear that mitochondria can increase in mass and divide by fission, they cannot be synthesized de novo and must be inherited by daughter cells during division.
In the budding yeast Saccharomyces cerevisiae, mitochondria form a network of tubular membranes located at the cell cortex (Hoffman and Avers, 1973). A portion of this mitochondrial network is transported into the emerging bud very early in the cell cycle (G1-S) (Stevens, 1981). Both genetic and biochemical analyses suggest that this polarized transport of mitochondria from mother to bud is an active process requiring functions of the cytoskeleton including actin (Drubin et al., 1993; Lazzarino et al., 1994; Simon et al., 1995; Hermann et al., 1997) and an intermediate filament-like protein (McConnell et al., 1990; McConnell and Yaffe, 1992, 1993; Berger and Yaffe, 1996). A number of studies indicate that transmission of mitochondrial genomes (nucleoids) is also regulated during yeast budding, and genes that affect this process have been isolated (Strausberg and Perlman, 1978; Zinn et al., 1987; Diffley and Stillman, 1991, 1992; Azpiroz and Butow, 1993; Chen et al., 1993; Guan et al., 1993; Campbell et al., 1994; Backer, 1995; Zelenaya-Troitskaya et al., 1995; Nunnari et al., 1997). Genetic screens have also identified molecules that play a role in establishing and/or maintaining mitochondrial morphology (Burgess et al., 1994; Sogo and Yaffe, 1994; Berger and Yaffe, 1996; Berger et al., 1997). In all of the mitochondrial inheritance and morphology mutants described to date, daughter cells that fail to receive a mitochondrial compartment do not separate from the mother cell and are unable to produce buds themselves.
Transmission of the mitochondrial network in wild-type yeast always begins immediately after bud emergence, suggesting that mitochondrial inheritance is tightly coordinated with the cell cycle (Stevens, 1981). This coordination could be achieved by attaching the mitochondrial network to structures at the incipient bud site and passively pulling the organelle into the growing bud. Alternatively (or in addition), molecules that control mitochondrial transport could be regulated in a cell cycle-dependent manner. Although mutations that completely block mitochondrial inheritance have been isolated (see above), genetic analyses performed to date have not uncovered genes that control the “timing” of mitochondrial movement into the bud. Here we report the isolation of a new mutation, mdm28 (mdm = mitochondrial distribution and morphology), that causes a pronounced delay in mitochondrial inheritance. mdm28-null cells initially produce buds that lack mitochondria, but these buds eventually receive part of the mitochondrial network from the mother cell. MDM28 is identical to PTC1, a gene encoding a serine/threonine phosphatase that is thought to regulate the high-osmolarity glycerol response (HOG) pathway (Maeda et al., 1994). Our analyses suggest that Ptc1p does not act through the HOG pathway kinases to influence the mitochondrial transport machinery in the cell. Rather, Ptc1p may be acting directly or through an alternative signaling pathway to affect this process. These studies identify a new role for the Ptc1p serine/threonine phosphatase in cells and provide the first evidence that mitochondrial inheritance is not physically linked to bud emergence in yeast.
MATERIALS AND METHODS
Yeast Strains
The S. cerevisiae strains used in this study are listed in Table 1. Plasmid pptc1-Δ1 (provided by E. Benson and G. Payne, University of California at Los Angeles, Los Angeles, CA) was used to construct the PTC1 disruption in strain JSY118. The plasmid pDHG12 (provided by M. Gustin, Rice University, Houston, TX) was used to disrupt HOG1 in FY250 to create AMY36. All disruptions were verified by Southern blot analysis. Strains AMY36 and JSY118 were mated to produce AMY38. AMY38 was sporulated to produce the strain AMY43. For some experiments, the strains JSY118 and JSY836 were transformed with the plasmid pOK29 (generously provided by O. Kerscher and R. Jensen, Johns Hopkins Medical School, Baltimore, MD) to allow visualization of the mitochondrial network by Cox4-GFP.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| JSY836 | Mata, ura3-52, his3Δ200, trp1Δ63 | This laboratory |
| JSY118 | Mata, ura3-52, his3Δ200, trp1Δ63, ptc1::TRP1 | This study |
| FY250 | Matα, Mal, Gal2+, ura3-52, his3Δ200, trp1Δ63, leu2Δ1 | F. Winston (Winston et al., 1995)a |
| AMY36 | Matα, Mal, Gal2+, ura3-52, his3Δ200, trp1Δ63, leu2Δ1 hog1::URA3 | This study |
| AMY38 | Mata/α, ura3-52/ura3-52, his3Δ200/his3Δ200, trp1Δ63/trp1Δ63, ptc1::TRP1/+, +/leu2Δ1, hog1::URA3/+ | This study |
| AMY43 | Mata, ura3-52, his3Δ200, trp1Δ63, ptc1::TRP1, hog1::URA3 | This study |
| SEY6210 | Mata, ura3-52, leu2-3, 112, his3-Δ200, trp1-Δ901, lys2-801 suc2-Δ9, GAL | S. Emr (Robinson et al., 1988) |
| JSY2090 | Mata, ura3-52, leu2-3, 112, his3-Δ200, trp1-Δ901, lys2-801 suc2-Δ9, GAL, ptc1::URA3 | T. Ketela and H. Bussey |
| JSY2092 | Matα, ura3-52, leu2-3, 112, his3-Δ200, trp1-Δ901, lys2-801 suc2-Δ9, GAL, pbs2::KANMX2 | T. Ketela and H. Bussey |
| JSY2093 | Mata, ura3-52, leu2-3, 112, his3-Δ200, trp1-Δ901, lys2-801 suc2-Δ9, GAL, pbs2::KANMX2, ptc1::URA3 | T. Ketela and H. Bussey |
| JSY2589 | Mata, ura3-52, leu2-3, 112, his3-Δ200, trp1-Δ901, lys2-801 suc2-Δ9, GAL, ptp2::KANMX2 | T. Ketela and H. Bussey |
Same genotype as FY834 but LYS2.
Culture Conditions and Media
The yeast strains were grown and maintained in liquid YP dextrose (YPD) or YP glycerol (YPG) media and on YPD or YPG plates at 25°C. The JSY836 and JSY118 strains containing the pOK29 plasmid were grown on SG minus histidine plates to select for maintenance of the plasmid. Liquid cultures were grown in Innova 3000 water bath shakers (New Brunswick Scientific) at shaking speeds between 200 and 240 rpm. To induce sporulation, cells were transferred to sporulation plates containing amino acids at 25% of supplemented minimal medium levels (Kaiser et al., 1994) and grown at 25°C.
Cloning and Disruption of MDM28 (PTC1)
Temperature-sensitive mitochondrial inheritance mutants were isolated and back-crossed as described by Hermann et al. (1997). The strain containing the mdm28 mutation was transformed with yeast genomic libraries contained within the YCp50 plasmid (Rose et al., 1987) (obtained from American Type Culture Collection, Rockville, MD) and the p366 plasmid (provided by T. Formosa, University of Utah, Salt Lake City, UT). Colonies were selected for growth at 37°C on SG minus leucine for p366 transformants and SG minus uracil for YCp50 transformants. Three colonies from the YCp50 library and eight colonies from the p366 library were found to contain plasmids that rescued the mdm28 temperature-sensitive growth defect on glycerol and the mitochondrial inheritance delay phenotype.
Restriction analysis and sequencing indicated that the YCp50 transformants contained three different overlapping inserts between 9.1 and 12.1 kb. These inserts were in a region of the left arm of chromosome IV that had previously been sequenced. The region of DNA responsible for complementing the mdm28 phenotype was located within the cosmids SCCHRIV42 and SC8119, which contained three genes, PTC1, YTA5, and D2930. Standard subcloning procedures were used to show that the PTC1 gene complemented the mutant phenotypes in mdm28. All eight p366 transformants were shown to contain PTC1 by Southern blotting. PTC1 (GenBank accession number L14593) was originally identified as the gene TPD1 in a screen for mutants in tRNA biosynthesis (van Zyl et al., 1989).
A ptc1 null mutation was generated using the pptc1-Δ1 plasmid. Briefly, a 1.7-kb fragment was released from pptc1–Δ1 by digestion with the restriction enzyme EcoRI. This fragment was then transformed into the yeast strain JSY836, and TRP+ colonies were selected. The ptc1::TRP1 disruption was verified by Southern blotting of genomic DNA from the TRP+ transformants.
Characterization of the ptc1Δ Phenotype
To determine the effects of the PTC1 deletion on growth rate, both wild-type (JSY836) and ptc1Δ (JSY118) cells were grown to log phase in liquid YPD or YPG medium at 25°C. Equal numbers of cells were serially diluted (10-fold dilutions) and spotted onto YPG or YPD solid medium. Plated cells were grown at 25, 30, or 37°C for 3 d. Growth rates were also analyzed in liquid medium. Cultures grown overnight in YPD or YPG medium were diluted to 0.1 OD600/ml in fresh YPD or YPG medium and grown at 25, 30, or 37°C. The densities of the cultures were measured at the indicated times.
Yeast mitochondrial networks were visualized by growing cells overnight in YPD at 25°C and staining with 100 nM 3,3′-dihexyloxacarbocyanine (DiOC6; Molecular Probes, Eugene, OR) as described previously (Hermann et al., 1997). Alternatively, JSY836 and JSY118 cells expressing the Cox4-GFP protein (targeted to the mitochondrial matrix; pOK29 plasmid provided by O. Kerscher and R. Jensen) were grown overnight under the same conditions and inspected microscopically. To quantify mitochondrial inheritance, fields of stained, dividing cells were analyzed for the presence or absence of the mitochondrial network in buds (n ≥ 100). Comparable results were obtained using both of these staining methods (Table 2), suggesting that the mitochondrial inheritance phenotype observed in ptc1Δ cells is not attributable to the presence of the Cox4-GFP fusion protein.
Table 2.
Comparison of the mitochondrial inheritance delay in DiOC6 and Cox4-GFP-stained ptc1Δ cells
| Strain | Temperature (°C) | Mitochondrial label | % of buds without mitochondria (n)a |
|---|---|---|---|
| JSY836 (WT) | 25 | DiOC6 | 1 (201) |
| 25 | Cox4-GFP | 1 (400) | |
| JSY118 (ptc1Δ) | 25 | DiOC6 | 24.9 (205) |
| 25 | Cox4-GFP | 27 (400) | |
| JSY836 (WT) | 37 | DiOC6 | 3 (202) |
| 37 | Cox4-GFP | 5.9 (407) | |
| JSY118 (ptc1Δ) | 37 | DiOC6 | 51.7 (201) |
| 37 | Cox4-GFP | 40 (403) |
n, Number of budded cells scored.
To synchronize cultures, cells grown to log phase in YPD medium at 25, 30, or 37°C were diluted to 0.5 OD600/ml, and α-factor (Sigma, St. Louis, MO) was added to a final concentration of 5 μM. After 3 h at the appropriate temperature, aliquots of each culture were analyzed microscopically to quantify the extent of synchronization (percentage of unbudded cells). The synchronized cultures were pelleted, washed two times in YPD medium, resuspended in YPD, and incubated at the indicated temperatures. Bud size and mitochondrial inheritance (DiOC6) were scored at 0.5-h intervals until the first round of budding was nearly completed in the ptc1Δ cultures.
Analysis of the Actin Cytoskeleton in ptc1Δ Cells by Indirect Immunofluorescence
Indirect immunofluorescence was used to analyze the organization of actin and the distribution of mitochondrial networks in wild-type and ptc1Δ cells. Log phase JSY836 and JSY118 cultures grown at 30°C in YPD medium were fixed essentially as described by Pringle et al. (1991). The cells were stained simultaneously with the following primary antibodies: 1) goat anti-actin antibody (1:50 dilution; Karpova, et al., 1993); and 2) mouse anti-porin monoclonal antibody (1:75 dilution; Molecular Probes). The primary antibodies were visualized by staining with the following secondary antibodies (applied sequentially): 1) rabbit anti-goat DTAF (1:1000 dilution, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA); and 2) goat anti-mouse LRSC (1:200 dilution, Jackson ImmunoResearch Laboratories, Inc.). Actin cytoskeletons and mitochondrial networks were visualized on a Zeiss epifluorescence microscope as described previously (Roeder and Shaw, 1996). Both mitochondrial distribution and actin organization were scored in budded cells. For actin organization, categories scored included no actin staining (approximately 1% in wild-type and ptc1Δ cells), wild-type actin organization (actin cables oriented toward the bud and actin patches clustered in the bud), or non–wild-type actin organization (absent or incorrect actin patch localization and actin cables absent or incorrectly oriented). For mitochondrial distribution, categories scored included the presence or absence of mitochondrial network in buds.
Analysis of the Effects of High-Osmolarity Medium on Mitochondrial Inheritance
JSY836 and JSY118 cells containing the pOK29 plasmid were grown overnight at 25°C in YPD and resuspended at a cell density of 0.5 OD600/ml in YPD containing 0.9 M NaCl to induce intracellular glycerol synthesis. Mitochondrial inheritance by buds was scored at 5 and 24 h after transfer to high-osmolarity medium. In control experiments, intracellular glycerol levels before and after growth in YPD plus 0.9 M NaCl were measured enzymatically as described previously (Jiang et al., 1995). When cells were grown in YPD medium, the glycerol concentration in the ptc1Δ strain (JSY118) was 1.3-fold higher than that of the isogenic wild-type strain (JSY836). The glycerol concentration of wild-type cells grown in YPD containing 0.9 M NaCl was 8.8-fold higher (after 5 h) and 6.5-fold higher (after 24 h) than that observed in YPD alone. The glycerol concentration of ptc1Δ cells grown in YPD containing 0.9 M NaCl was 6.6-fold higher (after 5 h) and 3.25-fold higher (after 24 h) than that observed in ptc1Δ cells grown in YPD alone.
Microscopy
In all experiments, the cells were viewed with a Zeiss Axioplan microscope equipped with differential interference contrast optics, epifluorescence capabilities, and a Zeiss Acroplan-Neofluar 100× (numerical aperture, 1.3) objective. LRSC (rhodamine), DiOC6/Cox4-GFP/DTAF and DAPI fluorescence were visualized using 546, 450–490, and 365 nm bandpass filters, respectively.
Images were captured using a video camera and assembled into figures as described previously (Roeder and Shaw, 1996). A Tektronix (Wilsonville, OR) Phaser IIsdx dye sublimation printer was used to print Figures 1, 2, and 4.
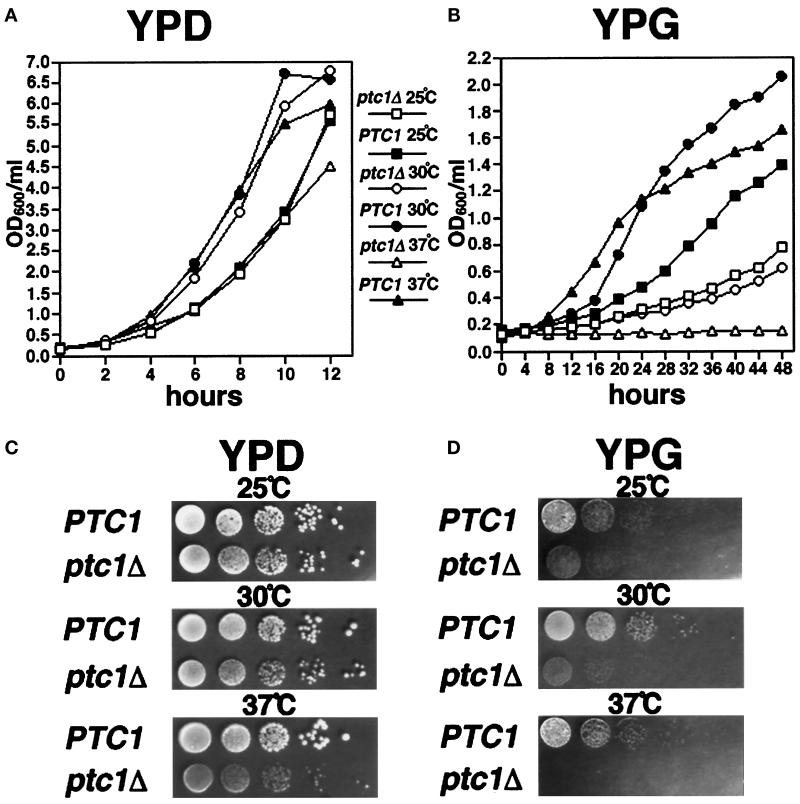
Figure 1.
Growth of wild-type (PTC1) and ptc1Δ cells at 25, 30, and 37°C. The OD600 values of PTC1 and ptc1Δ cells grown in YPD (A) or YPG (B) at 25, 30, or 37°C were measured at the indicated time intervals. PTC1 and ptc1Δ strains were serially diluted, spotted onto solid YPD (C) or YPG (D) medium, and grown at 25, 30, and 37°C for 3 d.
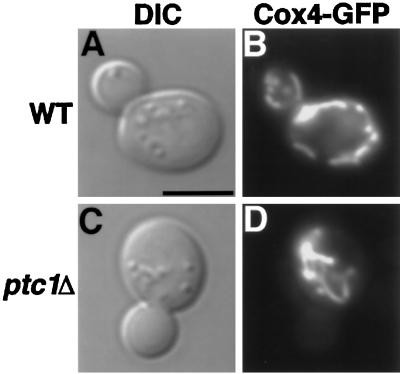
Figure 2.
ptc1Δ cells exhibit defects in mitochondrial inheritance. DIC images (A and C) and Cox4-GFP mitochondrial staining (B and D) of representative wild-type (PTC1) and ptc1Δ cells grown at 25°C are shown. Bar, 5 μm.
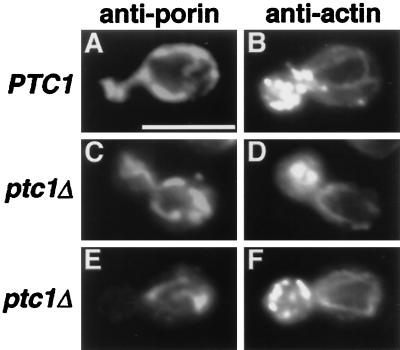
Figure 4.
Actin cables and patches are present and properly localized in PTC1 and ptc1Δ cells that contain mitochondria in buds (C and D) and in cells that have not transported mitochondria into buds (E and F). Wild-type (PTC1) and ptc1Δ cells grown in YPD medium at 30°C were fixed and stained with both actin (B, D, and F) and porin (A, C, and E) antibodies. Bar, 5 μm.
RESULTS
MDM28 Is Required for Mitochondrial Inheritance and Is Identical to the Serine/Threonine Phosphatase PTC1
The mdm28–1 mutant was originally identified in a screen for temperature-sensitive yeast strains exhibiting defects in mitochondrial inheritance (Hermann et al., 1997). In mdm28–1 mutant cells, 29.8% (25°C) or 43.9% (37°C) of growing buds failed to inherit mitochondrial networks stained with the potential-dependent fluorescent dye DiOC6. In contrast, only a small percentage of buds produced in a wild-type culture lack mitochondrial staining (4.9% at 25°C or 2.4% at 37°C). The mdm28 strain grew slowly compared with wild type on YPD solid medium at 37°C and failed to grow at 37°C on YPG medium (containing the nonfermentable carbon source glycerol). This recessive, temperature-sensitive growth defect on YPG medium segregated 2:2 in back-crosses with a wild-type strain and was linked to the mitochondrial inheritance phenotype observed in the original mdm28 isolate.
We cloned the MDM28 gene by complementation of the temperature-sensitive growth defect on glycerol in the mdm28–1 strain (see MATERIALS AND METHODS). Integrative mapping studies demonstrated that the cloned DNA contained the wild-type MDM28 gene. A combination of DNA sequence analysis and subcloning indicated that a fragment containing the previously identified gene, PTC1, rescued both the mitochondrial inheritance defect and the temperature-sensitive growth defect in the mdm28–1 mutant strain.
PTC1 encodes a type 2C serine/threonine phosphatase first isolated in a screen for mutants deficient in de novo tRNA biosynthesis (originally called TPD1; van Zyl et al., 1989). PTC1 was independently identified by Maeda et al. (1993) based on its synthetic lethal interaction with a tyrosine phosphatase mutant, ptp2. Subsequent studies showed that overexpression of Ptc1p could suppress the lethality caused by deletion of SLN1, a gene required for regulation of intracellular osmolarity (Maeda et al., 1994). Sln1p is a plasma membrane osmosensor that negatively regulates a downstream MAP kinase cascade in the HOG pathway. In yeast, the HOG pathway plays a critical role in regulating intracellular glycerol concentrations in response to osmotic stress. Both Ptp2p and Ptc1p are proposed to negatively regulate MAP kinases in the HOG pathway (see below).
A previous study showed that ptc1 null strains exhibited additional phenotypes including reduced sporulation efficiency and temperature-sensitive defects in cell separation during mitotic growth (reported as 80% multiply budded cells at 37°C; Robinson et al., 1994). The mutant also failed to grow at 37°C on YPD and at 28°C on a subset of nonfermentable carbon sources (glycerol, pyruvate, and acetate). When we generated a disruption of PTC1 (ptc1Δ) in our standard laboratory strain background (see MATERIALS AND METHODS), we observed similar growth defects on nonfermentable carbon sources (glycerol, ethanol, and acetate). In addition, in our strain background, the ptc1Δ mutant grew slower than wild type on solid YPG medium at 25 and 30°C and did not form colonies at 37°C (Figure 1D). Although our ptc1 mutant grew essentially as well as wild type on YPD plates at 25 and 30°C, colonies formed at 37°C were much smaller than wild type (Figure 1C). Similar differences in wild-type and ptc1Δ growth rates were also apparent in log phase cells grown in YPD and YPG liquid medium (Figure 1, A and B).
Mitochondrial Inheritance Is Delayed, But Not Blocked, In Cells Lacking PTC1
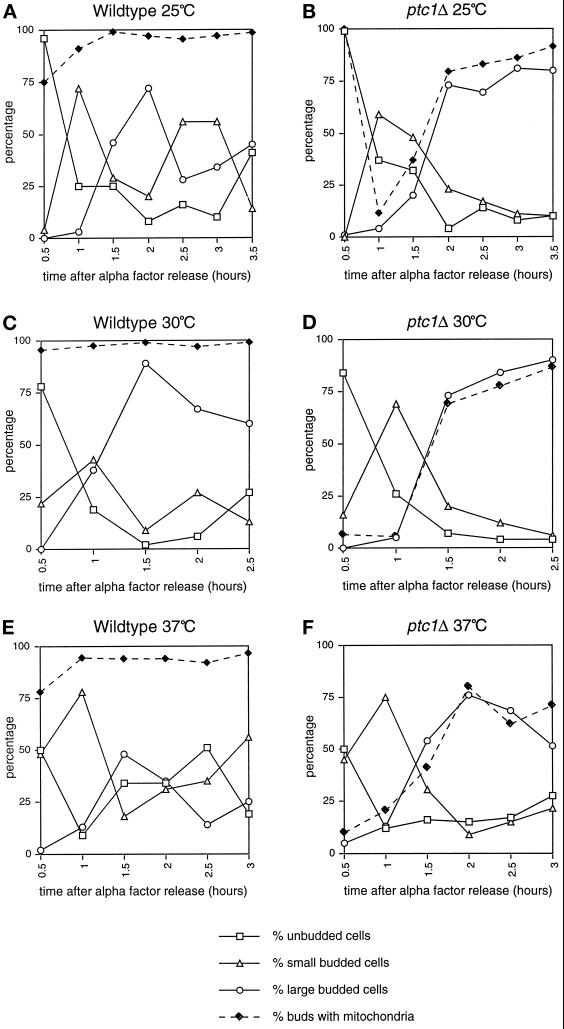
When a wild-type strain is grown at 25°C (Figure 2, A and B) or 37°C (Roeder, Hermann, Keegan, Thatcher, and Shaw, unpublished observations) in YPD liquid medium, mitochondrial networks visualized with a targeted form of the green fluorescent protein (Cox4-GFP) were easily detected in growing buds, regardless of bud size. In contrast, ptc1Δ mutant cells often produced buds that lacked this mitochondrial staining at 25°C (Figure 2, C and D) and at 37°C (Roeder, Hermann, Keegan, Thatcher, and Shaw, unpublished data). Despite this mitochondrial inheritance defect, the ptc1 mutant appeared to grow in YPD liquid culture at both temperatures (Figure 1A), suggesting that at least some buds produced by this strain were viable. Quantification of mitochondrial inheritance in the ptc1Δ strain revealed that a high percentage of small ptc1Δ buds lacked DiOC6-stained mitochondrial networks (63.0% at 25°C, 66.7% at 30°C, and 66.7% at 37°C). However, by the time buds were half the diameter of the mother cell (large buds), the defect was less severe (14.9% at 25°C, 16.7% at 30°C, and 5.6% at 37°C; Table 3). These results suggested that the timing of mitochondrial transfer to buds might be delayed, rather than blocked, in the ptc1 mutant strain. To test this possibility, we compared mitochondrial inheritance defects in wild-type and ptc1 cells during a single round of budding at 25, 30, and 37°C in YPD liquid medium after α-factor arrest and release. (This experiment could not be done in YPG medium, because ptc1Δ mutant cells never bud after α-factor arrest and release when glycerol is provided as the sole carbon source). As shown in Tables 4–6 and represented graphically in Figure 3, when wild-type cells arrested in the unbudded stage were released, the majority of newly formed small buds (Figure 3, A, C, and E, open triangles, 1-h time point) contained DiOC6-stained mitochondrial networks (Figure 3, A, C and E, closed diamonds, 1-h time point). As expected, at later time points the large buds also contained mitochondrial networks (Figure 3, A, C and E, open circles). In the ptc1Δ cells, the majority of small buds produced (Figure 3, B, D, and F, open triangles, see 1-h time point) did not contain mitochondrial networks (Figure 3, B, D, and F, closed diamonds, see 1-h time point). However, the proportion of buds containing mitochondria increased as bud size increased, indicating that mutations in PTC1 caused a delay, rather than a complete block, in mitochondrial inheritance (Figure 3, B, D, and F, open circles and closed diamonds). DAPI staining did not reveal a similar delay in nuclear segregation, suggesting that mutations in PTC1 do not cause a general delay in cytoplasmic organelle transfer to buds.
Table 3.
Mitochondrial inheritance in PTC1 and ptc1Δ cells grown in YPD
| Strain | Temperature (°C) | % of unbudded cells | % of smalla budded cells | % of small buds without mitochondria | % of largeb budded cells | % of large buds without mitochondria | % of total buds without mitochondria |
|---|---|---|---|---|---|---|---|
| JSY836 (WT) | 25 | 24 | 37 | 5.4 | 39 | 0 | 2.6 |
| JSY118 (ptc1Δ) | 25 | 26 | 27 | 63.0 | 47 | 14.9 | 32.4 |
| JSY836 (WT) | 30 | 39 | 21 | 0 | 40 | 0 | 0 |
| JSY118 (ptc1Δ) | 30 | 40 | 18 | 66.7 | 42 | 16.7 | 31.7 |
| JSY836 (WT) | 37 | 30 | 35 | 11.4 | 35 | 0 | 5.7 |
| JSY118 (ptc1Δ) | 37 | 22 | 24 | 66.7 | 54 | 5.6 | 24.4 |
The data shown correspond to the 6-h time point in Figure 1A. At least 100 cells were scored for each strain at each temperature.
Buds less than half the diameter of the mother cell.
Buds greater than half the diameter of the mother cell.
Table 4.
Mitochondrial inheritance is delayed in ptc1Δ cells after α-factor arrest and release at 25°C
| Strain | Time after release from α-factor (h) | % of unbudded cells | % of smalla budded cells | % of small buds without mitochondria | % of largeb budded cells | % of large buds without mitochondria | % of multiplyc budded cells | % of multiply budded cells without mitochondria in largest bud |
|---|---|---|---|---|---|---|---|---|
| WT | 0.5 | 96 | 4 | 25 | 0 | 0 | ||
| ptc1Δ | 0.5 | 99 | 0 | 0 | 1 | 0 | 0 | |
| WT | 1.0 | 25 | 72 | 9.7 | 3 | 0 | 0 | |
| ptc1Δ | 1.0 | 37 | 59 | 93.2 | 4 | 25 | 0 | |
| WT | 1.5 | 25 | 29 | 3.4 | 46 | 0 | 0 | |
| ptc1Δ | 1.5 | 32 | 48 | 87.5 | 20 | 5 | 0 | |
| WT | 2.0 | 8 | 20 | 10 | 71 | 1.4 | 1 | 0 |
| ptc1Δ | 2.0 | 4 | 23 | 78.3 | 72 | 2.9 | 1 | 0 |
| WT | 2.5 | 16 | 56 | 7.1 | 28 | 0 | 0 | |
| ptc1Δ | 2.5 | 13.9 | 16.8 | 88.2 | 60.4 | 0 | 8.9 | 0 |
| WT | 3.0 | 10 | 56 | 5.4 | 34 | 0 | 0 | |
| ptc1Δ | 3.0 | 8 | 11 | 81.8 | 26 | 7.7 | 55 | 3.6 |
| WT | 3.5 | 41 | 14 | 7.1 | 45 | 0 | 0 | |
| ptc1Δ | 3.5 | 10 | 10 | 80 | 19 | 0 | 61 | 0 |
At least 100 cells were scored for each strain at each time point.
Buds less than half the diameter of the mother cell.
Buds greater than half the diameter of the mother cell.
An increase in the percentage of multiply budded cells is observed in ptc1Δ cells after α-factor treatment. The percentage of unbudded wild-type cells upon α-factor release at 25°C was 99%. The percentage of unbudded ptc1Δ cells on α-factor release at 25°C was 90%.
Table 6.
Mitochondrial inheritance is delayed in ptc1Δ cells after α-factor arrest and release at 37°C
| Strain | Time after release from α-factor (h) | % of unbudded cells | % of smalla budded cells | % of small buds without mitochondria | % of largeb budded cells | % of large buds without mitochondria | % of multiplyc budded cells | % of multiply budded cells without mitochondria in largest bud |
|---|---|---|---|---|---|---|---|---|
| WT | 0.5 | 50 | 48 | 22.9 | 2 | 0 | 0 | |
| ptc1Δ | 0.5 | 50 | 45 | 93.3 | 5 | 60 | 0 | |
| WT | 1.0 | 9 | 78 | 6.4 | 13 | 0 | 0 | |
| ptc1Δ | 1.0 | 12 | 75 | 88 | 13 | 30.8 | 0 | |
| WT | 1.5 | 34 | 18 | 22.2 | 48 | 0 | 0 | |
| ptc1Δ | 1.5 | 15.7 | 30.4 | 90.3 | 53.9 | 41.8 | 0 | |
| WT | 2.0 | 34 | 31 | 12.9 | 35 | 0 | 0 | |
| ptc1Δ | 2.0 | 15 | 9 | 77.8 | 55 | 18.2 | 21 | 0 |
| WT | 2.5 | 51 | 35 | 11.4 | 14 | 0 | 0 | |
| ptc1Δ | 2.5 | 16.8 | 14.9 | 60 | 29.7 | 46.7 | 38.6 | 23.1 |
| WT | 3.0 | 19 | 56 | 5.4 | 24 | 0 | 1 | 0 |
| ptc1Δ | 3.0 | 27.3 | 21.2 | 76.2 | 7.1 | 14.3 | 44.4 | 9.1 |
At least 100 cells were scored for each strain at each time point.
Buds less than half the diameter of the mother cell.
Buds greater than half the diameter of the mother cell.
An increase in the percentage of multiply budded cells is observed in ptc1Δ cells after α-factor treatment. The percentage of unbudded wild-type cells upon α-factor release 37°C was 98%. The percentage of unbudded ptc1Δ cells on α-factor release at 37°C was 88%.
Figure 3.
Mitochondrial inheritance is delayed in ptc1Δ cells. After release from α-factor arrest, wild-type (A, C, and E) and ptc1Δ (B, D, and F) cells were scored for bud size (open squares, triangles, and circles) and mitochondrial inheritance (closed diamonds) by the bud at 25°C (A and B), 30°C (C and D), and 37°C (E and F). The percentages of unbudded wild-type cells upon α-factor release at 25, 30, and 37°C were 99, 99, and 98%, respectively. The percentages of unbudded ptc1Δ cells upon α-factor release at 25, 30, and 37°C were 90, 98, and 88%, respectively. Note that at 0.5 h in B, only one budded cell (Table 4; 1%, n = 100) was present, and this bud contained mitochondria.
ptc1Δ cells failed to grow in YPG medium at 37°C (Figure 1, B and D); however, this growth defect was not simply attributable to the failure of ptc1 buds to inherit mitochondria. Although ptc1Δ cells undergo an immediate cell cycle arrest upon transfer to 37°C in YPG medium (Figure 1B), the percentage of budded cells in the arrested cultures that contained DiOC6-stained mitochondrial networks was comparable to wild type (Table 7; wild type, 7.8%, vs. ptc1Δ, 7.0%). In addition, a delay in mitochondrial inheritance cannot completely explain the slow growth observed for ptc1Δ cells in YPG medium at 25 and 30°C (Figure 1, B and D). At 25°C, we observed only a slight mitochondrial inheritance delay in the ptc1 mutant relative to wild type (Table 7, 10.2 and 4.9%, respectively) and no delay in the ptc1 mutant relative to wild type at 30°C (Table 7, 7.7 and 10.3%, respectively). Although the doubling times of ptc1Δ cells grown in YPD medium at 25°C (2.5 h) and 30°C (2.2 h) are comparable to wild type (2.3 h at 25°C, 2.3 h at 30°C), the ptc1 mutant cells divide more slowly than wild-type cells in YPG medium (ptc1Δ: 18.9 h at 25°C, 22.7 h at 30°C; wild type: 10.1 h at 25°C, 5.9 h at 30°C). Thus, mitochondrial inheritance apparently “catches up” with slowly growing buds when ptc1 cells are cultured in glycerol at 25 and 30°C, and, as a result, no severe delay phenotype is detected.
Table 7.
Mitochondrial inheritance in PTC1 and ptc1Δ cells grown in YPG
| Strain | Temperature (°C) | % of unbudded cells | % of smalla budded cells | % of small buds without mitochondria | % of largeb budded cells | % of large buds without mitochondria | % ot total buds without mitochondria |
|---|---|---|---|---|---|---|---|
| JSY836 (WT) | 25 | 39 | 38 | 7.9 | 23 | 0 | 4.9 |
| JSY118 (ptc1Δ) | 25 | 51 | 29 | 17.2 | 20 | 0 | 10.2 |
| JSY836 (WT) | 30 | 42 | 33 | 18.2 | 25 | 0 | 10.3 |
| JSY118 (ptc1Δ) | 30 | 48 | 30 | 13.3 | 22 | 0 | 7.7 |
| JSY836 (WT) | 37 | 36 | 39 | 12.8 | 25 | 0 | 7.8 |
| JSY118 (ptc1Δ) | 37 | 43 | 35 | 11.4 | 22 | 0 | 7.0 |
The data shown correspond to the 16-h time point in Figure 1B. At least 100 cells were scored for each strain at each temperature.
Buds less than half the diameter of the mother cell.
Buds greater than half the diameter of the mother cell.
Increased Frequency of Petite Generation in ptc1Δ Cells
We also observed that ptc1Δ cells maintained on solid YPD medium generated petites (lost mitochondrial genome function) at a higher frequency than wild type. At 30°C, 24.4% of ptc1Δ cells became petite compared with 1.8% in an isogenic wild-type strain. This increased frequency of petite generation in ptc1Δ probably contributes to the growth defects observed for this strain on YPG medium (Figure 1). Although increases in the frequency of petite generation have been observed for other strains defective in mitochondrial distribution and morphology, the molecular basis for this phenomenon is unclear (reviewed in Berger and Yaffe, 1996). An increased frequency of petite formation could be attributable to the fact that buds sometimes inherit DNA-free mitochondrial compartments. To test this possibility for the ptc1 mutant, aliquots of fixed, wild-type, and ptc1Δ cultures (YPD, 30°C) were labeled with antiporin antibodies to quantify the inheritance of mitochondrial compartments. The samples were simultaneously labeled with DAPI to quantify the inheritance of mitochondrial nucleoids. In wild-type cultures, only 5.3% of small buds and 0% of large buds lacked anti-porin-stained mitochondrial networks. Similarly, only 11.4% of small and 0% of large wild-type buds lacked DAPI-stained mitochondrial nucleoids. In ptc1Δ cultures, 81.1% of small and 10.3% of large buds lacked anti-porin-stained networks. Again, similar results were observed when ptc1Δ cells were stained with DAPI. In this case, 87.2% of small and 3.3% of large ptc1Δ buds lacked DAPI-stained nucleoids. These results suggest that both the mitochondrial compartment and mitochondrial nucleoids are eventually inherited by large ptc1Δ buds. Although it seems likely that there is some relationship between the mitochondrial inheritance delay and the high frequency of petite generation in the ptc1Δ strain, further studies will be required to determine the mechanism of petite formation.
The Mitochondrial Inheritance Delay in ptc1Δ Cells Is Not Attributable to Changes in Actin Organization
In yeast, filamentous actin (F-actin) is organized into cortical patches and cables (bundles of F-actin) that undergo cell cycle-regulated changes in distribution (Adams and Pringle, 1984; Kilmartin and Adams, 1984; Winsor and Schiebel, 1997). Actin patches are required for cell expansion and are found randomly distributed in unbudded cells, clustered in the growing bud early in division, and assembled at the mother bud neck during cytokinesis. Actin cables are also randomly distributed in unbudded cells but become polarized along the mother bud axis during division. A number of studies suggest that the actin cytoskeleton plays an important role in regulating mitochondrial distribution, morphology, and inheritance, although the exact nature of this role is not yet clear. Mitochondrial membranes have been shown to align along some actin cables in yeast cells, and certain mutant actin alleles exhibit defects in mitochondrial morphology, distribution, and motility (Drubin et al., 1993; Lazzarino et al., 1994; Simon et al., 1995). In addition, cells lacking a novel protein, Mdm20p, completely lack actin cables and display severe defects in mitochondrial inheritance (Hermann et al., 1997). In vitro, yeast mitochondria can bind to phalloidin-stabilized actin filaments and display a myosin-like, actin-based motor activity on their surface (Lazzarino et al., 1994; Simon et al., 1995). This activity may be attributable to a novel motor protein, since mutations in the known S. cerevisiae myosin genes do not block mitochondrial movement in vitro (Simon et al., 1995).
An increase in external osmolarity and activation of the HOG kinase cascade induces yeast to accumulate glycerol (Brewster et al., 1993; Maeda et al., 1994; Posas et al., 1996; see below). Previous work indicted that this accumulation of intracellular glycerol is accompanied by a transient disassembly of the actin cytoskeleton (Brewster and Gustin, 1994). Because mutations in PTC1 have been shown to activate the HOG pathway (Jiang et al., 1995), we examined the possibility that the mitochondrial inheritance delay observed in the ptc1Δ strain was the result of a defect in organization of the actin cytoskeleton. After fixing ptc1 mutant cells grown at 30°C in YPD medium, actin cytoskeleton organization (visualized by indirect immunofluorescence with goat anti-actin antibodies) and mitochondrial distribution (visualized by mouse anti-porin antibody staining) were scored in individual cells. As summarized in Table 8, the organization of actin patches and cables was similar to wild type in dividing ptc1Δ cells that contained mitochondrial networks in growing buds (Figure 4, compare A and B with C and D). More importantly, polarized actin cables and clustered bud patches were also present in dividing ptc1Δ cells that had not yet transported anti-porin-stained mitochondrial networks into growing buds (Figure 3, E and F). Although we cannot rule out the possibility that subtle changes in actin organization are occurring in ptc1Δ cells, these results suggest that the mitochondrial inheritance delay in ptc1Δ does not result from global defects in actin organization.
Table 8.
Actin distribution is normal in ptc1Δ cells
| JSY836 (WT) (n = 200) | JSY118 (ptc1Δ) (n = 200) | |
|---|---|---|
| % of cells with mitochondria in budsa | 95.0 | 54.5 |
| % of cells with mitochondria in buds; wild-type actin organization | 98.4 | 96.3 |
| % of cells without mitochondria in buds; wild-type actin organization | 100.0 | 100.0 |
Double staining of the actin cytoskeleton and the mitochondrial network was observed in 100% of wild-type cells and 99% of ptc1Δ cells in these experiments. n, Number or budded cells scored.
Only budded cells were scored.
Elevating Intracellular Osmolarity Does Not Cause a Mitochondrial Inheritance Delay
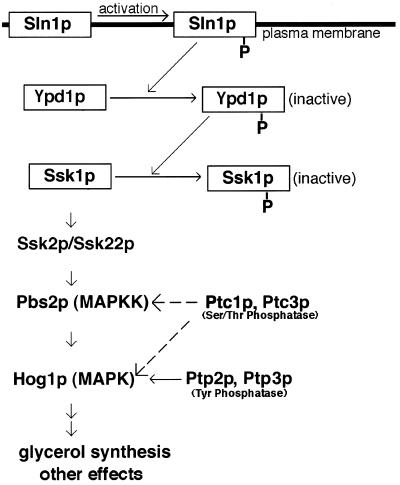
The HOG pathway plays a critical role in the yeast osmostress response and is composed of a signal transducer (Sln1p, Ypd1p, and Ssk1p) and an MAP kinase cascade (Ssk2p/Ssk22p, Pbs2p, and Hog1p) (Figure 5). Under conditions of normal extracellular osmolarity, the Sln1p histidine kinase is autophosphorylated, and a relay system sequentially transfers the phosphate from Sln1p to Ypd1p and finally to Ssk1p (Posas et al., 1996). Phosphorylated Ssk1p cannot activate the Ssk22p and Ssk2p MAPKKKs and, as a result, signaling via the Hog1p kinase is inhibited. When extracellular osmolarity is high, the Sln1p kinase is inhibited, the active, unphosphorylated form of Ssk1p interacts with the Ssk22p and Ssk2p MAPKKKs, and the HOG MAP kinase cascade is turned on. Activation of the Hog1p kinase at the end of this cascade results in a variety of cellular responses, including production of intracellular glycerol. Pbs2p (MAPKK) and Hog1p (MAPK) in the pathway are thought to be dephosphorylated and down-regulated by the serine/threonine phosphatases Ptc1p and Ptc3p (Maeda et al., 1994) and the tyrosine phosphatases Ptp2p and Ptp3p (Maeda et al., 1994; Wurgler-Murphy et al., 1997), as illustrated in Figure 5.
Figure 5.
Proposed regulatory role of Ptc1p in the HOG response pathway (Meada et al., 1994; Posas et al., 1996). When yeast cells are exposed to conditions of high extracellular osmolarity, the HOG MAP kinase cascade (shown above) is activated, producing a variety of cellular responses, including glycerol production. Under conditions of normal extracellular osmolarity, the kinase cascade is inactivated. Yeast cells also contain a second transmembrane osmosensor, Sho1p (not shown), which activates the Pbs2p kinase directly in response to high osmolarity (Meada et al., 1995; Posas et al., 1996).
If Ptc1p dephosphorylates and down-regulates Hog1p and/or Pbs2p, then cells lacking PTC1 should have elevated Hog1p kinase activity. As a result, intracellular glycerol concentrations should be elevated in the mutant even in low extracellular osmolarity (Jiang et al., 1995; see MATERIALS AND METHODS). To determine whether elevated glycerol concentrations account for the mitochondrial inheritance delay observed in the ptc1Δ mutant, wild-type and ptc1Δ cells expressing the Cox4-GFP protein were grown asynchronously overnight in YPD at 25°C and transferred into YPD medium supplemented with 0.9 M NaCl. Previous studies have shown that addition of NaCl to the medium induces glycerol synthesis in wild-type yeast (Edgley and Brown, 1983; Blomberg and Adler, 1989; see MATERIALS AND METHODS). To allow the strains to recover from the initial osmotic shock and resume budding, mitochondrial distributions were scored at 5- and 24-h time points. As summarized in Table 9, very few of the wild-type cells (6%) exhibited a mitochondrial inheritance defect after 5 h at 25°C in the high-osmolarity–producing medium. In contrast, 40.5% of the ptc1Δ cells produced buds without mitochondria (Table 9). Even after 24 h of culture, the percentage of wild-type buds without mitochondria remained low (3.5%), although ptc1Δ cells still produced buds lacking the organelle (20%). These results indicate that increasing intracellular osmolarity in a wild-type strain does not induce a delay in mitochondrial inheritance.
Table 9.
Mitochondrial inheritance is not affected by culture in high-osmolarity medium
| Strain | Growth medium | % of buds without mitochondriaa
|
|
|---|---|---|---|
| 5 h (n)b | 24 h (n)b | ||
| JSY836 (WT) | YPD + 0.9 M NaCl | 6.0 (402) | 3.5 (403) |
| JSY118 (ptc1Δ) | YPD + 0.9 M NaCl | 40.5 (400) | 20.0 (400) |
Only budded cells were counted.
Hours after transfer to high-osmolarity medium. n, Number of budded cells scored.
Ptc1p Is Not Acting Through the HOG Pathway to Affect the Mitochondrial Transport Machinery
Genetic evidence implicates Ptc1p as a negative regulator of the Hog1p and/or Pbs2p kinases in the HOG pathway (Maeda et al., 1993, 1994). Thus, it was formally possible that hyperactive Hog1p or Pbs2p kinases in this pathway were responsible for the mitochondrial inheritance delay we observed in the ptc1 mutant. If this model were correct, disruption of the HOG1 or PBS2 gene in a ptc1 strain should abolish (or at least reduce) the mitochondrial inheritance defect observed in the ptc1 mutant alone. To test this possibility, mitochondrial inheritance was compared in ptc1 hog1 and ptc1 pbs2 double mutants and isogenic control strains (wild type, ptc1Δ, hog1Δ, and pbs2Δ single mutant strains) in YPD medium at 25°C. As shown in Table 10, only a small percentage of the wild-type (JSY836) and hog1Δ (AMY36) cells produced buds lacking DiOC6-stained mitochondrial networks (4.7 and 2.3%, respectively). More importantly, the ptc1Δ hog1Δ double mutant (AMY43) did not exhibit a less pronounced mitochondrial inheritance defect than that observed in the ptc1Δ single mutant (JSY118) (Table 10; 26.9 and 29.6% of buds without mitochondrial staining, respectively). In addition, a mitochondrial inheritance defect was not observed in the pbs2Δ mutant (JSY2092, 1.3%) relative to the wild-type control (SEY6210, 2.7%), and comparable mitochondrial inheritance defects were displayed by the ptc1Δ single (JSY2090, 33.3%) and ptc1Δ pbs2Δ double (JSY2093, 29.6%) mutant strains (Table 10). These results indicate that: 1) Hog1p and Pbs2p are not required for mitochondrial inheritance in yeast; and 2) the serine/threonine phosphatase Ptc1p is not exerting its effect on the timing of mitochondrial transport through the activities of these two kinases. [Cells lacking Ptp2p, a tyrosine phosphatase known to down-regulate Hog1p activity, did not exhibit a delay in mitochondrial transport either (Table 10).] Whether Ptc1p is affecting the mitochondrial inheritance machinery directly or indirectly through some other cellular pathway remains to be determined.
Table 10.
Epistatis analysis between ptc1Δ and HOG1 pathway mutants
| Strain (25°C in YPD) | % of buds without mitochondria (n)a |
|---|---|
| JSY836 (WT) | 4.7 (301) |
| JSY118 (ptc1Δ) | 29.6 (301) |
| AMY36 (hog1Δ) | 2.3 (301) |
| AMY43 (ptc1Δ, hog1Δ) | 26.9 (305) |
| SEY6210 (WT) | 2.7 (300) |
| JSY2090 (ptc1Δ) | 33.3 (600) |
| JSY2092 (pbs2Δ) | 1.3 (300) |
| JSY2093 (ptc1Δ, pbs2Δ) | 29.6 (301) |
| JSY2589 (ptp2Δ) | 1.2 (85) |
| SEY6210 (ptc1Δ, ptp2Δ) | NDb |
Only budded cells were counted. n, Number of budded cells scored.
ND, Because of the severity of the synthetic growth defect in the ptc1Δ, ptp2Δ double mutant, mitochondrial inheritance could not be analyzed in this strain.
DISCUSSION
We have shown that cells lacking the serine/threonine phosphatase encoded by PTC1 exhibit a pronounced delay, but not a complete block, in mitochondrial inheritance during mitotic growth. The mitochondrial inheritance delay in ptc1Δ cells is not attributable to defects in the organization of the actin cytoskeleton, which is known to play a role in mitochondrial transport (Lazzarino et al., 1994; Simon et al., 1995; Hermann et al., 1997). Although Ptc1p is thought to be a negative regulator of the HOG pathway, we demonstrated that the mitochondrial inheritance defect in ptc1Δ strains does not result from changes in intracellular glycerol concentrations. Furthermore, Ptc1p is not acting through the HOG pathway kinases Hog1p or Pbs2p to control the timing of mitochondrial transfer to buds; the mitochondrial inheritance delay observed in the ptc1Δ strain does not change substantially in ptc1Δ hog1Δ or ptc1Δ pbs2Δ double mutants. Instead, Ptc1p appears to be acting either directly or through a different signaling pathway to affect the mitochondrial transport machinery in the cell.
Ptc1p is the first serine/threonine phosphatase reported to affect mitochondrial transport in yeast. Although it is possible that dephosphorylation by Ptc1p directly regulates the activities of one or more proteins required to move the mitochondrial network into buds, only a few proteins required for mitochondrial inheritance have been isolated to date, and none of them have been shown to be phosphorylated. Alternatively, Ptc1p may be affecting mitochondrial inheritance indirectly through a kinase cascade other than the HOG pathway. The protein kinase C (PKC) pathway is currently the only other signaling cascade proposed to be regulated by Ptc1p (Huang and Symington, 1995). Protein kinase C (Pkc1p) regulates an MAP kinase cascade (BCK1/SLK1, MKK1/2, and MPK1/SLT2) implicated in cell wall metabolism (Errede and Levin, 1993), DNA metabolism (Huang and Symington, 1994), and response to decreases in extracellular osmolarity (Davenport et al., 1995). Mutations in PTC1 were shown to suppress temperature-sensitive defects of a pkc1 allele and were also found to be lethal in combination with mutations in MPK1, the terminal MAP kinase in the PKC pathway (Huang and Symington, 1994). Due to the synthetic lethality of the ptc1 mpk1 double mutant, we were unable to determine whether a hyperactive Mpk1p kinase is responsible for the mitochondrial inheritance delay observed in the ptc1Δ mutant. Further analysis is required to determine whether Ptc1p is affecting mitochondrial transport through the upstream MKK1 and MKK2 encoded serine/threonine kinases in the PKC pathway or kinases in another signaling pathway.
Although PTC1 is the first single locus reported to control the timing of mitochondrial inheritance, a similar phenotype may occur in a strain carrying mutations in two different genes (BRO1 and CAF1; Nickas and Yaffe, 1996). Interestingly, BRO1 encodes a novel protein that also interacts genetically with Mpk1p, the terminal kinase in the PKC pathway. A detailed characterization of mitochondrial phenotypes in the BRO1 and CAF1 single and double mutants may provide additional insights regarding the link between the PKC kinase cascade and the temporal control of mitochondrial inheritance.
In principle, mitochondrial inheritance could be accomplished by at least three different mechanisms: 1) the mitochondrial network could diffuse into the bud as it forms; 2) the mitochondrial network could be transported into buds through a cytoskeletal-based motor activity; or 3) the mitochondrial network could attach to the incipient bud site and be passively “pulled” into the expanding daughter cell. Our observation that the mitochondrial network can move into large ptc1Δ buds well after they have already formed suggests that mitochondrial attachment to the bud site is not strictly required for inheritance or that multiple mechanisms are operating to transport this organelle into the bud. Although other interpretations are possible, we believe our findings eliminate the attachment model as the primary mechanism of mitochondrial inheritance in yeast.
Studies of living yeast cells suggest that mitochondrial inheritance routinely occurs early in the cell cycle, after inheritance of the endoplasmic reticulum and Golgi apparatus but before the segregation of the nucleus. Our studies of the ptc1Δ mutant provide the first genetic evidence that the timing of this mitochondrial transport is actively regulated and coordinated with the cell cycle. It is somewhat surprising that the mitochondrial transport delay in ptc1Δ is not accompanied by a more dramatic change in the growth properties of these cells. Apparently, the order in which some essential cytoplasmic organelles are inherited is not critical as long as some part of that compartment is received by the bud before cytokinesis. This flexibility may help ensure that small perturbations in organelle distribution during cell division do not lead to drastic effects on cell division.
Table 5.
Mitochondrial inheritance is delayed in ptc1Δ cells after α-factor arrest and release at 30°C
| Strain | Time after release from α-factor (h) | % of unbudded cells | % of smalla budded cells | % of small buds without mitochondria | % of largeb budded cells | % of large buds without mitochondria | % of multiplyc budded cells | % of multiply budded cells without mitochondria in largest bud |
|---|---|---|---|---|---|---|---|---|
| WT | 0.5 | 78 | 22 | 4.5 | 0 | 0 | ||
| ptc1Δ | 0.5 | 84 | 16 | 93.8 | 0 | 0 | ||
| WT | 1.0 | 19 | 43 | 4.7 | 38 | 0 | 0 | |
| ptc1Δ | 1.0 | 26 | 69 | 98.6 | 5 | 40 | 0 | |
| WT | 1.5 | 2 | 9 | 11.1 | 86 | 0 | 3 | 0 |
| ptc1Δ | 1.5 | 7 | 20 | 80 | 73 | 17.8 | 0 | |
| WT | 2.0 | 6 | 27 | 7.4 | 67 | 1.5 | 0 | |
| ptc1Δ | 2.0 | 4 | 12 | 91.7 | 55 | 18.2 | 29 | 3.4 |
| WT | 2.5 | 27 | 13 | 7.7 | 60 | 0 | 0 | |
| ptc1Δ | 2.5 | 4 | 6 | 83.3 | 26 | 7.7 | 64 | 9.4 |
At least 100 cells were scored for each strain at each time point.
Buds less than half the diameter of the mother cell.
Buds greater than half the diameter of the mother cell.
An increase in the percentage of multiply budded cells is observed in ptc1Δ cells after α-factor treatment. The percentage of unbudded wild-type cells upon α-factor release at 30°C was 99%. The percentage of unbudded ptc1Δ cells on α-factor release at 30°C was 98%.
ACKNOWLEDGMENTS
We are grateful to Eric Benson, Howard Bussey, John Cooper, Scott Emr, Mike Gustin, Robert Jensen, Troy Ketela, and Greg Payne for providing strains, plasmids, and antibodies. We also thank Greg Payne and members of the Shaw laboratory for stimulating discussions and careful review of the manuscript. This work was supported by National Institutes of Health grant GM53466 and American Cancer Society grant CB-97 to J.M.S. A.D.R. was supported by National Institutes of Health predoctoral genetics training grant 5 T32 GM07464.
REFERENCES
- Adams AEM, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiroz R, Butow RA. Patterns of mitochondrial sorting in yeast zygotes. Mol Biol Cell. 1993;4:21–36. doi: 10.1091/mbc.4.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer JS. New alleles of mgm1: a gene encoding a protein with a GTP-binding domain related to dynamin. Curr Genet. 1995;28:499–501. doi: 10.1007/BF00310822. [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J. Behavior of mitochondria in the living cell. Int Rev Cytol. 1990;122:1–63. doi: 10.1016/s0074-7696(08)61205-x. [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J, Vöth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech. 1994;27:198–219. doi: 10.1002/jemt.1070270303. [DOI] [PubMed] [Google Scholar]
- Berger KH, Sogo LF, Yaffe MP. Mdm12p, a component required for mitochondrial inheritance that is conserved between budding and fission yeast. J Cell Biol. 1997;136:545–553. doi: 10.1083/jcb.136.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger KH, Yaffe MP. Mitochondrial distribution and inheritance. Experentia. 1996;52:1111–1116. doi: 10.1007/BF01952109. [DOI] [PubMed] [Google Scholar]
- Blomberg A, Adler L. Roles of glycerol and glycerol-3-phosphate dehydrogenase (NAD+) in acquired osmotolerance of Saccharomyces cerevisiae. J Bacteriol. 1989;171:1087–1092. doi: 10.1128/jb.171.2.1087-1092.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Brewster JL, Gustin MC. Positioning of cell growth and division after osmotic stress requires a MAP kinase pathway. Yeast. 1994;10:425–439. doi: 10.1002/yea.320100402. [DOI] [PubMed] [Google Scholar]
- Burgess SM, Delannoy M, Jensen RE. MMM1 encodes a mitochondrial outer membrane protein essential for establishing and maintaining the structure of yeast mitochondria. J Cell Biol. 1994;126:1375–1391. doi: 10.1083/jcb.126.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CL, Tanaka N, White KH, Thorsness PE. Mitochondrial morphological and functional defects in yeast caused by yme1 are suppressed by mutation of a 26S protease subunit homologue. Mol Biol Cell. 1994;5:899–905. doi: 10.1091/mbc.5.8.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XJ, Guan MX, Clark-Walker GD. MGM101, a nuclear gene involved in maintenance of the mitochondrial genome in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:3473–3477. doi: 10.1093/nar/21.15.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport KR, Sohaskey M, Kamada Y, Levin DE, Gustin MC. A second osmosensing signal transduction pathway in yeast. J Biol Chem. 1995;270:30157–30161. doi: 10.1074/jbc.270.50.30157. [DOI] [PubMed] [Google Scholar]
- Diffley JF, Stillman B. A close relative of the nuclear, chromosomal high-mobility group protein HMG1 in yeast mitochondria. Proc Natl Acad Sci USA. 1991;88:7864–7868. doi: 10.1073/pnas.88.17.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley JF, Stillman B. DNA binding properties of an HMG1-related protein from yeast mitochondria. J Biol Chem. 1992;267:3368–3374. [PubMed] [Google Scholar]
- Drubin DG, Jones HD, Wertman KF. Actin structure and function: roles in mitochondrial organization and morphogenesis in budding yeast and identification of the phalloidin-binding site. Mol Biol Cell. 1993;4:1277–1294. doi: 10.1091/mbc.4.12.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley M, Brown AD. Yeast water relations: physiological changes induced by solute stress in Saccharomyces cerevisiae and Saccharomyces rouxii. J Gen Microbiol. 1983;129:3453–3463. [Google Scholar]
- Errede B, Levin DE. A conserved kinase cascade for MAP kinase activation in yeast. Curr Opin Cell Biol. 1993;5:254–260. doi: 10.1016/0955-0674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Guan K, Farh L, Marshall TK, Deschenes RJ. Normal mitochondrial structure and genome maintenance in yeast requires the dynamin-like product of the MGM1 gene. Curr Genet. 1993;24:141–148. doi: 10.1007/BF00324678. [DOI] [PubMed] [Google Scholar]
- Hermann GJ, King EJ, Shaw JM. The yeast gene, MDM20, is necessary for mitochondrial inheritance and organization of the actin cytoskeleton. J Cell Biol. 1997;137:141–153. doi: 10.1083/jcb.137.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman H, Avers CJ. Mitochondrion in yeast: ultrastructural evidence for one giant, branched organelle per cell. Science. 1973;181:749–751. doi: 10.1126/science.181.4101.749. [DOI] [PubMed] [Google Scholar]
- Huang KN, Symington LS. Mutation of the gene encoding protein kinase C 1 stimulates mitotic recombination in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:6039–6045. doi: 10.1128/mcb.14.9.6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KN, Symington LS. Suppressors of a Saccharomyces cerevisiae pkc1 mutation identify alleles of the phosphatase gene PTC1 and of a novel gene encoding a putative basic leucine zipper protein. Genetics. 1995;141:1275–1285. doi: 10.1093/genetics/141.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Ram AFJ, Sheraton J, Klis FM, Bussey H. Regulation of cell wall β-glucan assembly: PTC1 negatively affects PBS2 action in a pathway that includes modulation of EXG1 transcription. Mol Gen Genet. 1995;248:260–269. doi: 10.1007/BF02191592. [DOI] [PubMed] [Google Scholar]
- Kaiser C, Michaelis S, Mitchell A. Methods in yeast genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Karpova TS, Lepetit MM, Cooper JA. Mutations that enhance the cap2 null mutant phenotype in Saccharomyces cerevisiae affect the actin cytoskeleton, mophogenesis and pattern of growth. Genetics. 1993;135:693–709. doi: 10.1093/genetics/135.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin JV, Adams AEM. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces cerevisiae. J Cell Biol. 1984;98:922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarino DA, Boldogh I, Smith MG, Rosand J, Pon LA. Yeast mitochondria contain ATP-sensitive, reversible actin-binding activity. Mol Biol Cell. 1994;5:807–818. doi: 10.1091/mbc.5.7.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Takekawa M, Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Maeda T, Tsai AYM, Saito H. Mutations in a protein tyrosine phosphatase gene (PTP2) and a protein serine/threonine phosphatase gene (PTC1) cause a synthetic growth defect in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:5408–5417. doi: 10.1128/mcb.13.9.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Wurgler-Murphy SM, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- McConnell SJ, Stewart LC, Talin A, Yaffe MP. Temperature-sensitive yeast mutants defective in mitochondrial inheritance. J Cell Biol. 1990;111:967–976. doi: 10.1083/jcb.111.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SJ, Yaffe MP. Nuclear and mitochondrial inheritance in yeast depends on novel cytoplasmic structures defined by the MDM1 protein. J Cell Biol. 1992;118:385–395. doi: 10.1083/jcb.118.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SJ, Yaffe MP. Intermediate filament formation by a yeast protein essential for organelle inheritance. Science. 1993;260:687–689. doi: 10.1126/science.8480179. [DOI] [PubMed] [Google Scholar]
- Nickas ME, Yaffe MP. BRO1, a novel gene that interacts with components of the Pkc1p-mitogen-activated protein kinase pathway in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2585–2593. doi: 10.1128/mcb.16.6.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari J, Marshall WF, Straight A, Murray A, Sedat JW, Walter P. Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Mol Biol Cell. 1997;8:1233–1242. doi: 10.1091/mbc.8.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Pringle JR, Adams AEM, Drubin DG, Haarer BK. Immunofluorescence methods for yeast. In: Guthrie C, Fink GR, editors. Guide to Yeast Genetics and Molecular Biology. San Diego: Academic Press; 1991. pp. 565–601. [Google Scholar]
- Robinson JS, Klionsky DJ, Banta LM, Emr SD. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MK, van Zyl WH, Phizicky EM, Broach JR. TPD1 of Saccharomyces cerevisiae encodes a protein phosphatase 2C-like activity implicated in tRNA splicing and cell separation. Mol Cell Biol. 1994;14:3634–3645. doi: 10.1128/mcb.14.6.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder AD, Shaw JM. Vacuole partitioning during meiotic division in yeast. Genetics. 1996;144:445–458. doi: 10.1093/genetics/144.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MD, Novik P, Thomas JH, Botstein D, Fink GR. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60:237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Simon VR, Swayne TC, Pon LA. Actin-dependent mitochondrial motility in mitotic yeast and cell-free systems: identification of a motor activity on the mitochondrial surface. J Cell Biol. 1995;130:345–354. doi: 10.1083/jcb.130.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo LF, Yaffe MP. Regulation of mitochondrial morphology and inheritance by Mdm10p, a protein of the mitochondrial outer membrane. J Cell Biol. 1994;126:1361–1373. doi: 10.1083/jcb.126.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B. Mitochondrial structure. In: Strathern J, Jones NEW, Broach JR, editors. The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1981. pp. 471–504. [Google Scholar]
- Strausberg RL, Perlman PS. The effect of zygotic bud position on the transmission of mitochondrial genes in Saccharomyces cerevisiae. Mol Gen Genet. 1978;163:131–144. doi: 10.1007/BF00267404. [DOI] [PubMed] [Google Scholar]
- van Zyl WH, Wills N, Broach JR. A general screen for mutants of Saccharomyces cerevisiae deficient in tRNA biosynthesis. Genetics. 1989;123:55–68. doi: 10.1093/genetics/123.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsor B, Schiebel E. Review: an overview of the Saccharomyces cerevisiae microtubule and microfilament cytoskeleton. Yeast. 1997;13:399–434. doi: 10.1002/(SICI)1097-0061(199704)13:5<399::AID-YEA126>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Winston F, Dollard C, Ricupero-Hovasse SL. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S228C. Yeast. 1995;11:53–55. doi: 10.1002/yea.320110107. [DOI] [PubMed] [Google Scholar]
- Wurgler-Murphy SM, Maeda T, Witten EA, Saito H. Regulation of the Saccharomyces cerevisiae HOG1 mitogen-activated protein kinase by the PTP2 and PTP3 protein tyrosine phosphatases. Mol Cell Biol. 1997;17:1289–1297. doi: 10.1128/mcb.17.3.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenaya-Troitskaya O, Perlman PS, Butow RA. An enzyme in yeast mitochondria that catalyzes a step in branched-chain amino acid biosynthesis also functions in mitochondrial DNA stability. EMBO J. 1995;14:3268–3276. doi: 10.1002/j.1460-2075.1995.tb07330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn AR, Pohlman JK, Perlman PS, Butow RA. Kinetic and segregational analysis of mitochondrial DNA recombination in yeast. Plasmid. 1987;17:248–256. doi: 10.1016/0147-619x(87)90033-3. [DOI] [PubMed] [Google Scholar]