Abstract
Two outer capsid rotavirus proteins, VP3 and VP7, have been found to elicit neutralizing-antibody production, but the immunogenicity of these proteins during human rotavirus infection has not been determined. The relative amounts of serum neutralizing antibody against the VP3 and VP7 proteins of the CJN strain of human rotavirus were, therefore, determined in adult subjects before and after infection with this virus. Reassortant strains of rotavirus that contained the CJN gene segment for only one of these two neutralization proteins were isolated and used for this study. The geometric mean titer of serum neutralizing antibody to a reassortant virus (CJN-M) that contained VP7 of CJN and VP3 of another human rotavirus was 12.7 times less than that of antibody to CJN before infection and 20.3 times less after infection. This indicated that most neutralizing antibody was against the VP3 rather than the VP7 protein of CJN. This result was confirmed with other reassortants between CJN and animal rotavirus strains (EDIM and rhesus rotavirus). These findings suggest that VP3 is the primary immunogen that stimulates neutralizing antibody during at least some rotavirus infections of humans.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
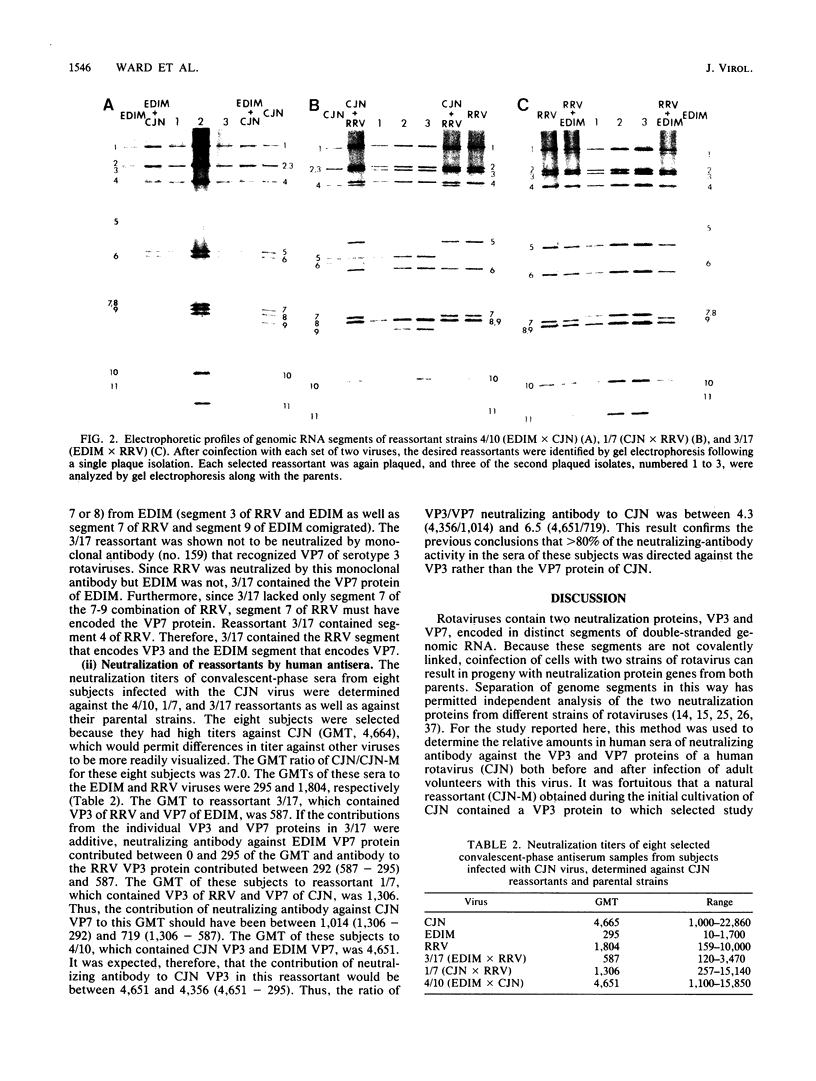
- Anderson E. L., Belshe R. B., Bartram J., Crookshanks-Newman F., Chanock R. M., Kapikian A. Z. Evaluation of rhesus rotavirus vaccine (MMU 18006) in infants and young children. J Infect Dis. 1986 May;153(5):823–831. doi: 10.1093/infdis/153.5.823. [DOI] [PubMed] [Google Scholar]
- Arias C. F., López S., Espejo R. T. Gene protein products of SA11 simian rotavirus genome. J Virol. 1982 Jan;41(1):42–50. doi: 10.1128/jvi.41.1.42-50.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Mattick J. S., Bellamy A. R. Serotype-specific glycoprotein of simian 11 rotavirus: coding assignment and gene sequence. Proc Natl Acad Sci U S A. 1983 May;80(10):3091–3095. doi: 10.1073/pnas.80.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Siegman L. J., Bellamy A. R., Atkinson P. H. Coding assignment and nucleotide sequence of simian rotavirus SA11 gene segment 10: location of glycosylation sites suggests that the signal peptide is not cleaved. J Virol. 1983 Nov;48(2):335–339. doi: 10.1128/jvi.48.2.335-339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Yokoyama T., Nakata S., Morita Y., Urasawa T., Taniguchi K., Urasawa S., Nakao T. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet. 1986 Aug 23;2(8504):417–421. doi: 10.1016/s0140-6736(86)92133-1. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Offit P. A., Dolan K. T., Tezza A., Gogalin K., Twist E. M., Plotkin S. A. Response of adult human volunteers to oral administration of bovine and bovine/human reassortant rotaviruses. Vaccine. 1986 Mar;4(1):25–31. doi: 10.1016/0264-410x(86)90094-0. [DOI] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Gene-coding assignments of rotavirus double-stranded RNA segments 10 and 11. J Virol. 1981 Jun;38(3):1099–1103. doi: 10.1128/jvi.38.3.1099-1103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson B. L., Graham D. Y., Mason B. B., Estes M. K. Identification, synthesis, and modifications of simian rotavirus SA11 polypeptides in infected cells. J Virol. 1982 Jun;42(3):825–839. doi: 10.1128/jvi.42.3.825-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Gonzalez M., Garcia D., Perez M., Daoud N., Cunto W., Chanock R. M., Kapikian A. Z. Protection against severe rotavirus diarrhoea by rhesus rotavirus vaccine in Venezuelan infants. Lancet. 1987 Apr 18;1(8538):882–884. doi: 10.1016/s0140-6736(87)92858-3. [DOI] [PubMed] [Google Scholar]
- Gombold J. L., Ramig R. F. Analysis of reassortment of genome segments in mice mixedly infected with rotaviruses SA11 and RRV. J Virol. 1986 Jan;57(1):110–116. doi: 10.1128/jvi.57.1.110-116.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Flores J., Kalica A. R., Wyatt R. G., Jones R. Gene coding assignments for growth restriction, neutralization and subgroup specificities of the W and DS-1 strains of human rotavirus. J Gen Virol. 1983 Feb;64(Pt 2):313–320. doi: 10.1099/0022-1317-64-2-313. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Vo P. T., Jones R. Cultivation and characterization of three strains of murine rotavirus. J Virol. 1986 Feb;57(2):585–590. doi: 10.1128/jvi.57.2.585-590.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Chanock R. M., Kapikian A. Z. Analysis by plaque reduction neutralization assay of intertypic rotaviruses suggests that gene reassortment occurs in vivo. J Clin Microbiol. 1987 Feb;25(2):290–294. doi: 10.1128/jcm.25.2.290-294.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Kantharidis P., Dyall-Smith M. L., Holmes I. H. Completion of the gene coding assignments of SA11 rotavirus: gene products of segments 7, 8, and 9. J Virol. 1983 Oct;48(1):330–334. doi: 10.1128/jvi.48.1.330-334.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton D. R., Ward R. L. Effect of mutation in immunodominant neutralization epitopes on the antigenicity of rotavirus SA-11. J Gen Virol. 1985 Nov;66(Pt 11):2375–2381. doi: 10.1099/0022-1317-66-11-2375. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Losonsky G. A., Rennels M. B., Kapikian A. Z., Midthun K., Ferra P. J., Fortier D. N., Hoffman K. M., Baig A., Levine M. M. Safety, infectivity, transmissibility and immunogenicity of rhesus rotavirus vaccine (MMU 18006) in infants. Pediatr Infect Dis. 1986 Jan-Feb;5(1):25–29. doi: 10.1097/00006454-198601000-00005. [DOI] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. Biochemical mapping of the simian rotavirus SA11 genome. J Virol. 1983 May;46(2):413–423. doi: 10.1128/jvi.46.2.413-423.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae M. A., McCorquodale J. G. The molecular biology of rotaviruses. II. Identification of the protein-coding assignments of calf rotavirus genome RNA species. Virology. 1982 Mar;117(2):435–443. doi: 10.1016/0042-6822(82)90482-2. [DOI] [PubMed] [Google Scholar]
- Midthun K., Greenberg H. B., Hoshino Y., Kapikian A. Z., Wyatt R. G., Chanock R. M. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985 Mar;53(3):949–954. doi: 10.1128/jvi.53.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Hoshino Y., Kapikian A. Z., Chanock R. M. Single gene substitution rotavirus reassortants containing the major neutralization protein (VP7) of human rotavirus serotype 4. J Clin Microbiol. 1986 Nov;24(5):822–826. doi: 10.1128/jcm.24.5.822-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Blavat G., Greenberg H. B. Reassortant rotaviruses containing structural proteins vp3 and vp7 from different parents induce antibodies protective against each parental serotype. J Virol. 1986 Nov;60(2):491–496. doi: 10.1128/jvi.60.2.491-496.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Shaw R. D., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986 May;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennels M. B., Losonsky G. A., Shindledecker C. L., Hughes T. P., Kapikian A. Z., Levine M. M. Immunogenicity and reactogenicity of lowered doses of rhesus rotavirus vaccine strain MMU 18006 in young children. Pediatr Infect Dis J. 1987 Mar;6(3):260–264. doi: 10.1097/00006454-198703000-00010. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Stoner-Ma D. L., Estes M. K., Greenberg H. B. Specific enzyme-linked immunoassay for rotavirus serotypes 1 and 3. J Clin Microbiol. 1985 Aug;22(2):286–291. doi: 10.1128/jcm.22.2.286-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. L., Lazdins I., Holmes I. H. Coding assignments of double-stranded RNA segments of SA 11 rotavirus established by in vitro translation. J Virol. 1980 Mar;33(3):976–982. doi: 10.1128/jvi.33.3.976-982.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuker G., Oshiro L. S., Schmidt N. J. Antigenic comparisons of two new rotaviruses from rhesus monkeys. J Clin Microbiol. 1980 Feb;11(2):202–203. doi: 10.1128/jcm.11.2.202-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L., Sheshberadaran H., Vesikari T., Norrby E., Wadell G. Immune response to rotavirus polypeptides after vaccination with heterologous rotavirus vaccines (RIT 4237, RRV-1). J Gen Virol. 1987 Jul;68(Pt 7):1993–1999. doi: 10.1099/0022-1317-68-7-1993. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Morita Y., Urasawa T., Urasawa S. Cross-reactive neutralization epitopes on VP3 of human rotavirus: analysis with monoclonal antibodies and antigenic variants. J Virol. 1987 May;61(5):1726–1730. doi: 10.1128/jvi.61.5.1726-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa S., Urasawa T. Preparation and characterization of neutralizing monoclonal antibodies with different reactivity patterns to human rotaviruses. J Gen Virol. 1985 May;66(Pt 5):1045–1053. doi: 10.1099/0022-1317-66-5-1045. [DOI] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K. Genetic reassortment between two human rotaviruses having different serotype and subgroup specificities. J Gen Virol. 1986 Aug;67(Pt 8):1551–1559. doi: 10.1099/0022-1317-67-8-1551. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., Delem A., D'Hondt E., André F. E., Zissis G. Immunogenicity and safety of live oral attenuated bovine rotavirus vaccine strain RIT 4237 in adults and young children. Lancet. 1983 Oct 8;2(8354):807–811. doi: 10.1016/s0140-6736(83)90734-1. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Bernstein D. I., Young E. C., Sherwood J. R., Knowlton D. R., Schiff G. M. Human rotavirus studies in volunteers: determination of infectious dose and serological response to infection. J Infect Dis. 1986 Nov;154(5):871–880. doi: 10.1093/infdis/154.5.871. [DOI] [PubMed] [Google Scholar]
- Wolf J. L., Cukor G., Blacklow N. R., Dambrauskas R., Trier J. S. Susceptibility of mice to rotavirus infection: effects of age and administration of corticosteroids. Infect Immun. 1981 Aug;33(2):565–574. doi: 10.1128/iai.33.2.565-574.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]