Abstract
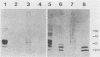
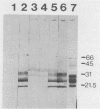
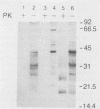
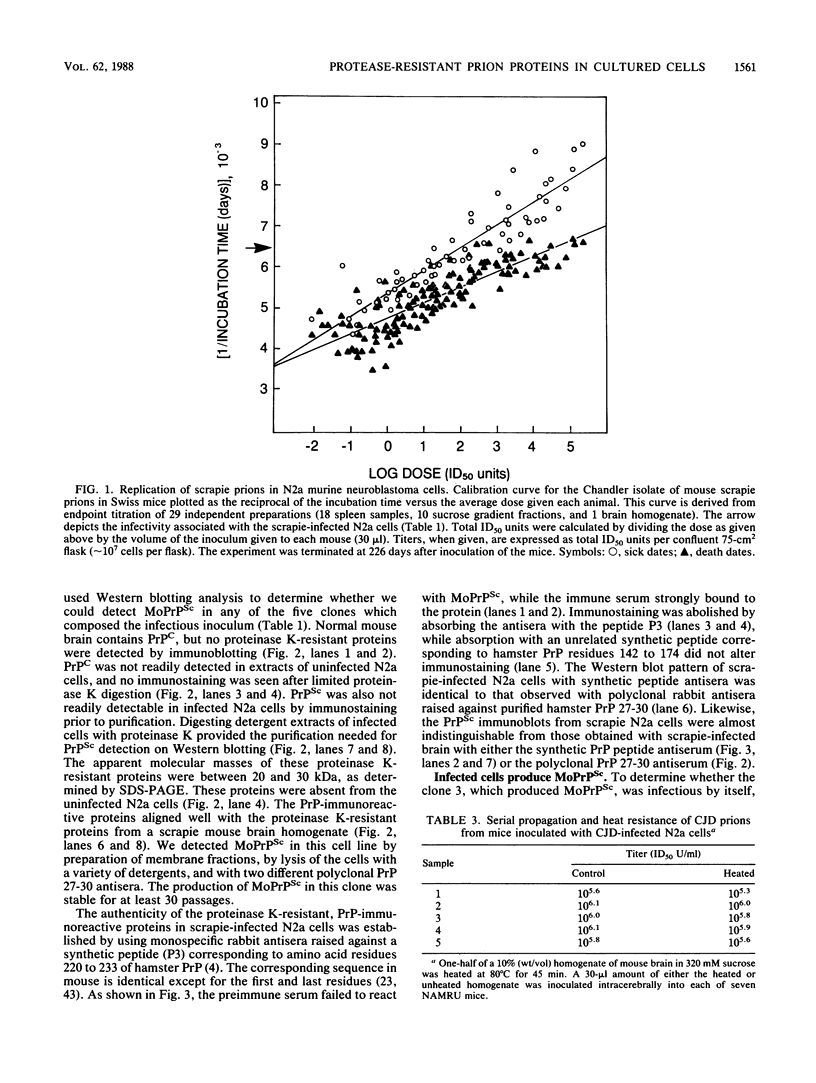
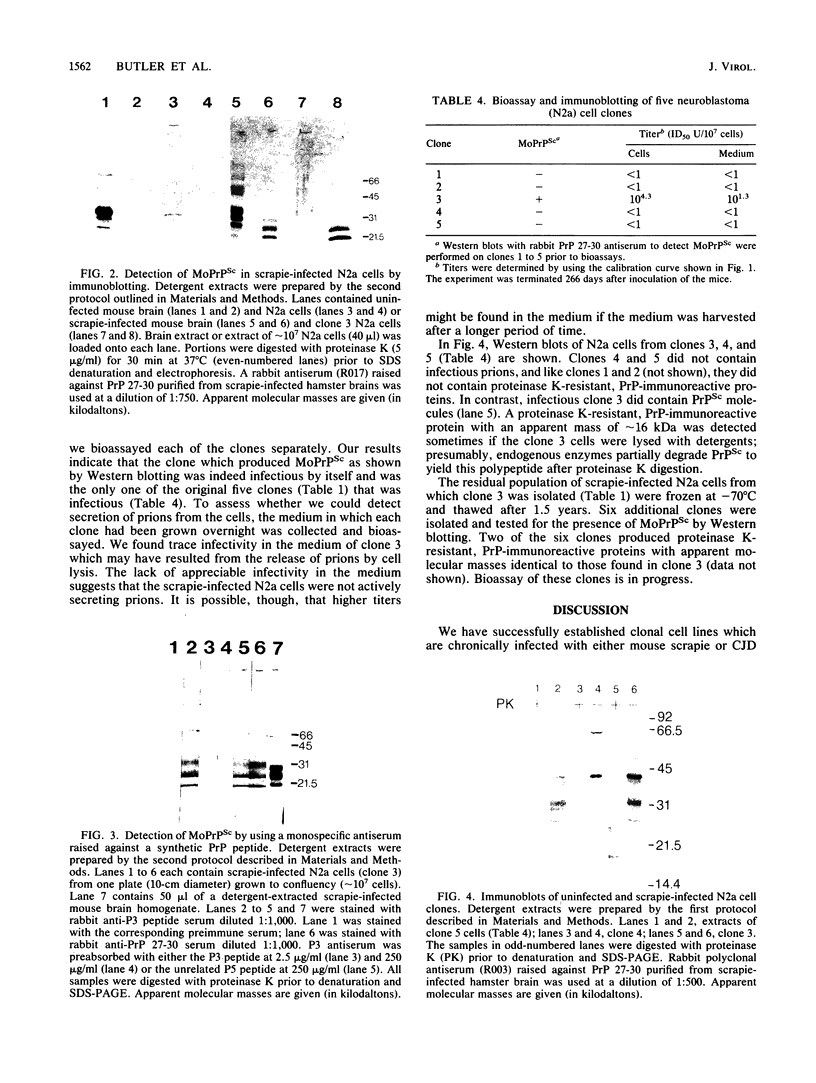
Scrapie and Creutzfeldt-Jakob disease are transmissible, degenerative neurological diseases caused by prions. Considerable evidence argues that prions contain protease-resistant sialoglycoproteins, designated PrPSc, encoded by a cellular gene. The prion protein (PrP) gene also encodes a normal cellular protein designated PrPC. We established clonal cell lines which support the replication of mouse scrapie or Creutzfeldt-Jakob disease prions. Mouse neuroblastoma N2a cells were exposed to mouse scrapie prions and subsequently cloned. After limited proteinase K digestion, three PrP-immunoreactive proteins with apparent molecular masses ranging between 20 and 30 kilodaltons were detected in extracts of scrapie-infected N2a cells by Western (immuno-) blotting. The authenticity of these PrPSc molecules was established by using monospecific antiserum raised against a synthetic peptide corresponding to a portion of the prion protein. Those clones synthesizing PrPSc molecules possessed scrapie prion infectivity as measured by bioassay; clones without PrPSc failed to demonstrate infectivity. Detection of PrPSc molecules in scrapie-infected N2a cells supports the contention that PrPSc is a component of the infectious scrapie particle and opens new approaches to the study of prion diseases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry R. A., Kent S. B., McKinley M. P., Meyer R. K., DeArmond S. J., Hood L. E., Prusiner S. B. Scrapie and cellular prion proteins share polypeptide epitopes. J Infect Dis. 1986 May;153(5):848–854. doi: 10.1093/infdis/153.5.848. [DOI] [PubMed] [Google Scholar]
- Barry R. A., McKinley M. P., Bendheim P. E., Lewis G. K., DeArmond S. J., Prusiner S. B. Antibodies to the scrapie protein decorate prion rods. J Immunol. 1985 Jul;135(1):603–613. [PubMed] [Google Scholar]
- Barry R. A., Prusiner S. B. Monoclonal antibodies to the cellular and scrapie prion proteins. J Infect Dis. 1986 Sep;154(3):518–521. doi: 10.1093/infdis/154.3.518. [DOI] [PubMed] [Google Scholar]
- Basler K., Oesch B., Scott M., Westaway D., Wälchli M., Groth D. F., McKinley M. P., Prusiner S. B., Weissmann C. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell. 1986 Aug 1;46(3):417–428. doi: 10.1016/0092-8674(86)90662-8. [DOI] [PubMed] [Google Scholar]
- Bellinger-Kawahara C., Cleaver J. E., Diener T. O., Prusiner S. B. Purified scrapie prions resist inactivation by UV irradiation. J Virol. 1987 Jan;61(1):159–166. doi: 10.1128/jvi.61.1.159-166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger-Kawahara C., Diener T. O., McKinley M. P., Groth D. F., Smith D. R., Prusiner S. B. Purified scrapie prions resist inactivation by procedures that hydrolyze, modify, or shear nucleic acids. Virology. 1987 Sep;160(1):271–274. doi: 10.1016/0042-6822(87)90072-9. [DOI] [PubMed] [Google Scholar]
- Bockman J. M., Kingsbury D. T., McKinley M. P., Bendheim P. E., Prusiner S. B. Creutzfeldt-Jakob disease prion proteins in human brains. N Engl J Med. 1985 Jan 10;312(2):73–78. doi: 10.1056/NEJM198501103120202. [DOI] [PubMed] [Google Scholar]
- Bockman J. M., Prusiner S. B., Tateishi J., Kingsbury D. T. Immunoblotting of Creutzfeldt-Jakob disease prion proteins: host species-specific epitopes. Ann Neurol. 1987 Jun;21(6):589–595. doi: 10.1002/ana.410210611. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., McKinley M. P., Prusiner S. B. Identification of a protein that purifies with the scrapie prion. Science. 1982 Dec 24;218(4579):1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., McKinley M. P., Prusiner S. B. Molecular characteristics of the major scrapie prion protein. Biochemistry. 1984 Dec 4;23(25):5898–5906. doi: 10.1021/bi00320a002. [DOI] [PubMed] [Google Scholar]
- Carlson G. A., Kingsbury D. T., Goodman P. A., Coleman S., Marshall S. T., DeArmond S., Westaway D., Prusiner S. B. Linkage of prion protein and scrapie incubation time genes. Cell. 1986 Aug 15;46(4):503–511. doi: 10.1016/0092-8674(86)90875-5. [DOI] [PubMed] [Google Scholar]
- Clarke M. C., Millson G. C. Infection of a cell line of mouse L fibroblasts with scrapie agent. Nature. 1976 May 13;261(5556):144–145. doi: 10.1038/261144a0. [DOI] [PubMed] [Google Scholar]
- Gabizon R., McKinley M. P., Prusiner S. B. Purified prion proteins and scrapie infectivity copartition into liposomes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4017–4021. doi: 10.1073/pnas.84.12.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek D. C. Unconventional viruses and the origin and disappearance of kuru. Science. 1977 Sep 2;197(4307):943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Gibbs C. J., Jr, Joy A., Heffner R., Franko M., Miyazaki M., Asher D. M., Parisi J. E., Brown P. W., Gajdusek D. C. Clinical and pathological features and laboratory confirmation of Creutzfeldt-Jakob disease in a recipient of pituitary-derived human growth hormone. N Engl J Med. 1985 Sep 19;313(12):734–738. doi: 10.1056/NEJM198509193131207. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Smeltzer D. A., Amyx H. L., Gibbs C. J., Jr, Gajdusek D. C. Evidence for an unconventional virus in mouse-adapted Creutzfeldt-Jakob disease. Infect Immun. 1982 Sep;37(3):1050–1053. doi: 10.1128/iai.37.3.1050-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T., Tateishi J., Tashima T., Takeshita I., Barry R. A., DeArmond S. J., Prusiner S. B. Amyloid plaques in Creutzfeldt-Jakob disease stain with prion protein antibodies. Ann Neurol. 1986 Aug;20(2):204–208. doi: 10.1002/ana.410200205. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Locht C., Chesebro B., Race R., Keith J. M. Molecular cloning and complete sequence of prion protein cDNA from mouse brain infected with the scrapie agent. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6372–6376. doi: 10.1073/pnas.83.17.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovits P., Dormont D., Delpech B., Court L., Latarjet R. Essais de propagation in vitro de l'agent scrapie dans des cellules nerveuses de souris. C R Seances Acad Sci III. 1981 Nov 2;293(8):413–417. [PubMed] [Google Scholar]
- Masters C. L., Gajdusek D. C., Gibbs C. J., Jr Creutzfeldt-Jakob disease virus isolations from the Gerstmann-Sträussler syndrome with an analysis of the various forms of amyloid plaque deposition in the virus-induced spongiform encephalopathies. Brain. 1981 Sep;104(3):559–588. doi: 10.1093/brain/104.3.559. [DOI] [PubMed] [Google Scholar]
- McKinley M. P., Bolton D. C., Prusiner S. B. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- McKinley M. P., Prusiner S. B. Biology and structure of scrapie prions. Int Rev Neurobiol. 1986;28:1–57. doi: 10.1016/s0074-7742(08)60105-1. [DOI] [PubMed] [Google Scholar]
- Meyer R. K., McKinley M. P., Bowman K. A., Braunfeld M. B., Barry R. A., Prusiner S. B. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Pattison I. H., Jones K. M. Modification of a strain of mouse-adapted scrapie by passage through rats. Res Vet Sci. 1968 Sep;9(5):408–410. [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bolton D. C., Kent S. B., Hood L. E. Purification and structural studies of a major scrapie prion protein. Cell. 1984 Aug;38(1):127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Prions and neurodegenerative diseases. N Engl J Med. 1987 Dec 17;317(25):1571–1581. doi: 10.1056/NEJM198712173172505. [DOI] [PubMed] [Google Scholar]
- Race R. E., Fadness L. H., Chesebro B. Characterization of scrapie infection in mouse neuroblastoma cells. J Gen Virol. 1987 May;68(Pt 5):1391–1399. doi: 10.1099/0022-1317-68-5-1391. [DOI] [PubMed] [Google Scholar]
- Roberts G. W., Lofthouse R., Brown R., Crow T. J., Barry R. A., Prusiner S. B. Prion-protein immunoreactivity in human transmissible dementias. N Engl J Med. 1986 Nov 6;315(19):1231–1233. doi: 10.1056/NEJM198611063151919. [DOI] [PubMed] [Google Scholar]
- Rubenstein R., Carp R. I., Callahan S. M. In vitro replication of scrapie agent in a neuronal model: infection of PC12 cells. J Gen Virol. 1984 Dec;65(Pt 12):2191–2198. doi: 10.1099/0022-1317-65-12-2191. [DOI] [PubMed] [Google Scholar]
- Stahl N., Borchelt D. R., Hsiao K., Prusiner S. B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987 Oct 23;51(2):229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- Tateishi J., Ohta M., Koga M., Sato Y., Kuroiwa Y. Transmission of chronic spongiform encephalopathy with kuru plaques from humans to small rodents. Ann Neurol. 1979 Jun;5(6):581–584. doi: 10.1002/ana.410050616. [DOI] [PubMed] [Google Scholar]
- Walker A. S., Inderlied C. B., Kingsbury D. T. Conditions for the chemical and physical inactivation of the K. Fu. strain of the agent of Creutzfeldt-Jakob disease. Am J Public Health. 1983 Jun;73(6):661–665. doi: 10.2105/ajph.73.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway D., Goodman P. A., Mirenda C. A., McKinley M. P., Carlson G. A., Prusiner S. B. Distinct prion proteins in short and long scrapie incubation period mice. Cell. 1987 Nov 20;51(4):651–662. doi: 10.1016/0092-8674(87)90134-6. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Green M., Mackey J. K., Martin J. D., Padgett B. L., Walker D. L. Integration pattern of human JC virus sequences in two clones of a cell line established from a JC virus-induced hamster brain tumor. J Virol. 1980 Mar;33(3):1225–1228. doi: 10.1128/jvi.33.3.1225-1228.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]