Abstract
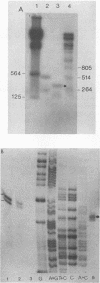
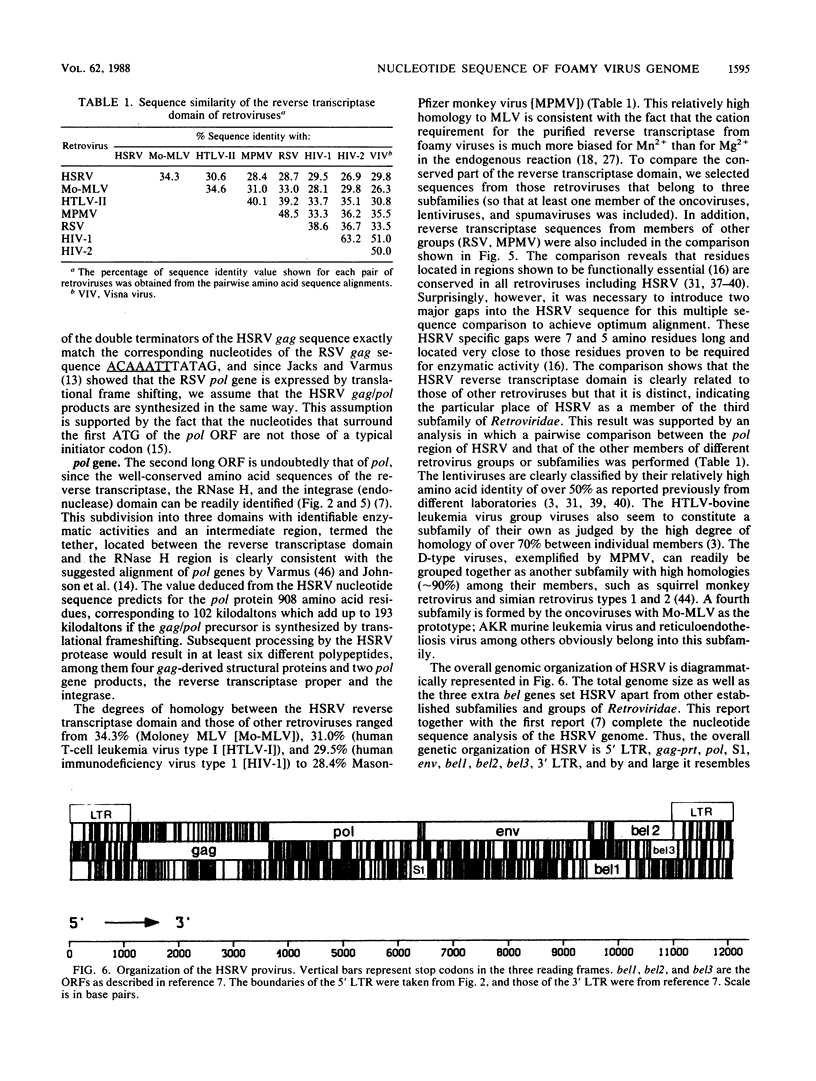
The nucleotide sequence of the human spumaretrovirus (HSRV) genome was determined. The 5' long terminal repeat region was analyzed by strong stop cDNA synthesis and S1 nuclease mapping. The length of the RU5 region was determined and found to be 346 nucleotides long. The 5' long terminal repeat is 1,123 base pairs long and is bound by an 18-base-pair primer-binding site complementary to the 3' end of mammalian lysine-1,2-specific tRNA. Open reading frames for gag and pol genes were identified. Surprisingly, the HSRV gag protein does not contain the cysteine motif of the nucleic acid-binding proteins found in and typical of all other retroviral gag proteins; instead the HSRV gag gene encodes a strongly basic protein reminiscent of those of hepatitis B virus and retrotransposons. The carboxy-terminal part of the HSRV gag gene products encodes a protease domain. The pol gene overlaps the gag gene and is postulated to be synthesized as a gag/pol precursor via translational frameshifting analogous to that of Rous sarcoma virus, with 7 nucleotides immediately upstream of the termination codons of gag conserved between the two viral genomes. The HSRV pol gene is 2,730 nucleotides long, and its deduced protein sequence is readily subdivided into three well-conserved domains, the reverse transcriptase, the RNase H, and the integrase. Although the degree of homology of the HSRV reverse transcriptase domain is highest to that of murine leukemia virus, the HSRV genomic organization is more similar to that of human and simian immunodeficiency viruses. The data justify classifying the spumaretroviruses as a third subfamily of Retroviridae.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achong B. G., Mansell P. W., Epstein M. A., Clifford P. An unusual virus in cultures from a human nasopharyngeal carcinoma. J Natl Cancer Inst. 1971 Feb;46(2):299–307. [PubMed] [Google Scholar]
- Cameron K. R., Birchall S. M., Moses M. A. Isolation of foamy virus from patient with dialysis encephalopathy. Lancet. 1978 Oct 7;2(8093):796–796. doi: 10.1016/s0140-6736(78)92691-0. [DOI] [PubMed] [Google Scholar]
- Chiu I. M., Yaniv A., Dahlberg J. E., Gazit A., Skuntz S. F., Tronick S. R., Aaronson S. A. Nucleotide sequence evidence for relationship of AIDS retrovirus to lentiviruses. 1985 Sep 26-Oct 2Nature. 317(6035):366–368. doi: 10.1038/317366a0. [DOI] [PubMed] [Google Scholar]
- Derse D. Bovine leukemia virus transcription is controlled by a virus-encoded trans-acting factor and by cis-acting response elements. J Virol. 1987 Aug;61(8):2462–2471. doi: 10.1128/jvi.61.8.2462-2471.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügel R. M., Maurer B., Bannert H., Rethwilm A., Schnitzler P., Darai G. Nucleotide sequence analysis of a cloned DNA fragment from human cells reveals homology to retrotransposons. Mol Cell Biol. 1987 Jan;7(1):231–236. doi: 10.1128/mcb.7.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügel R. M., Rapp U., Wells R. D. RNA-DNA covalent bonds between the RNA primers and the DNA products formed by RNA tumor virus DNA polymerase. J Virol. 1973 Dec;12(6):1491–1502. doi: 10.1128/jvi.12.6.1491-1502.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügel R. M., Rethwilm A., Maurer B., Darai G. Nucleotide sequence analysis of the env gene and its flanking regions of the human spumaretrovirus reveals two novel genes. EMBO J. 1987 Jul;6(7):2077–2084. doi: 10.1002/j.1460-2075.1987.tb02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Lobel L. I. Mutants of murine leukemia viruses and retroviral replication. Biochim Biophys Acta. 1987 Jul 8;907(2):93–123. doi: 10.1016/0304-419x(87)90001-1. [DOI] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G. A method for classification of 5' termini of retroviruses. Nature. 1978 Jun 1;273(5661):358–364. doi: 10.1038/273358a0. [DOI] [PubMed] [Google Scholar]
- Hirsch V., Riedel N., Mullins J. I. The genome organization of STLV-3 is similar to that of the AIDS virus except for a truncated transmembrane protein. Cell. 1987 May 8;49(3):307–319. doi: 10.1016/0092-8674(87)90283-2. [DOI] [PubMed] [Google Scholar]
- Jacks T., Varmus H. E. Expression of the Rous sarcoma virus pol gene by ribosomal frameshifting. Science. 1985 Dec 13;230(4731):1237–1242. doi: 10.1126/science.2416054. [DOI] [PubMed] [Google Scholar]
- Johnson M. S., McClure M. A., Feng D. F., Gray J., Doolittle R. F. Computer analysis of retroviral pol genes: assignment of enzymatic functions to specific sequences and homologies with nonviral enzymes. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7648–7652. doi: 10.1073/pnas.83.20.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Purifoy D. J., Powell K. L., Darby G. Site-specific mutagenesis of AIDS virus reverse transcriptase. 1987 Jun 25-Jul 1Nature. 327(6124):716–717. doi: 10.1038/327716a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Liu W. T., Natori T., Chang K. S., Wu A. M. Reverse transcriptase of foamy virus. Purification of the enzymes and immunological identification. Arch Virol. 1977;55(3):187–200. doi: 10.1007/BF01319905. [DOI] [PubMed] [Google Scholar]
- Loh P. C., Matsuura F., Mizumoto C. Seroepidemiology of human syncytial virus: antibody prevalence in the Pacific. Intervirology. 1980;13(2):87–90. doi: 10.1159/000149112. [DOI] [PubMed] [Google Scholar]
- Malmquist W. A., Van der Maaten M. J., Boothe A. D. Isolation, immunodiffusion, immunofluorescence, and electron microscopy of a syncytial virus of lymphosarcomatous and apparently normal cattle. Cancer Res. 1969 Jan;29(1):188–200. [PubMed] [Google Scholar]
- Maurer B., Flügel R. M. The 3'-orf protein of human immunodeficiency virus 2 shows sequence homology with the bel3 gene of the human spumaretrovirus. FEBS Lett. 1987 Oct 5;222(2):286–288. doi: 10.1016/0014-5793(87)80387-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure M. A., Johnson M. S., Doolittle R. F. Relocation of a protease-like gene segment between two retroviruses. Proc Natl Acad Sci U S A. 1987 May;84(9):2693–2697. doi: 10.1073/pnas.84.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H. K., Ball G., Epstein M. A., Achong B. G., Lenoir G., Levin A. The prevalence of naturally occurring antibodies to human syncytial virus in East African populations. J Gen Virol. 1980 Apr;47(2):399–406. doi: 10.1099/0022-1317-47-2-399. [DOI] [PubMed] [Google Scholar]
- Obara T., Bolognesi D. P., Bauer H. Ribosomal RNA in avian leukosis virus particles. Int J Cancer. 1971 May 15;7(3):535–546. doi: 10.1002/ijc.2910070320. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Todaro G. J., Scolnick E. M., Aaronson S. A. RNA dependent DNA polymerase in primate syncytium-forming (foamy) viruses. Nature. 1971 Jan 22;229(5282):258–260. doi: 10.1038/229258a0. [DOI] [PubMed] [Google Scholar]
- Prince A. M., Huima T., Williams B. A., Bardina L., Brotman B. Isolation of a virus from chimpanzee liver cell cultures inoculated with sera containing the agent of non-A, non-B hepatitis. Lancet. 1984 Nov 10;2(8411):1071–1075. doi: 10.1016/s0140-6736(84)91509-5. [DOI] [PubMed] [Google Scholar]
- Raba M., Limburg K., Burghagen M., Katze J. R., Simsek M., Heckman J. E., Rajbhandary U. L., Gross H. J. Nucleotide sequence of three isoaccepting lysine tRNAs from rabbit liver and SV40-transformed mouse fibroblasts. Eur J Biochem. 1979 Jun;97(1):305–318. doi: 10.1111/j.1432-1033.1979.tb13115.x. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Rethwilm A., Darai G., Rösen A., Maurer B., Flügel R. M. Molecular cloning of the genome of human spumaretrovirus. Gene. 1987;59(1):19–28. doi: 10.1016/0378-1119(87)90262-9. [DOI] [PubMed] [Google Scholar]
- Riggs J. L., Oshirls, Taylor D. O., Lennette E. H. Syncytium-forming agent isolated from domestic cats. Nature. 1969 Jun 21;222(5199):1190–1191. doi: 10.1038/2221190a0. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Schnitzer T. J. Characterization of a simian foamy virus isolated from a spontaneous primate lymphoma. J Med Primatol. 1981;10(6):312–328. doi: 10.1159/000460095. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Takahashi Y., Shimizu N., Gojobori T., Golde D. W., Chen I. S., Miwa M., Sugimura T. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc Natl Acad Sci U S A. 1985 May;82(10):3101–3105. doi: 10.1073/pnas.82.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Sonigo P., Alizon M., Staskus K., Klatzmann D., Cole S., Danos O., Retzel E., Tiollais P., Haase A., Wain-Hobson S. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell. 1985 Aug;42(1):369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Stancek D., Stanceková-Gressnerová M., Janotka M., Hnilica P., Oravec D. Isolation and some serological and epidemiological data on the viruses recovered from patients with subacute thyroiditis de Quervain. Med Microbiol Immunol. 1975;161(2):133–144. doi: 10.1007/BF02121755. [DOI] [PubMed] [Google Scholar]
- Taylor J. M. An analysis of the role of tRNA species as primers for the transcription into DNA of RNA tumor virus genomes. Biochim Biophys Acta. 1977 Mar 21;473(1):57–71. doi: 10.1016/0304-419x(77)90007-5. [DOI] [PubMed] [Google Scholar]
- Thayer R. M., Power M. D., Bryant M. L., Gardner M. B., Barr P. J., Luciw P. A. Sequence relationships of type D retroviruses which cause simian acquired immunodeficiency syndrome. Virology. 1987 Apr;157(2):317–329. doi: 10.1016/0042-6822(87)90274-1. [DOI] [PubMed] [Google Scholar]
- Toh H., Kikuno R., Hayashida H., Miyata T., Kugimiya W., Inouye S., Yuki S., Saigo K. Close structural resemblance between putative polymerase of a Drosophila transposable genetic element 17.6 and pol gene product of Moloney murine leukaemia virus. EMBO J. 1985 May;4(5):1267–1272. doi: 10.1002/j.1460-2075.1985.tb03771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E. Reverse transcriptase rides again. Nature. 1985 Apr 18;314(6012):583–584. doi: 10.1038/314583a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Baltimore D. Covalent linkage between ribonucleic Acid primer and deoxyribonucleic Acid product of the avian myeloblastosis virus deoxyribonucleic Acid polymerase. J Virol. 1972 Oct;10(4):622–627. doi: 10.1128/jvi.10.4.622-627.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner J., Gelderblom H. Isolation of foamy virus from patients with de Quervain thyroiditis. Lancet. 1979 Aug 4;2(8136):258–259. doi: 10.1016/s0140-6736(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Gallo R. C. Human T-lymphotropic retroviruses. Nature. 1985 Oct 3;317(6036):395–403. doi: 10.1038/317395a0. [DOI] [PubMed] [Google Scholar]
- Young D., Samuels J., Clarke J. K. A foamy virus of possible human origin isolated in BHK-21 cells. Arch Gesamte Virusforsch. 1973;42(3):228–234. doi: 10.1007/BF01265647. [DOI] [PubMed] [Google Scholar]