Abstract
Giant cell tumours are rare bone tumours that are characteristically benign but locally aggressive, most frequently occurring in the distal femur with pathological fractures being common. This paper investigates relationships between tumour size and cortical breach on initial X-rays and subsequent treatment. The X-rays of 54 patients with distal femoral giant cell tumours were reviewed. The volumes of the tumour, distal femur and a ratio between the two parameters were estimated. The presence of a cortical breach, discrete fracture and Campanacci grade was recorded. X-rays revealed intact cortical rim in 20 patients (37%), cortical breach in 22 patients (41%) and discrete fracture in 12 patients (22%). There was a significant difference in the ratio of tumour volume to distal femoral volume between the discrete fracture group and the cortical breach group. No significant differences in rates of local recurrence were demonstrated. Extended curettage was effective for intact and cortical breach groups; however, patients in the fracture group often required radical treatment.
Résumé
Les tumeurs à cellules géantes sont des tumeurs rares et dont la caractéristique est d’être à la fois bénigne et agressive localement. Elles surviennent le plus fréquemment au niveau de l’extrémité inférieure du fémur entraînant des fractures pathologiques. Ce travail a pour but de mettre en relation certaines caractéristiques de la tumeur, taille, effraction corticale, en fonction des radios initiales et du traitement pratiqué. 54 patients présentant une tumeur à cellules géantes de l’extrémité inférieure du fémur ont été revus. Une relation a été établie entre le volume de la tumeur et le volume de l’extrémité inférieure du fémur de même que la présence d’une effraction corticale ou d’une discrète fracture. La classification de Campanacci a été utilisée. Résultats : les radios ont révélé qu’il existait une corticale intacte chez 20 patients (37% des cas), une effraction corticale chez 22 patients (41% des cas) et une discrète fracture chez 12 patients (22% des cas). Il existe des différences significatives dans la relation du volume tumoral osseux et fémoral distal pour les patients présentant une effraction corticale ou une discrète fractur. Par contre, nous n’avons pas retrouvé de différences significatives de ces éléments pour les récidives locales. Un curetage extensif est nécessaire et suffisant pour les patients présentant une corticale intacte ou une effraction corticale par contre, chez les patients présentant une fracture, il est souvent nécessaire de faire un traitement radical.
Introduction
Giant cell tumour (GCT) remain one of the more poorly understood bone tumours despite being heavily investigated, with the World Health Organisation classifying GCT as “an aggressive, potentially malignant lesion” [1], implying a poor correlation between histological features and clinical course. GCTs represent 4–5% of primary bone tumours and 20% of biopsy-analysed benign bone tumours [2].
The majority of GCTs have a benign course, with a high rate of local recurrence, varying historically from 30% to 56% following simple curettage treatments, falling to 0–25% with extended debridement with a burr and adjuvant treatments. The rate of local recurrence is important, as the patient will require further treatment. Malignant transformation is rare, but 1–4% of patients will develop pulmonary metastases despite benign histological features at biopsy [3].
The tumour has been reported to show slight female predominance (1.2:1), with a peak incidence in young adults aged 20–40 years [4, 5]. Typically the tumour is eccentrically located at the end of long bones, with approximately 50–60% being located around the knee; the distal femur is the bone most often affected in most series [2, 3, 5] The tumour is locally aggressive, producing cystic osteolysis, causing the cortex to thin and sometimes be breached. The cortical breaching can be isolated or may weaken the bone sufficiently to allow a fracture, which may or may not displace and lead to collapse. The aim of the study was to identify the presence of a cortical breach or overt fracture on the initial presentation X-rays of patients with distal femoral GCTs and to analyse the treatment and outcome of these patients compared to patients without a cortical breach.
Methods
A prospective database is kept of all patients seen in our unit, containing patient, tumour, treatment and outcome details. We identified 54 consecutive patients with a GCT of the distal femur. It was possible to retrieve the initial X-ray taken at presentation to the unit for review. The X-rays taken prior to biopsy were used for the analysis.
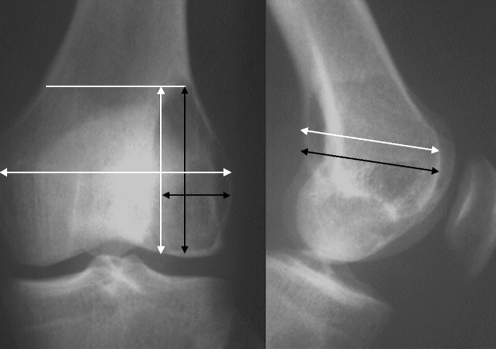
The size of the tumour was estimated using a facility of the database, by measuring the largest dimensions of the tumour (depth, breadth and height) and assuming the tumour was spherical in shape. The volume of the distal femur was estimated using the same X-ray and computer programme. The most proximal point of the tumour was used as a marker for the height of the distal femur, and the largest dimensions of width and breadth were used (Fig. 1). The calculated volumes were then used to calculate a ratio of tumour volume (TV) to distal femoral volume (DFV) for each tumour.
Fig. 1.
A schematic illustration of the measurement techniques from the X-ray, with the tumour volume measurements in black and femoral measurements in white
The X-rays were then carefully studied for evidence of a cortical breach (defined as incomplete cortical covering of the tumour) on antero-posterior (AP) and lateral views. The radiological grade of the tumour was estimated by two observers using the definitions by Campanacci et al. [12]. The database and clinical records were reviewed for details of treatment, subsequent local disease recurrence and metastases. The results were analysed using Kaplan–Meier survival analysis, Student t tests, univariate analysis and ANOVA, with the limit of significance set with α=0.05.
Results
Distal femoral GCTs were identified in 54 patients (29 male, 25 female) with a mean age of 36 years (median 31 years, range 18–72 years). The mean length of symptoms prior to biopsy was 36 weeks (median 20 weeks, range 1–220 weeks). All patients had a biopsy-proven GCT of the distal femur (34 left, 20 right side). The X-rays were classified by Campanacci grade (1–3) and the results are shown in Table 1. The mean TV increased with Campanacci grade, but this was not statistically significant (p=0.68).
Table 1.
The grade of GCT on presentation X-rays
| Campanacci grade | Count | Mean TV:DFV |
|---|---|---|
| 1 | 14 (26%) | 35% |
| 2 | 27 (50%) | 53% |
| 3 | 13 (24%) | 64% |
Initial X-rays showed that 20 (37%) patients had intact cortical margins to the tumour, 22 (41%) had an area of a cortical breach and 12 (22%) patients had a discrete fracture at presentation. There was no correlation between the duration of symptoms and presence of a cortical defect or fracture at presentation (univariate analysis, p=0.98).
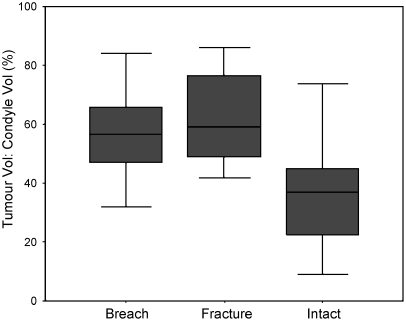
The TV:DFV ratios were distributed as shown in Fig. 2, and there was a significant correlation to the risk of fracture at presentation (p>0.001). Statistically significant differences were shown between the TV:DFV of those patients with and those without a fracture or cortical breach (univariate analysis, p<0.001) (Fig. 2); however, significant differences in TV:DFV between those patients with a cortical breach and those with a fracture were not demonstrated (p=0.43).
Fig. 2.
Box plot showing the range of values (T bars), 95% confidence intervals (grey box) and means (black line) for the TV:DFV ratio for the intact cortex, breached cortex and discrete fracture groups
When TV was used alone, this showed significant differences in mean volumes among groups (intact cortex=121 mm3, breach=186 mm3, fracture=258 mm3, p<0.001). On univariate analysis the difference in TV reached statistical significance only between the fracture group and the intact cortex group (p<0.001), with trends towards differences in TV between the intact cortex and cortical breach groups (p=0.12) and between the cortical breach and fracture groups (p=0.14).
There were 16 patients (29.6%) with local recurrent disease. However, there was no statistical difference (p=0.92) in subsequent local recurrence rates between those patients with a fracture, those with a cortical breach or those with an intact cortex on initial X-ray, when stratified by treatment. Neither was there any correlation between the TV:DFV ratio and local recurrence, though there was a trend towards local recurrence in smaller lesions (p=0.13, Table 2).
Table 2.
Relationship of TV:DFV ratio to local recurrence
| Local recurrence | Number | Mean TV:DFV ratio | Standard deviation | Significance (two-tailed) |
|---|---|---|---|---|
| Yes | 16 | 44.44 | 17.23 | p=0.13 |
| No | 38 | 52.95 | 19.11 |
There were no statistical differences in the number of operations among those who presented with a fracture, a cortical breach or an intact cortex (p=0.48). There was no evidence that more radical surgery, such as endoprosthetic replacement, was required if a patient presented with an intact cortex or cortical breach on initial X-ray; however, there was a strong trend towards prosthetic replacement if presenting with a fracture (p=0.06). It was possible to treat 70% of patients (n=38/54) by extended curettage alone, and there was no evidence that the extended curettage was less effective in the intact cortex or cortical breach group; however, there was a strong trend (p=0.07) that curettage was more likely to fail in the fracture group. The relationship between cortex group and the success of treatment is seen in Table 3.
Table 3.
Success of treatment stratified by cortex category
| Group | Number | Mean number of operations | Percentage initial curettage successful | Percentage requiring EPR | Percentage local recurrence |
|---|---|---|---|---|---|
| Intact cortex | 20 | 1.30 | 80% (n=16) | 15% (n=3) | 30% (n=6) |
| Cortex breach | 22 | 1.45 | 73% (n=16) | 23% (n=5) | 32%(n=7) |
| Fracture | 12 | 1.67 | 42% (n=5) | 50% (n=6) | 25% (n=3) |
Two patients (3.7%) subsequently developed pulmonary metastases following local recurrence. Both patients initially had an intact cortex at presentation and relatively small TV:DFV ratio (mean=35.5).
Discussion
GCT has long been an enigmatic tumour, leading to difficulty in predicting its clinical course; features used in proposed classification systems have included histological, clinical and radiological. The histological grading system of Jaffe et al. [16] attempts to classify the tumour as benign, aggressive or malignant, whilst Dahlin [17] dropped the aggressive category, but both have poor correlation with clinical course. Enneking [18] classified GCTs by clinical course, and Campanacci et al. [12] attempted to classify GCTs by radiological appearance. In grade I tumours there is a well-defined margin border and a thin rim of mature bone. Grade II tumours have a relatively well-defined margin but no radio-opaque rim. The combined rim of reactive bone and cortex is thin and moderately expanded but still present. A separate grade II F represents a grade II appearance with a fracture. In grade III the tumour has indistinct borders, suggesting rapid and possibly permeative growth, the tumour bulges into the soft tissues, but the soft tissue mass does not follow the contour of the bone and is not limited by an apparent shell of bone.
Relatively little has been published on the relationship of cortical breaching and pathological fractures of GCTs in weight bearing bones. Pathological fractures through a GCT are not unusual, often presenting with features of non-specific pain, swelling and warmth. The literature suggests that overt pathological fractures at presentation are seen in 4–32% of cases, with a 12% incidence in the largest series of 677 GCTs [6–15] The fractures often occur with minimal trauma and on a background of discomfort in the affected limb. In our series of GCTs in a major weight-bearing joint, a cortical breach on initial X-ray occurred in 41% of patients (n=22/54) and a pathological fracture was present in 22% (n=12/54).
Isolated cortical breaches per se did not significantly alter the efficacy of initial treatment, which was by simple curettage and burr debridement. However, the presence of a fracture through the tumour at presentation made simple treatment less effective and increased the rate of prosthetic reconstruction, but fortunately did not increase the rate of local recurrence or metastases.
In our study, the risk of cortical breach and fracture in patients with GCTs of the distal femur is statistically dependent upon the TV:DFV ratio. If the ratio is above 54% then the GCT is likely to present with a cortical breach or fracture on X-ray (lower 95% confidence interval); conversely, if the ratio is less than 44% then a cortical breach on initial X-ray is unlikely. The use of TV alone was not as predictive of the risk of fracture as the TV:DFV ratio.
The use of TV to predict impending fracture is not a new concept, being widely used in metastatic bone disease. Initial work by Harrington et al. [19] suggested that metastatic destruction of more than 50% of the proximal femoral cortex leads to an increased risk of pathological fracture. However, Keene et al. [20] subsequently questioned these findings following a retrospective review of metastases from the breast to the proximal end of the femur, suggesting that there was no reliable relationship between the risk of fracture and the size of the area involved, the degree of pain, or the nature of the lesion (lytic, blastic, or mixed). Mirels [21] developed a scoring system for the prediction of fracture risk in which each lesion is evaluated on the basis of four factors: site, size, type, and pain. This system is in wide use in many orthopaedic oncology units internationally. Our findings add further weight to the principle that if more than 54% of the bone is destroyed by tumour, a pathological fracture is likely.
The authors feel that cortical breach and pathological fracture are on the same spectrum and are probably sequential. We feel our results support the treatment rationale of extended debridement for patients with intact and small breaches in the cortex of the distal femur; however, patients with pathological fractures may require more aggressive surgery. Smaller lesions appear, paradoxically, to have a higher rate of local recurrence, and it is essential that adequate debridement is performed.
References
- 1.Schajowicz F (1993) Histological typing of bone tumours. Springer, Berlin Heidelberg New York, pp 20–22
- 2.Turcotte RE, Wunder JS, Isler MH, Bell RS, Schachar N, Masri BA, Moreau G, Davis AM (2002) Giant cell tumor of long bone: a Canadian sarcoma group study. Clin Orthop Relat Res 1(397):248–258 [DOI] [PubMed]
- 3.Szendröi M (2004) Giant-cell tumour of bone. J Bone Jt Surg [Br] 86-B:5–12 [PubMed]
- 4.Campanacci M (1990) Giant cell tumor. In: Gaggi A (ed) Bone and soft-tissue tumors. Springer, Bologna, pp 117–153
- 5.Unni KK (1998) Dahlin’s bone tumors: general aspect and data on 11087 cases, 5th edn. Lippincott-Raven, Philadelphia
- 6.Goldenberg RR, Campbell CJ, Bonfiglio M (1970) Giant cell tumour of bone: an analysis of 218 cases. J Bone Jt Surg [Am] 52-A:619–664 [PubMed]
- 7.Larsson SE, Lorentzon R, Boquist L (1975) Giant cell tumor of bone: a demographic, clinical and histopathological study of all cases recorded on the Swedish Cancer Registry for the years 1958 to 1968. J Bone Jt Surg [Am] 57-A:167–173 [PubMed]
- 8.McInerny DP, Middlemiss JH (1978) Giant cell tumour of bone. Skelet Radiol 2:195–204
- 9.Sung HW, Kuo DP, Shu WP et al (1982) Giant cell tumour of bone: an analysis of 218 cases in Chinese patients. J Bone Jt Surg [Am] 64-A:755–761 [PubMed]
- 10.Komiya S, Inoue A, Nakashima M et al (1986) Prognostic factors in giant cell tumour of bone: a modified histological grading system as a useful guide to prognosis. Arch Orthop Trauma Surg 105:67–72 [DOI] [PubMed]
- 11.MacDonald DJ, Sim FH, McLeod RA, Dahlin DC (1986) Giant cell tumour of bone. J Bone Jt Surg [Am] 68-A:235–242 [PubMed]
- 12.Campanacci M, Baldini N, Boriani S, Sudanese A (1987) Giant cell tumour of bone. J Bone Jt Surg [Am] 69-A:106–114 [PubMed]
- 13.Szendroi M (1990) Giant cell tumour of the radius: aggressiveness and soft tissue recurrence. Chir Organi Mov 75[Ssuppl 1]:241–243 [PubMed]
- 14.Miller G, Bettelli G, Fabbri N, Campanna R (1990) Curettage of giant cell tumor of bone: introduction, material and methods. Chir Organi Mov 75[Suppl 1]:203 [PubMed]
- 15.Dreinfhöfer KE, Rydholm A, Bauer HCF, Kreicbergs A (1995) Giant-cell tumours with fracture at diagnosis. J Bone Jt Surg [Br] 77-B:189–193 [PubMed]
- 16.Jaffe HL, Lichtenstein L, Portis RB (1940) Giant cell tumor of bone: its pathologic appearance, grading, supposed variants and treatment. Arch Pathol 30:993–1031
- 17.Dahlin DC (1978) Bone tumours, 3rd edn. Charles C Thomas, Springfield, Illinois, pp 99–115
- 18.Enneking WF (1983) Musculoskeletal tumor surgery, vol 1. Churchill Livingstone, New York, pp 1436–1476
- 19.Harrington KD, Sim FH, Enis JE, Johnston JO, Dick HM, Gristina AG (1976) Methylmethacrylate as an adjunct in internal fixation of pathological fractures. Experience with three hundred and seventy-five cases. J Bone Jt Surg 58-A:1047–1055 Dec [PubMed]
- 20.Keene JS, Sellinger DS, McBeath AA, Engber WD (1986) Metastatic breast cancer in the femur. A search for the lesion at risk of fracture. Clin Orthop 203:282–288 [PubMed]
- 21.Mirels H (1989) Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop 249:256–264 [PubMed]