Abstract
Osteoid osteoma is a benign bone tumour usually occurring in young individuals (10–30 years). It presents with intense pain (typically nocturnal), which can be alleviated by salicylates. Treatment consists of surgical excision or destroying the nidus and it is curative. In the past, surgery was performed in an “open” fashion and the nidus had to be removed with a bone block. This extensive type of surgery could be associated with some rates of both failure and complication. There is growing evidence to suggest that percutaneous CT-guided removal or destruction of the nidus is a good alternative and it is indeed gaining worldwide popularity. We present a series of 18 consecutive patients with osteoid osteoma of the pelvis, femur, and tibia, treated percutaneously under CT guidance. Removal of the nidus was performed using a 4.5-mm cannulated drill and a cannulated curette of our own design. Tissue samples for histological evaluation were obtained in the same way. The mean follow-up time was 29 months. Sixteen patients were initially cured. The procedure had to be repeated in two patients and was eventually successful (primary and secondary success rates 88 and 100% respectively). The diagnosis was histologically confirmed in 14 cases out of 18 (77%). In four cases no histological confirmation of osteoid osteoma could be achieved. There were only two minor complications, one case of femoral neuropraxia and one case of skin abrasion. Percutaneous CT-guided removal seems to be efficient and safe for the treatment of osteoid osteoma. The use of a cannulated drill and a cannulated curette facilitates efficient removal of the tumour and procurement of tissue for diagnosis.
Résumé
L’ostéome ostéoïde est une tumeur bénigne survenant chez le sujet jeune (10–30 ans), se manifestant par des douleurs intenses, typiquement nocturnes calmées par les salicylés. Classiquement la chirurgie était réalisée à ciel ouvert et le nidus retiré avec un bloc osseux. Les éléments convergent pour préférer un abord percutané, détruisant ou enlevant le nidus, guidé par scanner. Nous présentons une série de 18 cas consécutifs d’ostéomes ostéoïdes du bassin du fémur et du tibia traités de cette façon en utilisant une mèche canulée de 4,5 mm et une curette canulée, avec prélèvements à visée histologique. Le délai moyen de suivi est de 29 mois. Seize patients furent traités initialement et le procédé fut répété chez 2 patients (avec un taux de succés primaire de 88% et secondaire de 100%). Le diagnostic a été confirmé histologiquement 14 fois sur les 18. Il n’y a eu que 2 complications mineures (neurapraxie crurale et érosion cutanée). En conclusion, l’ablation de l’ostéome ostéoïde par voie percutanée sous contrôle scanner est un traitement efficace. L’utilisation d’un matériel spécial facilite le traitement et l’obtention d’un fragment pour biopsie.
Introduction
The management of osteoid osteoma has changed in recent years from open “en bloc” resection of the area containing the nidus to more accurate and sophisticated ways of treatment: percutaneous drilling [11], trepanation, laser photocoagulation or chemical destruction of the nidus, all of them by way of CT guidance. These modalities have been shown to have many advantages over the traditional “open” method, including a higher success rate and fewer associated complications [8, 16].
Ongoing efforts are being made in an attempt to preserve the tissue while the nidus is removed in order to achieve a proper diagnosis [2, 12]. We present our experience using a procedure with a strong emphasis upon obtaining proper histological proof using a cannulated drill and curette.
Patients and methods
All patients diagnosed as suffering from osteoid osteoma who were treated by the method described were retrospectively evaluated. In all, 18 patients (four males and 14 females) were included. The clinical diagnosis was based upon patients’ histories, X-rays, and CT scans or MRI. A clear demonstration of the typical nidus by preoperative imaging modalities was an absolute prerequisite for entering this study group, and all patients with atypical findings in whom we had any doubts concerning the diagnosis were excluded. In all patients the nidus was not larger than 10 mm in diameter. Patients’ ages ranged between 11 and 35 years with an average age of 18. The distribution of tumour location was as follows: three cases in the pelvis (one in the iliac bone, one in the sacrum, and one in the acetabular roof), seven cases in the proximal femur, three in the femoral shaft, and five in the tibia. The operation was performed under either local or general anesthesia. We used a 2400 Elite Spiral CT apparatus (by Elcint, Haifa, Israel), with interval cuts of 2 mm. A 0.3 partially threaded K-wire was initially driven percutaneously into the nidus under CT guidance. The track up to the nidus was next drilled and enlarged by a 4.5-mm cannulated drill bit. At this point, a cannulated curette of our own design was used. The curette was inserted along the K-wire into the nidus. When in place, it was used to remove the nidus mechanically. Two goals were thus simultaneously achieved: the nidus was removed and tissue biopsy was obtained for histological evaluation. Tissues were preserved in a 4% buffered formalin solution and decalcified in 10% formic acid. They were then embedded in paraffin wax and cut to obtain 4-μm thick sections. The sections were stained with hematoxylin and eosin and were subjected to microscopic analysis.
Computed tomography reconfirmed that the nidus was in fact removed. The tiny skin wound was closed with a single suture. All patients remained in hospital for observation for 24 h. All patients apart from the one with osteoid osteoma in the sacrum and another patient with a tumour located in the iliac crest were limited to partial weight-bearing for 6 weeks. Follow-up consisted of a repeat clinical evaluation as well as plain X-rays taken 3 months and 1 year postoperatively. All patients were followed for a period of at least 12 months, with an average follow-up of 29 months (range 12 to 42 months).
Results
Sixteen patients were initially cured; the procedure resulted in immediate and permanent pain relief. In none of these patients could a nidus seen on their follow-up X-rays. No other imaging modality was used for the follow-up of these 16 “cured” patients. The nidus was missed in two patients with osteoid osteoma of the pelvis, one located in the acetabular roof and the other in the iliac crest. In both cases the operators misinterpreted the control CT scan at the end of the procedure, and the follow-up demonstrated no clinical improvement as well as the presence of the nidus on the repeated CT scan. These two patients required a second procedure which was carried out in the same way and was successful in both cases (primary and secondary rates of success 88% and 100% respectively). There were no other cases of recurrence. Other complications included one case of mild femoral neurapraxia in the patient with osteoid osteoma of the acetabular roof, which spontaneously resolved over a few weeks, and another case of abrasion of the skin caused by the drill, which responded to local treatment. No other complications were recorded. A histological diagnosis of an interlacing network of osteoid trabeculae with variable mineralization and richly vascular stroma, consistent with osteoid osteoma, was obtained in 14 cases out of 18 (77%). Histological reports were inconclusive in the remaining four cases, and the diagnosis of osteoid osteoma could not be obtained.
All patients fully resumed their previous activities.
Discussion
Osteoid osteoma is a benign, extremely painful and well-localized bone tumour. Pain is often worse at night and it can be relieved by salicylates. The most common anatomic sites of this tumour are the femur and the tibia, although any bone can be involved.
Radiologically, osteoid osteoma consists of a round or elliptic, lucent lesion of up to 10 mm in diameter, called the “nidus”, surrounded by a zone of reactive sclerosis. The position relative to the cortex, periosteum, and spongiosa determines the radiographic appearance; detection and localization of the lesion are difficult. Bone scintigraphy may be helpful and it demonstrates a markedly increased uptake of contrast medium. CT is also helpful in localizing the nidus and thus aids in planning the surgical approach. It may also sometimes reveal a nidus that cannot be seen on plain X-rays, either because it is obscured by an intense sclerotic reaction or because the sclerotic bone is absent, such as with an intramedullary osteoid osteoma. MRI was compared with CT and was found to be inferior to it, and even misleading in correctly diagnosing and localizing osteoid osteoma [1, 3, 21].
The natural history of this bone tumour is self-limiting and pharmacomedical therapy using NSAIDS can be recommended if the diagnosis is doubtless and a close follow-up is established.
Osteoid osteoma has traditionally been treated surgically in an “open” fashion by removing a bone block containing the nidus and the sclerotic reactive bone. Pain is dramatically resolved postoperatively if the nidus has been removed. The main difficulty with this technique is in the intraoperative identification of the nidus, and misjudgment often results in failure of the procedure due to incomplete resection. Incomplete removal usually results in a clinical recurrence. Attempts to overcome this problem have led to the development of numerous methods of allowing accurate intraoperative localization of the nidus [10, 15, 20, 23]. Moreover, the removal of large portions of cortical bone weakens the affected long bone considerably and for long periods of time, with a significant risk of pathological fractures, which do in fact occur.
In view of these disadvantages, the trend is presently changing worldwide toward minimally invasive procedures that lower failure rates and avoid potential complications. Much experience has been gained in recent years with percutaneous CT-guided treatment of osteoid osteoma. The nidus is located under CT imaging and is then marked by a thin K-wire. A cannulated drill is then used over the K-wire to create a small diameter canal that reaches the nidus (Fig. 1), which may then be destroyed by one of several existing methods (Fig. 2). One of these consists of simply drilling through the nidus at high power and speed, thereby combining mechanical trauma with thermal necrosis, with or without removing a block cylinder. Results obtained with this technique are excellent; Kohler et al. have treated 27 patients, 24 of whom healed initially [12]. D’erme et al. reported 10 primary cures out of 11 patients [6]. Similar results were described by Voto et al., who use a CORB biopsy system [22]. Baunin and co-workers had a 100% success rate in eight children with osteoid osteoma by using a 7-mm diameter toothed drill [4].
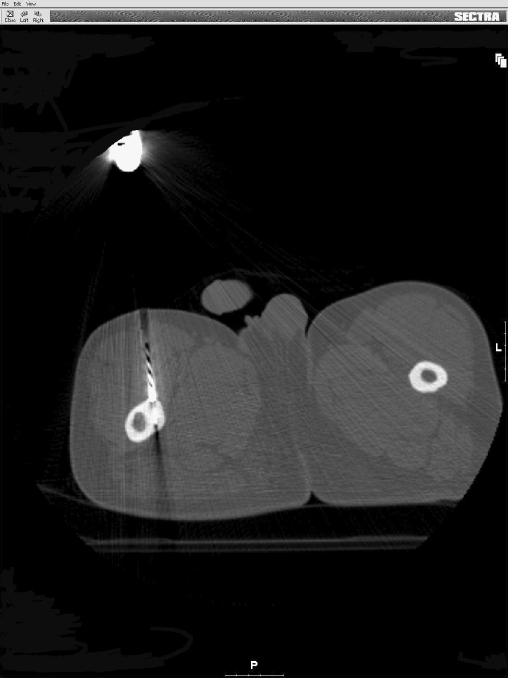
Fig. 1.
Drilling the cortex of the femur using a 4.5-mm cannulated drill over a K-wire, which is positioned inside the nidus
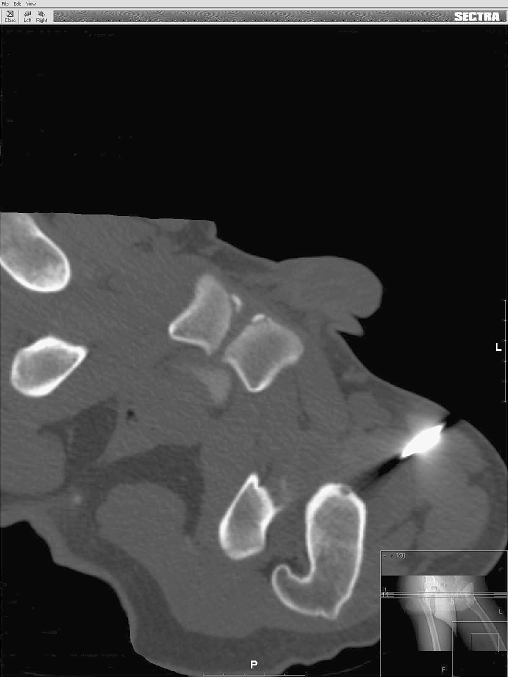
Fig. 2.
After drilling and curettage of the nidus located in the femoral head. The K-wire and the cannulated curette are removed
Other ways of ablating the nidus make use of electro cauterization [5], LASER Photo-coagulation [9], 96% ethanol injection [7, 19], and radiofrequency ablation [17, 18], with similarly successful results.
The first research employing the CT-guided technique relied solely on the clinical diagnosis and was thus concerned with ablation of the nidus followed by pain relief. Tissue diagnosis of osteoid osteoma is, however, an important issue as it is with any other bone tumour. The clinical diagnosis of osteoid ostoma is not always accurate and other lesions may be considered in the differential diagnosis. This places emphasis on the importance of correct tissue diagnosis, as with any other bone tumour. Histological proof may also certainly have medico-legal implications. These arguments led to recent attempts to obtain tissue for histological evaluation and different methods have been used with varying degrees of success: Kohler et al., as mentioned, successfully used a special self-manufactured device (the “Kohler” trephine), but at the beginning the diagnosis was histologically correctly approved in only 50% of cases [12]. Assoun and colleagues used the “Kohler” trephine for retrieving tissue samples with success in 19 out of 24 patients. All authors using the Kohler method [2, 4, 6, 11, 12, 13] achieved approximately 70–80% positive histological findings.
Peyser et al. also used a hollow drill system in their series. However, they reported a much lower success rate at obtaining a tissue diagnosis in only 38% (15 patients out of 42 overall).
The potential advantages of percutaneous CT-guided resection of osteoid osteoma was also shown by Muscolo et al., who treated seven patients with a nidus located in the hip [14]. Histological confirmation was obtained in five of the patients and after follow-ups between 12 and 40 months, all seven patients remain asymptomatic.
Our results in terms of cure rates are consistent with the aforementioned studies using CT guidance for treating osteoid osteoma. The use of a cannulated curette was found to be an effective tool as it yielded a 77% success rate at confirming the diagnosis by histology. These results are in accordance with those obtained with other hollow drill systems. There were no major complications associated with this procedure; using a 4.5-mm drill and the cannulated curette did not act as stress risers for the development of pathological fractures.
In conclusion, percutaneous CT-guided removal or destruction of osteoid osteoma seems to be both safe and reliable. The cannulated curette that we use allows us to remove the nidus and retrieve adequate tissue samples for histology in most cases.
References
- 1.Assoun J, De-Haldat F, Richardi G et al (1993) Magnetic resonance imaging in osteoid osteoma. Rev Rhum Ed Fr 60:28–36 [PubMed]
- 2.Assoun J, Railhac JJ, Bonevialle P et al (1993) Osteoid osteoma: percutaneous resection with CT guidance. Radiology 188:541–547 [DOI] [PubMed]
- 3.Assoun J, Richardi G, Reilhac JJ et al (1994) Osteoid osteoma: MR imaging versus CT. Radiology 191:217–223 [DOI] [PubMed]
- 4.Baunin C, Puget C, Sales-de Gauzy J et al (1994) Percutaneous resection of osteoid osteoma under CT guidance in eight children. Pediatr Radiol 24:185–188 [DOI] [PubMed]
- 5.Berning W, Freyschmidt J, Wiens J (1997) Percutaneous therapy of osteoid osteoma. Unfallchirurg 100:536–540 [DOI] [PubMed]
- 6.D’erme M, Del-Popolo P, Pasquali- Lasagni M et al (1995) CT guided percutaneous treatment of osteoid osteoma. Radiol Med Torino 90:84–87 [PubMed]
- 7.Duda SH, Schnatterbeck P, Claussen CD et al (1997) Treatment of osteoid osteoma with CT guided drilling and ethanol instillation. Dtsch Med Wochenschr 122:507–510 [DOI] [PubMed]
- 8.Erdtmann B, Duda SH, Claussen CD et al (2001) CT-guided therapy of osteoid osteoma by drill trepanation of the nidus. Clinical follow-up results. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 173:708–713 [DOI] [PubMed]
- 9.Gangi A, Dietermann JL, Guth S et al (1998) Percutaneous laser photocoagulation of spinal osteoid osteomas under CT guidance. Am J Neuroradiol 19:1955–1958 [PMC free article] [PubMed]
- 10.Healey JH, Ghelman B (1986) Osteoid osteoma and osteoblastoma. Current concept and recent advances. Clin Orthop 204:76–85 [PubMed]
- 11.Klose KC, Forst R, Günter RW et al (1991) The percutaneous removal of osteoid osteoma via CT guided drilling. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 155:532–537 [DOI] [PubMed]
- 12.Kohler R, Rubini J, Archimbaud F et al (1995) Treatment of osteoid osteoma by CT-controlled percutaneous drill resection. Apropos of 27 cases. Rev Chir Orthop Reparatrice Appar Mot 81:317–325 [PubMed]
- 13.Mazoyer JF, Kohler R, Bossard D (1991) Osteoid osteoma: CT guided percutaneous treatment. Radiology 181:269–271 [DOI] [PubMed]
- 14.Muscolo DL, Velan O, Santini Araujo E et al (1995) Osteoid osteoma of the hip. Percutaneous resection guided by computed tomography. Clin Orthop Relat Res 310:170–175 [PubMed]
- 15.O’Brien TM, Murray TE, Malone LA et al (1984) Osteoid osteoma: excision with scintimetric guidance. Radiology 153:543–544 [DOI] [PubMed]
- 16.Parlier-Cuau C, Champsaur P, Laredo JD et al (1998) Percutaneous removal of osteoid osteoma. Radiol Clin North Am 36:559–566 [DOI] [PubMed]
- 17.Rosenthal DI, Alexander A (1992) Ablation of osteoid osteoma with a percutaneous placed electrode, a new procedure. Radiology 183:29–33 [DOI] [PubMed]
- 18.Rosenthal DI, Springfield DS (1995) Osteoid osteoma: percutaneous radio-frequency ablation. Radiology 197:451–454 [DOI] [PubMed]
- 19.Sanhaji L, Gharbaoui IS, Boukhrissi N et al (1996) A new treatment of osteoid osteoma: percutaneous sclerosis with ethanol under scanner guidance. J Radiol 77:37–40 [PubMed]
- 20.Thomazeau H, Langlais F, Lancien G et al (1996) Contribution of nidus fluorescence in the surgical treatment of osteoid osteoma. Rev Chir Orthop Reparatrice Appar Mot 82:737–742 [PubMed]
- 21.Thompson GH, Wong KM, Vibhakar S et al (1990) Magnetic resonance imaging of an osteoid osteoma of the proximal femur: a potentially confusing appearance. J Pediatr Orthop 10:800–804 [DOI] [PubMed]
- 22.Voto SJ, Cook AJ, Arrington LE et al (1990) Treatment of osteoid osteoma by computed tomography guided excision in the pediatric patient. J Pediatr Orthop 10:510–513 [PubMed]
- 23.Wang NH, Ma HL, Yang DJ et al (1990) Osteoid osteoma: clinical and investigative features. J Formos Med Assoc 89:366–372 [PubMed]