Abstract
We randomly assigned 17 patients with scaphoid non-union at the proximal pole to three treatment groups: (1) autologous iliac graft (n=6), (2) autologous iliac graft + osteogenic protein-1 (OP-1; n=6), and (3) allogenic iliac graft + OP-1 (n=5). Radiographic, scintigraphic, and clinical assessments were performed throughout the follow-up period of 24 months. OP-1 improved the performance of both autologous and allogenic bone implants and reduced radiographic healing time to 4 weeks compared with 9 weeks in group 1. Helical CT scans and scintigraphy showed that in OP-1-treated patients sclerotic bone was replaced by well-vascularised bone. The addition of OP-1 to allogenic bone implant equalised the clinical outcome with the autologous graft procedure. Consequently the harvesting of autologous graft can be avoided.
Résumé
Nous avons réparti 17 malades avec une pseudarthrose du pôle proximal du scaphoide en trois groupes aléatoires de traitement: (1) greffe autologue iliaque (n=6), (2) greffe autologue iliaque + protéine osteogenique-1 (OP-1; n=6), et (3) greffe allogène iliaque + OP-1 (n=5). L’estimation radiographique, scintigraphique et clinique a été exécutée pendant une période de suivi de 24 mois. L’OP-1 a amélioré la performance des autogreffes et des allogreffes osseuses et a réduit le temps curatif radiographique à 4 semaines, comparé à 9 semaines dans le groupe traité uniquement avec de l’os autologues. Chez les malades traités avec l’OP-1, la tomodensitométrie hélicoïdale et la scintigraphie ont montré que l’os scléreux était remplacé par de l’os bien vascularisé. L’addition d’OP-1 à la greffe allogène a égalisé le résultat clinique avec la procédure de greffe autologue et a permis de supprimer le temps de prise de greffe.
Introduction
Bone graft with or without internal fixation is the standard treatment for symptomatic scaphoid non-unions without osteoarthritis [8, 17, 19, 21]. Cancellous bone grafting, first described by Matti and modified by Russe is the most common surgical treatment [5, 7]. Union is achieved in 70–90% and the type of fracture and the vascularity of the proximal pole are important determinants [9]. Recent meta-analysis of scaphoid non-union literature indicated a 94% success rate in unstable non-unions treated with screw fixation and grafting, but only 47% in patients with concomitant avascular necrosis [12].
Bone morphogenetic proteins (BMPs) or osteogenic proteins (OPs) are growth and differentiation factors capable of initiating the recruitment, attachment, proliferation, and differentiation of mesenchymal cells, leading to new bone formation [11, 20]. Extensive clinical and preclinical research has been undertaken to investigate the role of OP-1 (BMP-7) in bone and cartilage regeneration [14]. OP-1 heals critical-sized diaphyseal defects and non-unions of long bones, enhances bone graft incorporation and implant fixation, and increases remodelling and bone ingrowth of bone grafts and bone substitutes [3, 4, 6, 11].
The aim of this pilot study was to test if the recombinant BMP-7 (OP-1), a signalling molecule for promoting osteogenesis [11], could enhance radiographic and clinical healing and removal of sclerosis in scaphoid non-unions containing sclerotic bone.
Materials and methods
The study design
The cases included in the study comprised male or female patients between ages of 15 and 30 years with a symptomatic proximal pole scaphoid non-union of at least 9 months’ duration with no evidence of progressive healing over the previous 3 months, and the presence of 100 mm2 or more of pre-existing sclerotic bone in the proximal scaphoid pole, as determined preoperatively by spiral CT. Study exclusion criteria included prior surgical treatment, carpal collapse, immature skeleton, and being unable or unwilling to fulfill the follow-up requirements. One hundred and thirty-nine patients were screened for scaphoid non-unions in the period from January 1999 to January 2003 in the Department of Orthopedic Surgery, University of Zagreb.
Patients were randomly assigned to one of the treatment groups using computer-generated randomisation. The cohorts were defined as follows: group 1, autologous iliac graft only (n=6); group 2, autologous iliac graft + OP-1 (n=6); and group 3 allogenic iliac graft + OP-1 (n=6). The study was performed in compliance with the Helsinki Declaration and fulfilled the CONSORT statement requirements [13]. The bone grafts were prepared according to a modified Matti-Russe procedure by Bilic [1] using compressed iliac bone. Surgeons were unaware of the treatment group to which each patient was assigned after the random selection process. Two months following operation and removal of the cast physiotherapy was started and included active exercise of the wrist and fingers, as well as supported and passive exercise.
Demographics
The demographics of the study groups are presented in Table 1. Three randomly assigned groups of young men were similar (p>0.05) in many respects, including age, body mass index, duration of non-union, fracture site, direction of fracture line, and area of proximal pole sclerosis. The mean time from original injury to treatment of scaphoid non-union was 14 months (range 9–20 months). All patients had pain and weakness, loss of motion, decreased grip strength, and fatigue with repetitive work. In most patients, preoperative motion was restricted and grip strength was decreased to a mean of 62%.
Table 1.
Patient demographics
| Study groups | |||
|---|---|---|---|
| OP-1 + autograft, group 2 (n=6) | Autograft only, group 1 (n=6) | OP-1 + allograft, group 3 (n=6) | |
| Non-union duration (months)a | 15±5 | 14±5 | 13±4 |
| Fracture site (proximal/middle/distal) | 1/3/2 | 1/4/1 | 1/3/1 |
| Proximal pole sclerosis CT area (>100 mm2) | 6 | 6 | 5 |
| Age (years)a | 23±5 | 22±5 | 19±4 |
| Body mass index (kg/m2)a | 20.1±1.5 | 21.3±2.1 | 19.8±1.3 |
| Tobacco | 3 | 3 | 2 |
OP-1 osteogenic protein-1
ANOVA analyses of duration, age, and body mass index were not different among patient cohorts
aMean ± SD
OP-1 Implant
The OP-1 Implant (Osigraft) composed of 3.5 mg recombinant human OP-1 (BMP-7) and 1 g of collagen as a carrier was obtained from Stryker Biotech (Hopkinton, MS, USA). The OP-1 implant was applied to both scaphoid fragments and subsequently autogenous or allogenic bone implants were positioned as per the Matti-Russe technique.
Methods of clinical assessment
The clinical evaluation included pain assessment by visual analogue scale during rest, at maximal grip strength, and at maximal dorsal flexion of the wrist. Additionally, measurements of movements in the radiocarpal joint, strength of the grip, and strength of the pinch were assessed in both hands. Patients were clinically evaluated after removal of a postoperatively applied cast, no later than 2 months postoperatively, and thereafter at 2, 4, 5, 9, 12, and 24 months. Physiotherapy started following removal of the cast. In addition, date and details of original injury, previous treatments, body mass index, and smoking status were recorded.
Radiographic analysis
Union was assessed on X-rays using plain film in four views.
Radiographs of both hands were taken prior to operation, of the affected hand immediately following operation, and then 1, 2, 2.5 or 3 (2.5 if the healing was more than 75% and 3 months if less than 75%), 4, 9, and 24 months following operation. X-rays were evaluated by two radiologists who were blinded to the treatment and time following the surgical procedure, and who assessed whether 0–33%, 34–66% or 67–100% of the scaphoid bone surface that had been remodelled had healed. Healing of the regenerating scaphoid bone reflects a gradual replacement of the graft by newly formed and well-incorporated bone, including its full mineralisation by the end of follow-up.
Computerized tomography
Helical CT scans were used to assess the amount of proximal pole sclerotic bone preoperatively, since only patients with more than 100 mm2 of sclerotic bone area were included in the study. Pre-existing sclerotic bone from the proximal scaphoid pole was measured using the helical CT software (AW 4.2 General Electric Medical System—GEMS) and then manually confirmed by a planimeter on multiple CT sections taken immediately following the operation. Bone was also tested during the operation and was considered sclerotic and avascular by demonstration of the absence of punctuate bleeding from the fragments of exposed bone. CT of the affected hand was taken prior to operation, immediately following operation, and then 3, 9, 12, and 24 months following operation. Proximal pole sclerotic bone removal was assessed by blinded interpretation of CT scans to assess the efficacy of a single application of BMP-7 (administered with an auto- or allograft) compared with autograft treatment alone. Sections were made at 0.6-mm intervals [15].
Three-phase bone scintigraphy
To further assess removal of pre-existing sclerotic bone by new blood vessels, patients were subjected to three-phase 99m Technetium-dicarboxypropane diphosphonate (99mTc-DPD) scintigraphy prior to operation and then 24 months following operation. The angiographic phase (0–60 s post injection) was acquired in dorsal projection after bolus injection of 600 MBq of 99mTc-DPD. The blood pool phase of the tracer was investigated 10–20 min following injection and the bone uptake phase 3 h following injection. Bone scans of the radiocarpal joint of the operated and healthy hands were obtained with a LFoV gamma camera (DIACAM; Siemens) in the dorsal position. The affected area was measured planimetrically.
Data analysis
Distributions of all quantitative data were tested with the Kolmogorov-Smirnov test. In spite of the small sample sizes, all distributions conform to normal distribution; hence, parametric analytical procedures were applied. Demographic data and functional measurement data within the same time point were analysed using a one-way ANOVA with the one-sided Dunnett t post hoc test against group 1 patients. Sclerotic bone area changes were analysed using a two-way ANOVA for repeated measurements with the Scheffe post hoc test. Analysis was performed using the STATISTICA computer program, version 6.0 (StatSoft, Tulsa, OK, USA).
Results
Radiographic results
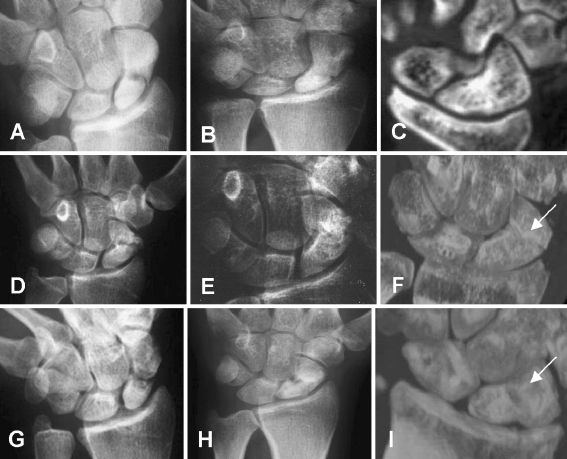
One patient who received the allogenic bone and OP-1 (group 3) was lost to follow-up (Table 1). The scaphoid non-union in the remaining 17 patients healed as assessed by conventional X-rays and CT analysis (Fig. 1). Four weeks following the operation 70–95% of bone in group 2 patients demonstrated radiographic bridging, compared with 60–80% in both group 3 and group 1 patients (p<0.05). At 4 weeks radiographic data could not be correlated to clinical parameters since the hand was still immobilized. At 8 weeks following operation 90–100% of group 2 patients showed radiographic bridging compared with 75–90% in both group 3 and group 1 patients (not significant).
Fig. 1.
Radiograph of a patient (number 1) treated with autologous bone implant a before and b 8 weeks following operation. c CT scan 9 months following operation. d, e Radiograph of a patient (number 7) treated with autologous bone and OP-1 before (d) and 8 weeks following operation (e). f CT scan 9 months following operation. g, h Radiograph of a patient (number 13) treated with allogenic bone and OP-1 before (g) and 8 weeks following operation (h). i CT scan 9 months following operation
Between all follow-up visits within each treatment group a statistically significant decrease in sclerotic bone area was observed (Fig. 2, Table 2). A greater loss of sclerotic bone area was demonstrated in patients treated with OP-1 compared with autograft only in the first and last follow-up periods. On the contrary, between 3 and 12 months’ follow-up the loss of sclerotic bone area was similar in all groups of patients. At 24 months’ follow-up non-union had healed in all 17 patients. However, the sclerotic bone almost fully disappeared in group 2 patients (Fig. 2e, Table 2) compared with group 1 patients (Fig. 2b, Table 2), indicating an accelerated process of sclerotic bone resorption following full restoration of bone integrity (Fig. 2e, Table 2). In patients treated with allogenic bone and OP-1 (group 3) an increased and transient radiolucency along the graft was observed 8 weeks following operation (Fig. 1g–i), which did not influence the clinical outcome as evidenced by functional clinical tests (Table 3). Twenty-four months following operation (Fig. 2f) the pre-existing sclerotic bone of group 3 patients was also significantly reduced compared with group 1 patients (Table 2).
Fig. 2.
Late bone scintigraphy of a a patient (number 1) treated with autologous bone, b a patient (number 7) treated with autologous bone and OP-1, and c a patient (number 13) treated with allogenic bone and OP-1 24 months following operation. Higher uptake of 99mTc is present in surgically treated scaphoids (arrow), compared with opposite healthy hand (h). Increased vascular spaces in OP-1-treated patients (b, c) 24 months following operation indicate its permanent presence. 3D reconstruction of CT scans of d patient number 1, e patient number 7, and f patient number 13 24 months following operation. Minimal amounts of sclerotic bone (OS) are present in the proximal pole of patient number 7
Table 2.
Sclerotic bone areas as assessed by CT
| Sclerotic area (mm2; range) | ||||
|---|---|---|---|---|
| Immediately following operation | 3 months following operation | 9 months following operation | 24 months following operation | |
| Autograft only (group 1) | 148.3±11.9 (136–168) | 138.3±15.1* (122–160) | 118.8±19.0 (95–141) | 111.5±8.6 (103–126) |
| Autograft + OP-1 (group 2) | 121.5±12.5 (111–140) | 74.0±14.1* (59–93) | 44.7±11.3** (31–62) | 31.7±6.8*** (25–40) |
| Allograft + OP-1 (group 3) | 133.7±9.2 (121–144) | 103.6±13.2* (89–122) | 77.2±7.8** (69–86) | 55.6±11.7*** (42–69) |
*p<0.05 vs. before operation
**p<0.05 vs. 3 months following operation and p<0.01 vs. before operation
***p<0.05 vs. autograft only
Table 3.
Functional measurement of scaphoid non-union healing 4 and 12 months following operation
| Functional test | Healthy hand | 4 months | 12 months | ||||
|---|---|---|---|---|---|---|---|
| Autograft only (group 1) | Autograft + OP-1 (group 2) | Allograft + OP-1 (group 3) | Autograft only (group 1) | Autograft + OP-1 (group 2) | Allograft + OP-1 (group 3) | ||
| Ulnar deviation (°) | 47±6* | 38±5 | 49±5* | 40±4 | 50±5 | 57±5 | 58±6 |
| Radial deviation (°) | 30±4* | 20±4 | 29±3* | 26±3* | 32±3 | 35±4 | 34±3 |
| Palmar flexion (°) | 74±9* | 52±6 | 60±6 | 59±6 | 70±7 | 78±8 | 76±9 |
| Dorsal flexion (°) | 66±7* | 46±6 | 60±5* | 55±5 | 67±6 | 69±6 | 68±7 |
| Grip strength (kg) | 44±5* | 28±4 | 36±4 | 31±3 | 35±4 | 41±5 | 37±3 |
| Pinch strength (kg) | 9±2* | 6±1 | 8±2* | 6±1 | 9±2 | 10±2 | 9±2 |
| Pain at resta | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pain during maximal gripa | 0* | 5±1 | 0* | 3±1* | 6±1 | 3±1 | 0* |
| Pain in maximal dorsiflexiona | 0* | 15±2 | 0* | 11±2 | 11±2 | 6±1 | 3±1 |
*p<0.05 vs. autograft only, ANOVA Dunnett test
aPatients were asked to draw a point on a line showing pain from 0 (no pain) to 100 (maximal pain); analogous measurement scale
Clinical outcome
Patients in both group 1 and group 2 had pain at a donor site following operation. Those treated with allogenic bone and OP-1 had approximately 50 ml less blood loss due to the lack of a second operation and had no pain at the pelvic site. Additionally, these patients were exposed to anesthesia for 95 min compared with 140 min in patients undergoing both operations. There were no reported adverse events. Patients in all three groups showed improvement of functional measurements and clinical outcome throughout the period of 24 months (Table 3). Four months following operation, patients in group 2 reached about 91% of the functional values of the non-affected hand and had a statistically significant improvement in functional measurements of ulnar and radial deviation, palmar and dorsal flexion, pinch strength, and pain during maximal dorsiflexion and during maximal grip compared with group 1 patients (Table 3). Group 3 patients showed about 85% of the function of the healthy hand and had significantly improved radial deviation and pain during maximal grip 4 months following operation compared with patients treated with autologous bone only. There were no statistically significant differences in functional tests between patients in group 2 and those in group 3. Patients treated with autologous bone graft had 75% of healthy hand function. Two years following operation patients in all three treatment groups had good functional results. Patients in group 3 had reduced pain during maximal grip compared with group 1 patients.
Scintigraphic results
Patients subjected to scintigraphic analyses by 99mTc had normal perfusion during the arterial phase, with no signs of infection. Blood pool image showed increased uptake in patients treated with OP-1 corresponding to the formation of new vascular spaces and bone marrow, while in control patients a normal blood pool image probably corresponds with a stable phase. The late bone scans showed higher tracer uptake in patients treated with OP-1 and bone graft, compared with patients treated only with autologous bone (4.86 in group 2, 4.57 in group 3 and 3.26 cm2 in group 1 patients, p<0.05; Fig. 2), suggesting increased bone vascularity and metabolic activity in scaphoids treated with OP-1. Both groups of patients showed increased tracer uptake in the affected hand compared with the healthy hand (Fig. 2). These results confirm that OP-1 added to both autologous and allogenic bone graft accelerated vascular ingrowth and subsequent replacement of pre-existing sclerotic bone (Fig. 2e,f).
Discussion
The results of this pilot study demonstrate that recombinant human OP-1 (BMP-7) supports proximal pole scaphoid non-union healing via increased bone vascularisation and replacement of pre-existing proximal pole sclerotic bone as a consequence of avascular necrosis. OP-1 enhances radiological healing of scaphoid non-unions in autologous bone-treated patients, reducing the healing time to 4 weeks compared with 9 weeks in patients treated with autologous bone only. Potential shortening of the immobilization period by OP-1 may considerably reduce the incidence of arthritic changes in radioscaphoid and radiocarpal joints, contractures of immobilized joints, and eventually Sudeck’s dystrophy. This is also a way of preventing muscular atrophy of the forearm and hand, enabling patients to return to work earlier. Patients treated with allogenic bone implant and OP-1 (group 3) showed radiographic healing 8 weeks following operation, which was comparable to patients treated with autologous bone only (group 1), suggesting that autologous bone implant could be avoided when allogenic bone implant is used in combination with OP-1. The use of autogenous iliac graft is linked to donor site complications including increased operative blood loss, postoperative pain and increased probability of infection. Furthermore, the addition of OP-1 to both autologous and allogenic bone significantly improves functional performance of the affected hand compared with autologous grafting alone.
Despite extensive experience the treatment of scaphoid fractures is still a difficult problem. The worst healing rate has been described in patients with avascular necrosis of the proximal fragment (47%), with an option of vascularised bone grafting implantation, which leads to questionable results [12, 18]. Since OP-1 enhances vascular ingrowth into the scaphoid, more precursor cells become available leading to accelerated removal of sclerotic bone from the proximal pole. Up to now, BMPs have been successfully tested in clinical trials and subsequently approved for treating long bone non-unions [6], acute fractures [10], and spinal fusions [2].
The likelihood of healing scaphoid non-unions was decreased in our patients due to delay between injury and operation for non-union, and avascular necrosis of the proximal pole (Table 1). As scaphoid branches of the radial artery supply about 70–80% of bone, fractures separating the proximal pole, which is completely covered with hyaline cartilage, leads to roughly 30–40% necrosis [16]. Successful outcome in these patients due to the addition of OP-1 was achieved in part by increased vascular ingrowth resulting in accelerated removal of sclerotic bone from the proximal scaphoid poles. Enhanced vascularisation enabled a greater number of both preosteoblasts and preosteoclasts to reach this specific environment. Contrary to the outcome of this study, it has been reported that purified native BMP and iliac crest bone along with a biocoral or screw fixation in the treatment of scaphoid non-unions [9] gives poor clinical results, possibly due to the compromised avascular environment, which prevented access of the native BMP and stem cells, and resulted in inadequate resorption of biocoral implants. The controlled dosing and pronounced angiogenic effect with recombinant human BMP compared with purified bovine BMP may explain the different outcomes in the two studies.
The timely application of OP-1 in treating scaphoid fractures may thus prevent the development of avascular necrosis and subsequent osteosclerosis, and help to restore normal bone structure by simulating removal and replacement of sclerotic bone.
This study has also shown that application of OP-1 with an allograft results in healing of the scaphoid non-union as well. Use of an iliac crest allograft avoids autograft donor site and results in a shorter procedure.
In conclusion, this clinical study demonstrated that recombinant BMP resulted in a radiological and clinical repair of scaphoid avascular and necrotic proximal pole non-unions. The results of this study may also raise hope for patients with avascular necrosis of the femoral head, which is one of the most challenging, as yet unsolved orthopedic problems.
References
- 1.Bilic R, Korzinek K (1987) Results of scaphoid non-union treatment by Matti-Russe procedure using compressed cancellous bone. Unfallchirurg 90:134–138 [PubMed]
- 2.Burkus JK, Gornet MF, Dickman CA, Zdeblick TA (2002) Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech 15:337–349 [DOI] [PubMed]
- 3.Cook SD, Rueger DC (2002) Preclinical models of recombinant BMP induced healing of orthopedic defects. In: Vukicevic S, Sampath K (eds) Bone morphogenetic proteins: from laboratory to clinical practice. Birkhäuser, Basel, pp 121–144
- 4.Djapic T, Kusec V, Jelic M, Vukicevic S, Pecina M (2003) Compressed homologous cancellous bone and bone morphogenetic protein (BMP)-7 or bone marrow accelerate healing of long-bone critical defects. Int Orthop 27:326–330 [DOI] [PMC free article] [PubMed]
- 5.Fisk GR (1984) The wrist. J Bone Joint Surg Br 66:396–407 [DOI] [PubMed]
- 6.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF et al (2001) Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am 83:S151–S158 [PMC free article] [PubMed]
- 7.Green DP (1985) The effect of avascular necrosis on Russe bone grafting for scaphoid nonunion. J Hand Surg [Am] 10:597–605 [DOI] [PubMed]
- 8.Inoue G, Shionoya K, Kuwahata Y (1997) Ununited proximal pole scaphoid fractures. Treatment with Herbert screw in 16 cases followed for 0.5–8 years. Acta Orthop Scand 68:124–127 [DOI] [PubMed]
- 9.Kujala S, Raatikainen T, Ryhänen J, Kaarela O, Jalovaara P (2002) Composite implant of native bovine bone morphogenetic protein (BMP) and biocoral in the treatment of scaphoid nonunions—a preliminary study. Scand J Surg 91:186–190 [DOI] [PubMed]
- 10.Lieberman JR, Daluiski A, Einhorn TA (2002) The role of growth factors in the repair of bone: biology and clinical applications. J Bone Joint Surg Am 84:1032–1044 [DOI] [PubMed]
- 11.Martinovic S, Simic P, Borovecki F, Vukicevic S (2004) Biology of bone morphogenetic proteins. In: Vukicevic S, Sampath K (eds) Bone morphogenetic proteins: regeneration of bone and beyond. Birkhäuser, Basel, pp 45–72
- 12.Merrell GA, Wolfe SW, Slade JF III (2002) Treatment of scaphoid nonunions: quantitative meta-analysis of the literature. J Hand Surg [Am] 27:685–691 [DOI] [PubMed]
- 13.Moher D, Schulz KF, Altman DG (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 357:1191–1194 [DOI] [PubMed]
- 14.Pecina M, Giltaij LR, Vukicevic S (2001) Orthopaedic applications of osteogenic protein-1 (BMP-7). Int Orthop 25:203–208 [DOI] [PMC free article] [PubMed]
- 15.Sanders WE (1988) Evaluation of the humpback scaphoid by computed tomography in the longitudinal axial plane of the scaphoid. J Hand Surg [Am] 13:182–187 [DOI] [PubMed]
- 16.Sherman SB, Greenspan SB, Norman A (1983) Osteonecrosis of the distal pole of the carpal scaphoid following fracture—a rare complication. Skeletal Radiol 9:189–191 [DOI] [PubMed]
- 17.Stark A, Brostrom LA, Svartengren G (1987) Scaphoid nonunion treated with the Matti-Russe technique. Long-term results. Clin Orthop 214:175–180 [PubMed]
- 18.Tambe AD, Cutler L, Stilwell J, Murali SR, Trail IA, Stanley JK (2005) Scaphoid non-union: the role of vascularized grafting in recalcitrant non-unions of the scaphoid. J Hand Surg [Br] http://www.dx.doi.org/10.1006.2005.09.09.012 [DOI] [PubMed]
- 19.Trezies AJ, Davis TR, Barton NJ (2000) Factors influencing the outcome of bone grafting surgery for scaphoid fracture non-union. Injury 31:605–607 [DOI] [PubMed]
- 20.Vukicevic S, Stavljenic A, Pecina M (1995) Discovery and clinical applications of bone morphogenetic proteins. Eur J Chem Clin Biochem 33:661–671 [DOI] [PubMed]
- 21.Warren-Smith CD, Barton NJ (1988) Non-union of the scaphoid: Russe graft vs Herbert screw. J Hand Surg 13B:83–86 [DOI] [PubMed]