Abstract
Background
Emerging evidence indicates that Group I metabotropic glutamate receptors (mGluR1 and mGluR5) differentially regulates ethanol self-administration in several rodent behavioral models. The purpose of this work was to further characterize involvement of Group I mGluRs in the reinforcing effects of ethanol using a progressive ratio schedule of reinforcement.
Methods
Alcohol-preferring (P) rats were trained to self-administer ethanol (15% v/v) versus water on a concurrent schedule of reinforcement, and the effects of the Group I mGluR antagonists were evaluated on progressive ratio performance. The rats were then trained to self-administer sucrose (0.4% w/v) versus water, and the effects of the antagonists were tested on progressive ratio performance.
Results
The mGluR1 antagonist, 3,4-dihydro-2H-pyrano[2,3]b quinolin-7-yl (cis-4-methoxy-cyclohexyl) methanone (JNJ 16259685; 0 to 1 mg/kg) and the mGluR5 antagonist, 6-methyl-2-(phenylethynyl) pyridine (MPEP; 0 to 10 mg/kg) dose-dependently reduced ethanol break point. In separate locomotor activity assessments, the lowest effective dose of JNJ 16259685 (0.3 mg/kg) produced a motor impairment, whereas the lowest effective dose of MPEP (3 mg/kg) did not. Thus, the reduction in ethanol break point by mGluR1 antagonism was probably a result of a motor impairment. JNJ 16259685 (0.3 mg/kg) and MPEP (10 mg/kg) reduced sucrose break point and produced motor impairments. Thus, the reductions in sucrose break point produced by both Group I antagonists were probably because of nonspecific effects on motor activity.
Conclusions
Together, these results suggest that glutamate activity at mGluR5 regulates motivation to self-administer ethanol.
Keywords: Ethanol Self-Administration, mGluR5, mGluR1, Glutamate, Progressive Ratio, Motivation
THE METABOTROPIC GLUTAMATE receptor sub-type1(mGluR1)and subtype 5 (mGluR5) belong to the Group I family of mGluRs. This family of receptors share common agonist pharmacological profiles and are coupled to similar signal transduction pathways (Conn and Pin, 1997). However, these receptor subtypes are abundant in different brain regions, with greater expression of mGluR5 in corticolimbic regions than mGluR1, which is highly abundant in the cerebellum (Romano et al., 1995; Shigemoto et al., 1992). Pharmacological antagonists of the Group I mGluRs show positive effects in models of nociception (Bhave et al., 2001; Sevostianova and Danysz, 2006) and anxiety (Spooren et al., 2000; Steckler et al., 2005a), and may possess neuroprotective properties (Baskys et al., 2005; Makarewicz et al., 2006); for reviews of Group I mGluR therapeutic potential, see (Bordi and Ugolini, 1999; Gasparini et al., 2002).
There is a growing interest in the role of mGluR5 in drug taking behaviors, whereas mGluR1 has been less studied. For example, mGluR5 antagonism has been shown to attenuate self-administration of cocaine (Kenny et al., 2003; Lee et al., 2005; Tessari et al., 2004), nicotine (Kenny et al., 2003; Paterson et al., 2003; Tessari et al., 2004), as well as relapse to cocaine (Backstrom and Hyytia, 2006) and nicotine seeking (Tessari et al., 2004). mGluR5 antagonism has also been implicated in mediating ethanol reinforcement as the mGluR5 antagonist 6-methyl-2-(phenylethynyl) pyridine (MPEP) has been shown to reduce ethanol self-administration in mice (Hodge et al., 2006; Lominac et al., 2006), alcohol-preferring P rats (Schroeder et al., 2005), and Long Evans rats (Backstrom et al., 2004), and relapse to ethanol seeking (Backstrom et al., 2004; Schroeder et al., 2005). The findings of mGluR1 antagonism on ethanol self-administration are mixed with a reduction (Lominac et al., 2006), and no change (Hodge et al., 2006; Schroeder et al., 2005) in self-administration reported to date. In sum, glutamate activity at mGluR5, and perhaps mGluR1, appears to be an important factor mediating ethanol reinforcement.
To further examine the role of Group I mGluRs in ethanol reinforcement, the present study examined ethanol self-administration under a progressive ratio (PR) schedule of reinforcement in selectively bred alcohol-preferring P rats (Li et al., 1979). Under PR schedules, the response requirement for reinforcer delivery increases systematically. The point at which the animal ceases to respond within a determined period is defined as the break point. Thus, the break point serves as an index of an animal’s motivation to work (e.g., respond) for the reinforcer (e.g., ethanol) and the reinforcing efficacy of the drug (for reviews, see Markou et al., 1993; Richardson and Roberts, 1996; Stafford et al., 1998).
In the present work, the role of Group I receptors (mGluR1 and mGluR5) was examined on ethanol and sucrose reinforced responding under a PR schedule of reinforcement. First, P-rats were trained to self-administer 15% ethanol (v/v) with water concurrently available. The mGluR1 antagonist JNJ 16259685 (0 to 1 mg/kg) and the mGluR5 antagonist MPEP (0 to 10 mg/kg) were then tested for ethanol self-administration under a PR schedule of reinforcement. Second, to determine if antagonism of Group I mGluRs alters motivation to self-administer a nondrug rewarding substance, the rats were subsequently trained to self-administer sucrose (0.4%, w/v) with water concurrently available.The same dose ranges of JNJ 16259685 and MPEP were tested on sucrose self-administration under the same PR schedule of reinforcement. After each antagonist assessment on ethanol and sucrose reinforcement, the lowest effective dose of the antagonist that reduced ethanol and sucrose break point responding was examined for potential effects on locomotor activity.
METHODS
Subjects
Male inbred alcohol-preferring rats (n = 11) weighing 480 ± 5 g (mean ± SEM) at the beginning of testing were used in this work. This stock of inbred P rats were derived from breeders of the selected line of P rats originally provided in 1999 by Indiana University (courtesy of Dr. T.K. Li, Indianapolis, Indiana) and has been bred on-site at the University of North Carolina at Chapel Hill. P-rats were selectively bred (Li et al., 1979) to voluntarily consume ethanol in quantities that produce significant blood alcohol concentrations and develop tolerance and dependence through voluntary drinking (Kampov-Polevoy et al., 2000; Lumeng and Li, 1986; Murphy et al., 2002). The rats were housed in pairs in Plexiglas cages with water and food available continuously unless otherwise mentioned. The colony room was maintained on a 12 hour light/dark cycle and experiments were conducted during the light portion of the cycle. All procedures were carried out in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Research Council, National Academy Press, 1996) and institutional guidelines.
Apparatus
The chambers used for the self-administration sessions measured 30.5 × 24.1 × 21.0 cm (Med Associates, Georgia, VT) and were located within sound-attenuating cubicles. Each cubicle was equipped with an exhaust fan that provided ventilation and masked external sounds. The left and right wall of each chamber contained a liquid receptacle and a response lever (Med Associates). Lever press responses activated a syringe pump (Med Associates) that delivered 0.1 ml of solution into the receptacle in a 1.66-second period. A stimulus light located above each response lever was illuminated during pump activation. Lever presses during reinforcer delivery were recorded, but produced no programmed consequence. The chambers were interfaced (Med Associates) to a computer that was programmed to control sessions and record data.
Four clear Plexiglas chambers (48 × 26 × 20 cm) were used to assess locomotor activity. Each chamber was divided into 4 equal sections by markings on the outside wall along the length of the chamber. The locomotor sessions were video-taped and the number of line crosses were subsequently quantified by an observer blind to drug condition.
Ethanol Reinforcement
Ethanol Self-Administration Training
On the first 2 days of training, rats were placed in the self-administration chambers for 16-hour training sessions to establish reliable lever pressing behavior. Water was removed from the home cage 24 hours before the first training session. After completion of the first training session, returned for 1 hour. During these 2 consecutive sessions, both levers were active on a concurrent fixed ratio 1 (CONC FR1 FR1) schedule with sucrose (10%, w/v) and water presented as the reinforcers. That is, 1 lever response resulted in the presentation of 0.1 ml of the solution paired with that lever. After the 16-hour training sessions, the rats received one 30-minute self-administration session each day (Monday to Friday). From this point onwards, water was continuously available in the home cage. As in lever press training, both levers were maintained on a CONC FR1 FRI schedule of reinforcement with sucrose (10%, w/v) and water. When sucrose and water response patterns stabilized, the rats were trained to self-administer ethanol (10% v/v) using a sucrose-fading procedure (Samson et al., 1986). Ethanol was gradually added to the 10% sucrose solution and the sucrose was gradually faded out so that ethanol (10% v/v) maintained lever pressing. The exact order of exposure was as follows: 10% sucrose/2% ethanol (10S/2E; 4 sessions), 10S/5E (5 sessions), 10S/10E (4 sessions), 5S/10E (3 sessions), and 2S/10E (3 sessions). After sucrose fading, self-administration sessions with ethanol (10% v/v) and water as the reinforcers continued for 18 sessions. The ethanol concentration was then increased to 15% (v/v) for the remainder of the study. At the time of testing, the rats had 23 sessions of ethanol 15% (v/v) versus water self-administration. Ethanol reinforcement was paired with the left lever for half of the rats and with the right lever for the other half.
Progressive Ratio Baseline
To establish baseline PR responding and to test the feasibility of repeated testing, rats experienced 2 sessions under the PR schedule of reinforcement (each session separated by 2 days), interspersed with the regular training sessions. Under the PR schedule, the response requirement for ethanol delivery increased by 1, each time a reinforcer was delivered (PR1; i.e., first reinforcer delivered after 1 response, second reinforcer delivered after 2 responses, third reinforcer delivered after 3 responses, etc). Thus, the number of reinforcers received also reflects the highest response ratio completed (i.e. break point). The PR1 schedule was in effect for the entire 30-minute session. The water lever remained on the FR1 schedule of reinforcement. One week following these sessions, another PR1 session was conducted with saline administered intraperitoneally (i.p.) 40 minutes prior to the session to habituate the animals to the injection protocol to be used for the mGluR antagonist assessments.
Effects of mGluR Antagonists on Progressive Ratio Performance
The mGluR1 antagonist JNJ 16259685 was tested on responding, under the PR schedule of reinforcement (PR1). Rats were administered JNJ 16259685 [0, 0.1, 0.3, 1.0 mg/kg (i.p.)] and were returned to the home cage. After 40 minutes, rats were placed in the self-administration chambers for the PR1 test session. After completion of this PR assessment, potential locomotor effects of JNJ 16259685 were assessed. The mGluR5 antagonist MPEP [0, 1, 3, 10 mg/kg (i.p.)] was also assessed in PR1 test sessions. The same testing protocol as described for JNJ 16259685 was used. After completion of this assessment, the MPEP locomotor assessment was conducted.
For each antagonist evaluation, the antagonist doses were tested using a Latin-square design. There were at least 2 drug-free intervening ethanol self-administration sessions between the PR1 test sessions. We found that self-administration quickly recovered to baseline levels by the self-administration session that followed a test day, but 2 intervening sessions were allowed to ensure stable responding. Also, tests occurred from Tuesday through Friday (tests were not conducted on Mondays because of a slight rebound in responding after the weekend; Schroeder et al., 2005). There were 11 and 12 intervening ethanol self-administration sessions upon completion of the entire JNJ 16259685 and MPEP assessments (including locomotor testing), respectively, and 9 drug-free self-administration sessions between the JNJ 16259685 and MPEP assessments. Responding under the PR1 was assessed again to determine if break points had changed after mGluR antagonist testing.
Locomotor Activity
After each mGluR antagonist was tested on PR1 performance, the lowest effective dose that altered ethanol break point was tested for potential effects on locomotor activity, to determine whether the reduction in break points was because of a motor impairment. Locomotor activity was assessed over 2 days for each antagonist with half of the rats receiving vehicle/saline on the first day and the antagonist dose on the second day; and the other half receiving the reverse order. Rats were injected with the assigned dose, returned to the home cage and 40 minutes later placed in the locomotor activity chamber. The locomotor assessments were for a duration of 10 minutes to correspond to the time of the greatest responding during the PR tests and to correspond to the emergence of the antagonist-induced reductions during those tests. These locomotor assessments were interspersed with self-administration training sessions and self-administration sessions were withheld on the days of the locomotor assessments.
Blood Ethanol Concentrations
Tail blood was collected immediately after the 30-minute ethanol self-administration training session for determination of blood ethanol concentration. This self-administration session was the fourth session after the final locomotor test. Individual blood samples were centrifuged and 5 μl of plasma from each sample was used to determine ethanol concentration using the AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA).
Sucrose Reinforcement
Sucrose Self-Administration Training
Once testing of the antagonists (PR and locomotor activity) on ethanol self-administration was complete, sucrose self-administration training began. That is, sucrose replaced ethanol as the reinforcer and from this point onwards, the rats no longer received ethanol. All parameters for the sucrose self-administration sessions were identical to the ethanol self-administration sessions and sucrose reinforcement was paired with the same lever with which ethanol had been paired. In an effort to equate sucrose responses to baseline ethanol responses, sucrose concentration was manipulated: 0.5% (w/v) for the initial 2 sessions, 0.3% (w/v) for the next 14 sessions, and 0.4% (w/v; for the remaining part of the experiment). At the time of testing, rats had 17 sessions of sucrose (0.4%, w/v) self-administration.
Progressive Ratio Baseline and Effects of mGluR Antagonists on Progressive Ratio Performance
Two PR (PR1) tests, interspersed with sucrose self-administration sessions were conducted to determine a break point baseline for sucrose (0.4% w/v) reinforcement. JNJ 16259685 [0, 0.1, 0.3, 1 mg/kg, (i.p)] and MPEP [(0, 1, 3, 10 mg/kg, (i.p.)] were tested in PR1 tests using the same parameters as described for ethanol reinforcement tests. Locomotor activity was tested after completion of each antagonist PR1 assessment. Responding under the PR1 was assessed again to determine if break points had changed after mGluR antagonist testing. There were 11 and 13 intervening sucrose self-administration sessions upon completion of the entire JNJ 16259685 and MPEP assessments (including locomotor testing), respectively.
Locomotor Activity
After each antagonist evaluation on PR responding was completed, the lowest effective dose of JNJ 16259685 or MPEP that altered PR responding was tested to determine if that dose produced changes in locomotor activity. The same parameters as described previously were used.
Drugs
Ethanol (95%) was diluted in distilled water. MPEP (Sigma-Aldrich, St Louis, MO) a selective antagonist of mGluR5 (Gasparini et al., 1999) was dissolved in saline. JNJ 16259685 [3,4-dihydro-2H-pyrano[2,3]b quinolin-7-yl (cis-4-methoxycyclohexyl) methanone] (Tocris, Ellisville, MO) a selective antagonist of mGluR1 (Lavreysen et al., 2004) was suspended in a 0.1% carboxymethylcellulose vehicle. Both antagonists were injected i.p. at a volume of 1 ml/kg and the dose selection for both compounds was made based on published work (Schroeder et al., 2005; Steckler et al., 2005a,b). The MPEP and JNJ 16259685 pretreatment interval (40 minutes) was chosen based on studies showing maximal receptor occupancy corresponding to this pretreatment time and the 30-minute self-administration test sessions (Anderson et al., 2003; Lavreysen et al., 2004).
Data Analysis
The number of responses on the ethanol and water levers and the sucrose and water levers for the baseline sessions were compared using a paired t-test. The PR break point was determined to be the final response ratio completed before the end of the session. Thus, if an animal did not respond during these sessions, the break point was 0. Break point, latency to the first ethanol/sucrose response, total responses on the ethanol and water levers, and ethanol intake (g/kg) were analyzed by a one-way repeated measures analysis of variance (RM ANOVA). Ethanol intake (g/kg) was determined from body weight and the number of reinforcers delivered. Cumulative reinforcers received during the PR tests, and number of line crosses during the locomotor assessments were analyzed using a two-way RM ANOVA. Tukey’s post hoc comparisons were conducted to extract significant main effects and interactions. Statistical significance was declared at p ≤ 0.05.
RESULTS
Ethanol Reinforcement
Baseline Ethanol (15% v/v) Self-Administration Performance
Average total responses, ethanol preference, and ethanol intake for the 5 consecutive sessions preceding testing of PR responding are shown in Fig. 1A. There were significantly greater responses on the ethanol-paired lever, relative to the water lever, [t(10) = 13.29, p < 0.001; left panel]. This corresponded to greater than 80% preference for the ethanol paired lever (middle panel) with greater than 0.6 g/kg ethanol intake in the 30-minute sessions (right panel). Average (±SEM) ethanol and water reinforcers received were 26.27 ± 1.35 and 5.93 ± 0.94, respectively. A reliable ethanol PR1 baseline was established as break point values did not differ with repeated testing or under the various conditions (i.e., no injection, saline injection, and posttest; Fig.1B).
Fig. 1.
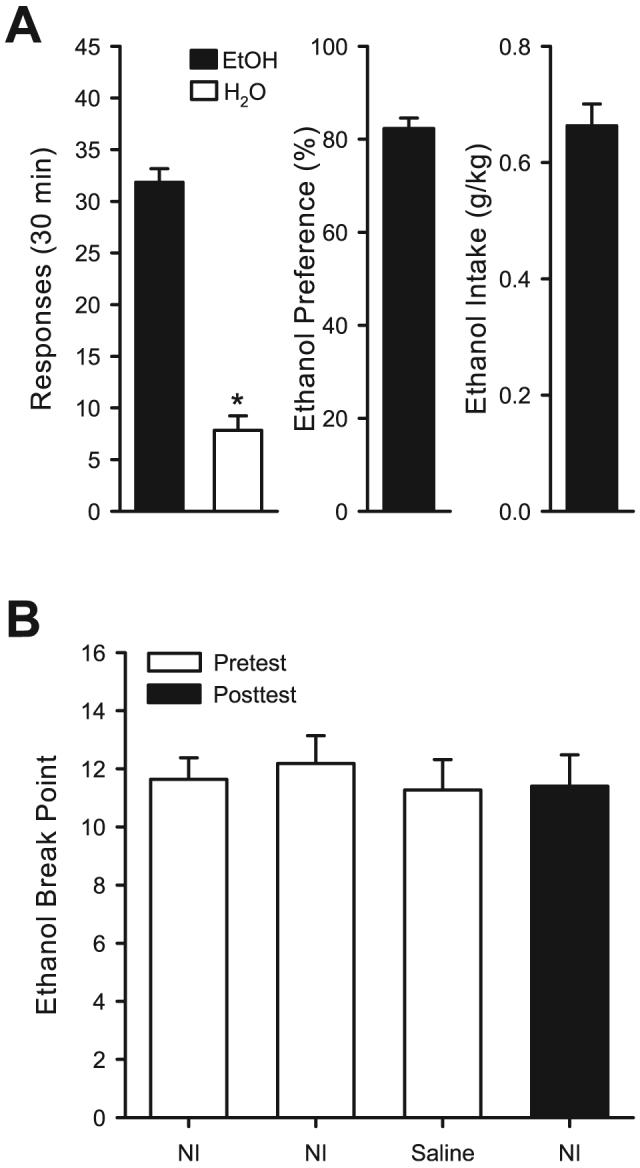
Panel (A) Left Panel: Mean (±SEM) responses on the ethanol (EtOH, 15% v/v) and water (H2O) levers during the 30-minute baseline sessions. Middle Panel: Mean (±SEM) ethanol preference in percent (i.e., ethanol responses/(ethanol + water responses ×100)), Right Panel: Mean (±SEM) ethanol intake (g/kg) during the 30-minute baseline session. All the mean (±SEM) values represent the average of 5 consecutive self-administration sessions before the start of progressive ratio (PR1) testing. Panel (B): Mean (±SEM) ethanol (15% v/v) break point with repeated testing after no injection and saline injection. Filled bar represents the ethanol (15% v/v) break point after the testing of metabotropic glutamate receptor antagonists on ethanol (15% v/v) PR1 and locomotor assessments. *Indicates significant difference from ethanol (Paired t-test, p < 0.05).
Progressive Ratio Testing (ethanol)
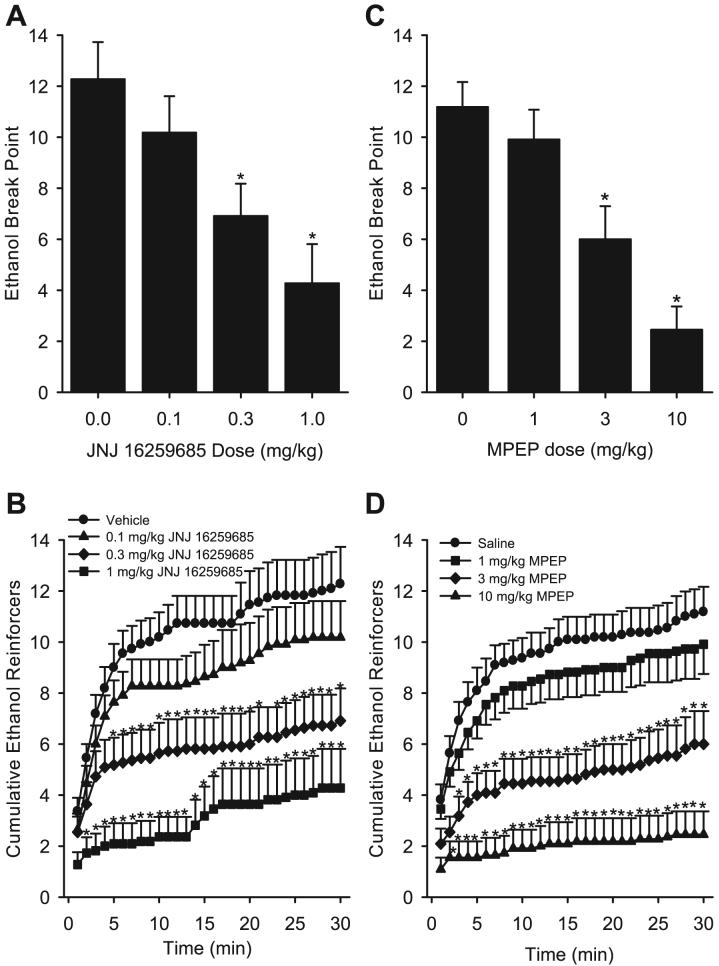
The mGluR1 antagonist JNJ 16259685 induced a significant dose-dependent decrease in the ethanol break point [F(3,30) = 13.06, p < 0.001; Fig. 2A]. This reduction in ethanol reinforced break point was accompanied by a dose- and time-dependent decrease in the number of cumulative reinforcers delivered (Fig. 2B). The RM ANOVA showed a significant main effect of dose [F(3,30) = 18.27, p < 0.001], a main effect of time [F(29,290) = 26.79, p < 0.001], and a significant interaction [F(87,870) = 3.77, p < 0.001]. Consequently, JNJ 16259685 significantly reduced ethanol intake [F(3,30) = 13, p < 0.001] by 0.3 and 1 mg/kg JNJ 16259685 relative to vehicle (p < 0.003; Table 1). Total session responses on the ethanol and water levers are shown in Table 2. The significant reduction in break point responding by JNJ 16259685 pretreatment was accordingly accompanied by a significant reduction in total session ethanol responses [F(3,30) = 9.39, p < 0.001; Table 2]. Further, JNJ 16259685 pretreatment also resulted in a reduction in total session water responses [F(3,30) = 5.26, p = 0.005; Table 2]. Latency to the first response on the ethanol lever was significantly increased by 1 mg/kg JNJ 16259685 [F(3,30) = 6.02, p = 0.002; Table 3].
Fig. 2.
Panel (A): Mean (±SEM) ethanol (15% v/v) break point achieved after JNJ 16259685 administration. Panel (B): Mean (±SEM) number of cumulative ethanol (15% v/v) reinforcers delivered across the 30-minute progressive ratio 1 (PR1) test sessions after 3,4-dihydro-2H-pyrano[2,3]b quinolin-7-yl (cis-4-methoxycyclohexyl) methanone (JNJ 16259685) pretreatment. Panel (C): Mean (±SEM) ethanol (15% v/v) break point achieved after 6-methyl-2-(phenylethynyl) pyridine (MPEP) administration. Panel (D): Mean (±SEM) number of cumulative ethanol (15% v/v) reinforcers delivered across the 30-minute PR1 test sessions after MPEP pretreatment. *Indicates significant difference from vehicle or saline (Tukey’s post hoc test, p < 0.05).
Table 1.
Ethanol Intake (g/kg) for the Antagonist Tests (mean ± SEM)
| JNJ 16259685 dose (mg/kg) | |||
| 0 | 0.1 | 0.3 | 1 |
| 0.3 ± 0.04 | 0.25 ± 0.04 | 0.17 ± 0.03* | 0.10 ± 0.04* |
| MPEP dose (mg/kg) | |||
| 0 | 1 | 3 | 10 |
| 0.28 ± 0.03 | 0.24 ± 0.03 | 0.15 ± 0.03* | 0.06 ± 0.02* |
JNJ 16259685, 3,4-dihydro-2H-pyrano[2,3]b quinolin-7-yl (cis-4-methoxycyclohexyl) methanone; MPEP, 6-methyl-2-(phenylethynyl) pyridine.
p < 0.05 relative to 0 (Tukey’s post hoc test).
Table 2.
Total Session Lever Responses for the Antagonist Tests (mean ± SEM)
| JNJ 16259685 dose (mg/kg) | ||||
|---|---|---|---|---|
| Reinforcer | 0 | 0.1 | 0.3 | 1 |
| Ethanol | 102.18 ± 18.34 | 75.00 ± 19.95 | 40.46 ± 12.13* | 26.36 ± 14.72* |
| Water | 8.09 ± 1.46 | 3.46 ± 0.84 | 1.46 ± 0.47* | 1.91 ± 1.81* |
| MPEP dose (mg/kg) | ||||
| 0 | 1 | 3 | 10 | |
| Ethanol | 81.09 ± 11.64 | 67.36 ± 12.94 | 32.64 ± 11.46* | 9.46 ± 5.12* |
| Water | 9.91 ± 2.34 | 5.55 ± 1.70 | 1.64 ± 1.16* | 1.27 ± 0.62* |
| JNJ 16259685 dose (mg/kg) | ||||
| 0 | 0.1 | 0.3 | 1 | |
| Sucrose | 43.36 ± 3.720 | 36.73 ± 7.24 | 13.73 ± 2.99* | 15.82 ± 9.47* |
| Water | 2.18 ± 0.35 | 2.91 ± 1.05 | 1.36 ± 0.58 | 0.82 ± 0.82 |
| MPEP dose (mg/kg) | ||||
| 0 | 1 | 3 | 10 | |
| Sucrose | 38.18 ± 7.66 | 44.55 ± 13.83 | 23.73 ± 6.49 | 7.73 ± 3.53* |
| Water | 4.09 ± 0.96 | 3.82 ± 2.08 | 0.91 ± 0.32 | 0.64 ± 0.31 |
JNJ 16259685, 3,4-dihydro-2H-pyrano[2,3]b quinolin-7-yl (cis-4-methoxycyclohexyl) methanone; MPEP, 6-methyl-2-(phenylethynyl) pyridine.
p < 0.05 relative to 0 (Tukey’s post hoc test).
Table 3.
Latency to First Response on Ethanol or Sucrose Lever in Minutes (mean ± SEM)
| JNJ 16259685 dose (mg/kg) | ||||
|---|---|---|---|---|
| 0 | 0.1 | 0.3 | 1 | |
| Ethanol | 0.26 ± 0.09 | 0.49 ± 0.22 | 2.16 ± 1.81 | 11.13 ± 4.13* |
| MPEP dose (mg/kg) | ||||
| 0 | 1 | 3 | 10 | |
| Ethanol | 0.30 ± 0.16 | 0.19 ± 0.05 | 3.31 ± 2.69 | 11.29 ± 4.32* |
| JNJ 16259685 dose (mg/kg) | ||||
| 0 | 0.1 | 0.3 | 1 | |
| Sucrose | 0.35 ± 0.15 | 2.12 ± 1.65 | 3.09 ± 2.69 | 10.32 ± 3.82* |
| MPEP dose (mg/kg) | ||||
| 0 | 1 | 3 | 10 | |
| Sucrose | 0.51 ± 0.22 | 3.37 ± 2.67 | 1.23 ± 0.23 | 10.75 ± 4.00* |
JNJ 16259685, 3,4-dihydro-2H-pyrano[2,3]b quinolin-7-yl (cis-4-methoxycyclohexyl) methanone; MPEP, 6-methyl-2-(phenylethynyl) pyridine.
p < 0.05 relative to 0 (Tukey’s post hoc test).
The mGluR5 antagonist MPEP dose-dependently reduced the ethanol break point [F(3,30) = 16.81, p < 0.001; Fig. 2C]. MPEP produced a dose- and time-dependent reduction in the cumulative number of reinforcers received as determined by a significant main effect of MPEP dose [F(3,30) = 19.40, p < 0.001], a main effect of time [F(29,290) = 40.77, p < 0.001], and a significant interaction [F(87,870) = 6.08, p < 0.001; Fig. 2D]. Accordingly, MPEP reduced ethanol intake [g/kg; F(3,30) = 16.65, p < 0.001] with significant reductions observed at the 3 and 10 mg/kg MPEP doses relative to saline (ps < 0.003; Table 1). The significant reduction in break point responding by MPEP pretreatment was accordingly accompanied by a significant reduction in total session ethanol responses [F(3,30) = 12.04, p < 0.001; Table 2]. MPEP pretreatment also resulted in a significant reduction in water responses [F(3,30) = 7.34, p < 0.001; Table 2]. Latency to the first response on the ethanol lever was significantly increased by 10 mg/kg MPEP [F(3,30) = 5.01, p = 0.006; Table 3].
Locomotor Activity
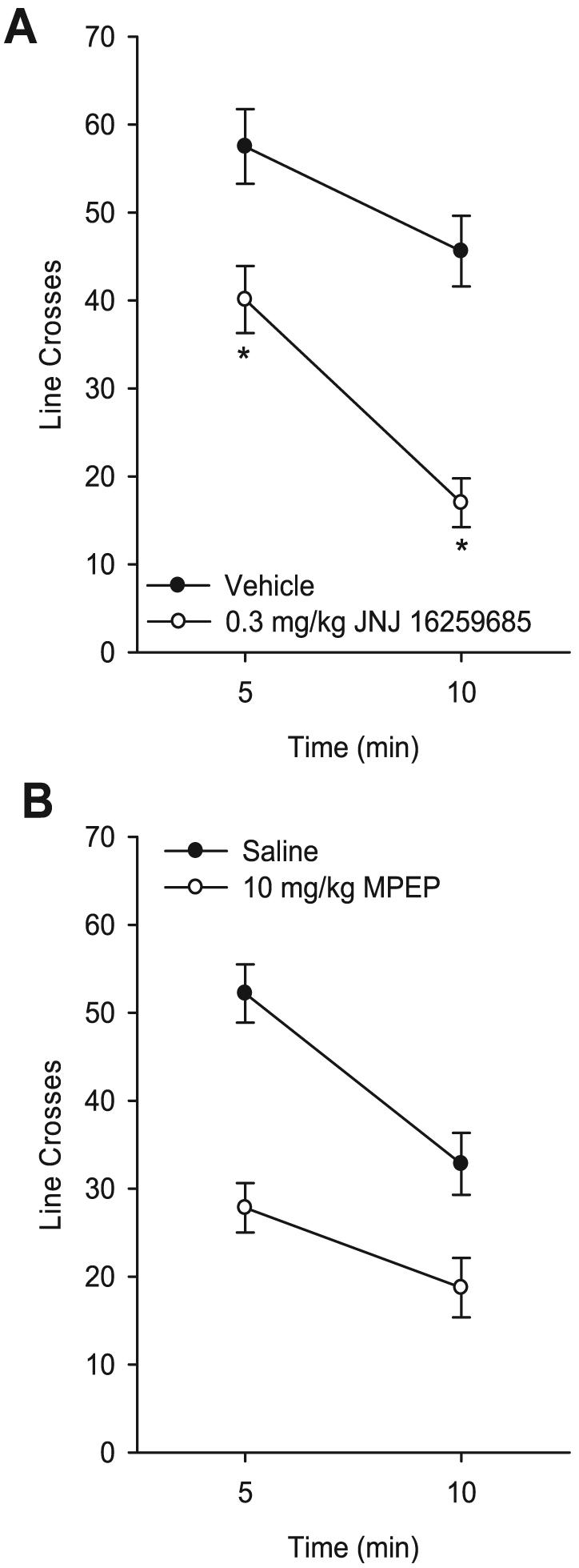
The mGluR1 antagonist JNJ 16259685 (0.3 mg/kg) produced a time-dependent reduction in locomotor activity as shown in Fig. 3A. The two-way RM ANOVA showed a significant main effect of time [F(1,10) = 49.45, p < 0.001], a significant main effect of drug treatment [F(1,10) = 9.32, p = 0.01], and a significant interaction [F(1,10) = 5.30, p = 0.04]. After vehicle and JNJ 16259685 (0.3 mg/kg) treatment, a significant reduction in line crosses was observed at 10 minutes relative to the first 5 minutes of the session, ps < 0.02, which is indicative of habituation to the environment. However, JNJ 16259685 (0.3 mg/kg) treatment significantly reduced the number of line crosses during the final 5 minutes of the session relative to saline treatment, ps < 0.01. This difference indicates the possibility of an emergence of a motor impairment, given that activity during the first 5 minutes of the session was similar.
Fig. 3.
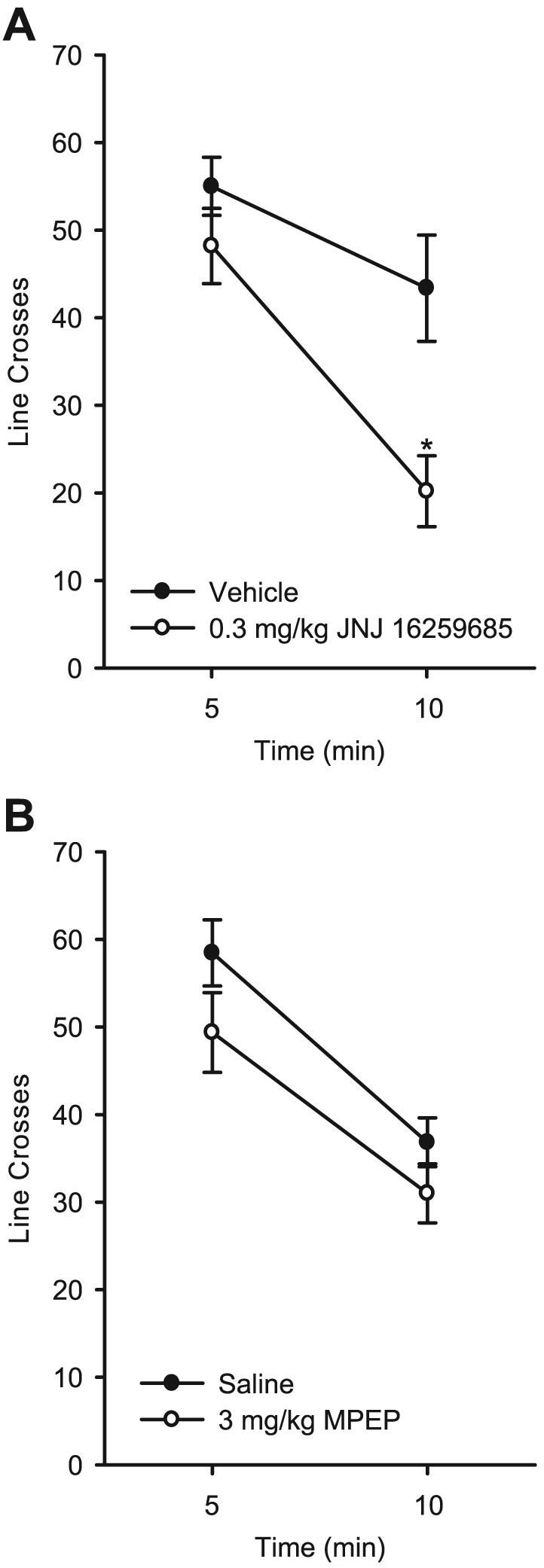
Panel (A): Mean (±SEM) number of line crosses during the 10-minute locomotor assessment after pretreatment with 3,4-dihydro-2H-pyrano[2,3]b quinolin-7-yl (cis-4-methoxycyclohexyl) methanone (JNJ 16259685; 0.3 mg/kg). Panel (B): Mean (±SEM) number of line crosses during the 10-minute locomotor assessment after pretreatment with 6-methyl-2-(phenylethynyl) pyridine (MPEP; 3 mg/kg). *Indicates significant difference from vehicle at 10 minutes (Tukey’s post hoc test, p < 0.05).
Locomotor activity after administration of the mGluR5 antagonist MPEP (3 mg/kg) is illustrated in Fig. 3B. MPEP treatment did not affect locomotor activity. A main effect of time was observed [F(1,10) = 215.69, p < 0.001], with significantly fewer line crosses in the second 5 minutes of the session, probably indicating habituation to the environment. There was no significant interaction between MPEP and time.
Blood Ethanol Concentrations
The total number of ethanol reinforced responses from the 30-minute training session was 38.55 ± 5.11 (mean ± SEM) with the corresponding ethanol intake at 0.73 ± 0.1 g/kg. This resulted in blood ethanol concentrations of 79.97 ± 6.44 mg/dl.
Sucrose Reinforcement
Baseline Sucrose (0.4%, w/v) Self-Administration Performance
Average responses and sucrose preference for the 5 consecutive sessions preceding testing of PR responding are shown in Fig. 4A. There were significantly greater responses on the sucrose-paired lever, relative to the water lever, [t(10) = 5.44, p < 0.001; left panel] which corresponded to greater than 85% preference for the sucrose-paired lever (right panel). Average (±SEM) sucrose and water reinforcers received were 37.51 ± 6.25 and 5.82 ± 1.59, respectively. PR performance with sucrose reinforcement did not vary with repeated testing (Fig. 4B, open bars). In addition, PR performance was maintained after mGluR antagonist tests (Fig. 4B, filled bar).
Fig. 4.
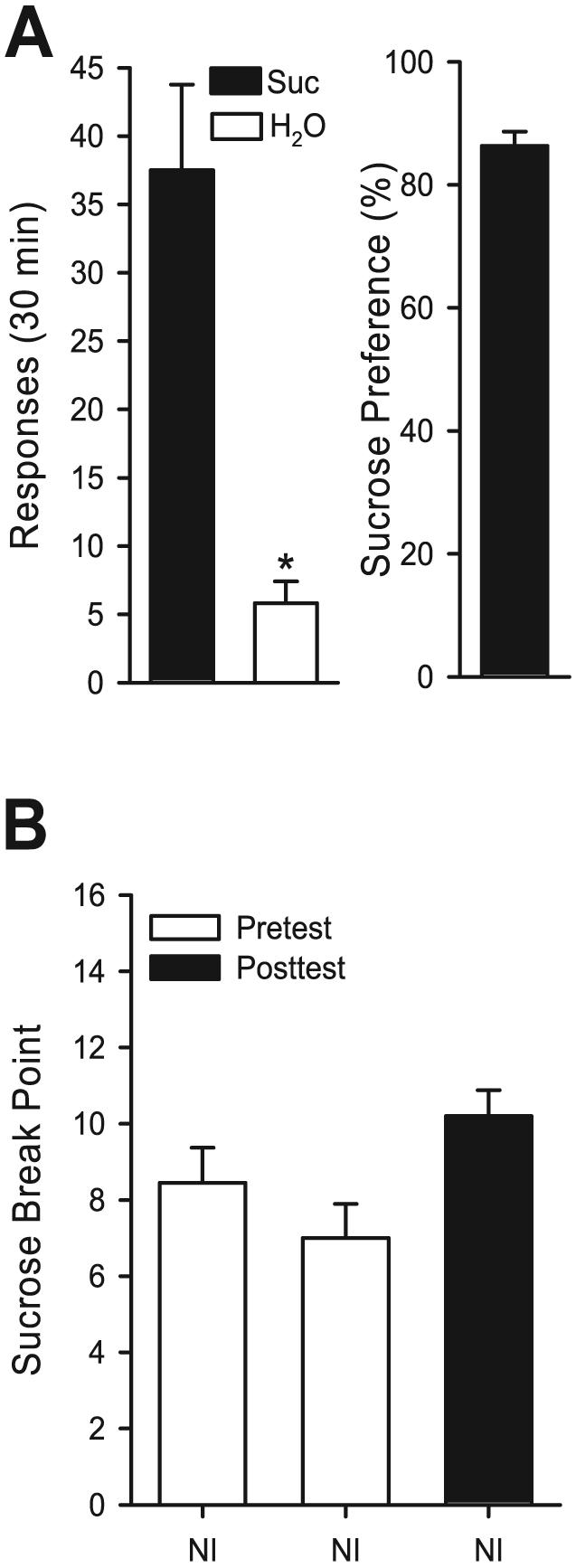
Panel (A) Left Panel: Mean (±SEM) responses on the sucrose (Suc, 0.4% w/v) and water (H2O) levers during the 30-minute baseline sessions. Middle Panel: Mean (±SEM) sucrose preference in percent (i.e., sucrose responses /(sucrose + water responses × 100)). All the mean (±SEM) values represent the average of 5 consecutive sucrose self-administration sessions before the start of antagonist testing. Panel (B): Mean (±SEM) sucrose (0.4% w/v) break point after repeated testing after no injection. Filled bar represents the sucrose break point after the testing of mGluR antagonists on sucrose progressive ratio 1 and locomotor assessments. *Indicates significant difference from sucrose (Paired t-test, p < 0.05).
Progressive Ratio Testing (Sucrose)
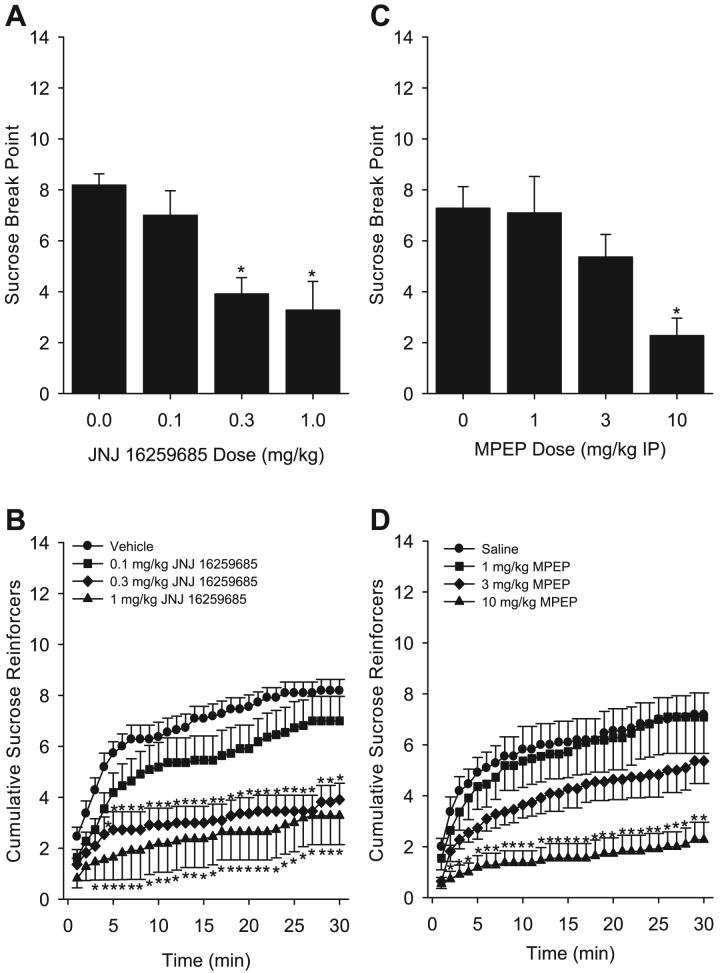
The mGluR1 antagonist JNJ 16259685 significantly reduced sucrose break point [F(3,30) = 11.72, p < 0.001; Fig. 5A]. Accordingly, JNJ 16259685 significantly reduced the cumulative number of reinforcers delivered in a dose- and time-dependent manner (Fig. 5B). There was a significant main effect of dose [F(3,30) = 14.04, p < 0.001], a significant main effect of time [F(29,290) = 44.17, p < 0.001], and a significant interaction [F(87,870) = 3.20, p < 0.001]. Total sucrose and water responses during the test sessions are shown in Table 2. Total session sucrose responses were significantly reduced by JNJ 16259685 [F(3,30) = 8.16, p < 0.001]. Water responses were not significantly altered by JNJ 16259685 treatment. Latency to the first response on the sucrose lever was significantly increased by 1 mg/kg JNJ 16259685 relative to vehicle [F(3,30) = 3.35, p = 0.03; Table 3].
Fig. 5.
Panel (A): Mean (±SEM) sucrose (0.4% w/v) break point achieved after 3,4-dihydro-2H-pyrano[2,3]b quinolin-7-yl (cis-4-methoxycyclohexyl) methanone (JNJ 16259685) administration. Panel (B): Mean (±SEM) number of cumulative sucrose (0.4% w/v) reinforcers received across the 30-minute progressive ratio 1 (PR1) test sessions after JNJ 16259685 pretreatment. Panel (C): Mean (±SEM) sucrose (0.4% w/v) break point achieved after 6-methyl-2-(phenylethynyl) pyridine (MPEP) administration. Panel (D): Mean (±SEM) number of cumulative sucrose (0.4% w/v) reinforcers received across the 30-minute PR1 test sessions after MPEP pretreatment. *Indicates significant difference from vehicle or saline (Tukey’s post hoc test, p < 0.05).
Figure 5C shows the reduction in sucrose break point by the mGluR5 antagonist MPEP [10 mg/kg; F(3,30) = 9.94, p < 0.001]. As shown in Fig. 5D, MPEP altered the pattern of cumulative reinforcers delivered. There was a significant main effect of MPEP dose [F(3,30) = 12.17, p < 0.001], a significant main effect of time [F(29,290) = 29.86, p < 0.001], and a significant interaction [F(87,870) = 3.04, p < 0.001]. Total session sucrose responses were significantly reduced by MPEP [F(3,30) = 5.16, p = 0.005; Table 2]. Water responses were not significantly altered by MPEP treatment (Table 2). Latency to the first response on the sucrose lever was significantly increased by 10 mg/kg MPEP relative to saline [F(3,30) = 4.46, p = 0.01; Table 3].
Locomotor Activity
The mGluR1 antagonist JNJ 16259685 significantly reduced the number of line crosses (Fig. 6A). There was a significant main effect of JNJ 16259685 dose [F(1,9) = 53.32, p < 0.001], a significant main effect of time [F(1,9) = 41.60, p < 0.001], and a significant interaction [F(1,9) = 5.01, p = 0.05]. JNJ 16259685 (0.3 mg/kg) reduced locomotor activity during both time intervals, ps < 0.001. Interestingly, there was a difference in the pattern of activity induced by 0.3 mg/kg JNJ 16259685 following the ethanol PR1 tests and the present assessment after sucrose PR1 tests (i.e., JNJ 16259685 reduced line crosses only during the last 5 minutes after the ethanol PR tests and throughout the entire 10 minutes test after the sucrose PR tests). To investigate the difference between the two locomotor assessments, the number of line crosses during the first 5 minutes and for the entire session (i.e., total number of line crosses) following both JNJ 16259685 (0.3 mg/kg) treatments were compared by paired t-tests. No significant differences were found between the two drug-only tests (first 5 minutes: t = 1.57; total: t = 1.19), suggesting that the difference in the pattern of results between the two assessments was probably because of minor shifts in activity after vehicle and JNJ 16259685 treatments.
Fig. 6.
Panel (A): Mean (±SEM) number of line crosses during the 10-minute locomotor assessment after pretreatment with JNJ 16259685 (0.3 mg/kg). Panel (B): Mean (±SEM) number of line crosses during the 10-minute locomotor assessment after pretreatment with 6-methyl-2-(phenylethynyl) pyridine (10 mg/kg). *Indicates significant difference from vehicle at 5 and 10 minutes (Tukey’s post hoc test, p < 0.05).
Figure 6B shows the locomotor activity after administration of the mGluR5 antagonist MPEP (10 mg/kg). MPEP treatment resulted in a significant reduction in the number of line crosses as evidenced by a significant main effect of dose [F(1,10) = 36.95, p < 0.001], and a significant main effect of time interval [F(1,10) = 40.73, p < 0.001]. The interaction was not significant.
DISCUSSION
The purpose of this study was to further characterize involvement of Group I GluR in the reinforcing effects of ethanol using a PR schedule of reinforcement in alcohol-preferring P-rats. Antagonism of mGluR5 by MPEP reduced ethanol break point at a dose (3 mg/kg) that did not affect motor behavior. In contrast, the mGluR1 antagonist JNJ 16259685 showed nonselective effects, as the same dose (0.3 mg/kg) that reduced ethanol break point produced a motor impairment. Both JNJ 16259685 (0.3 mg/kg) and MPEP (10 mg/kg) reduced sucrose break point; however, these doses also produced motor deficits. This study suggests that glutamate activity at mGluR5 is involved in regulating motivation to self-administer ethanol.
In the present work, the average ethanol break point (i.e., final FR completed) was approximately 12. As compared to break points achieved for other drugs of abuse (i.e., psychostimulants; see Richardson and Roberts, 1996), ethanol break points are considerably lower (Economidou et al., 2006; Pickering et al., 2007; Rodd et al., 2003; Walker and Koob, 2007; results of present study), and the response requirements for reinforcement delivery under the PR schedule tend to increase in a slower manner (Economidou et al., 2006; Pickering et al., 2007; Rodd et al., 2003;Walker and Koob, 2007). Further, the ethanol break point in the present study corresponds to an ethanol intake of approximately 0.3 g/kg. This is almost half of the ethanol dose received during a regular training session which is consistent with other work (Pickering et al., 2007; Rodd et al., 2003), and eliminates satiation as a possible mechanism for the cessation of responding. Importantly, consistent ethanol break points were observed during repeated baseline tests and following the mGluR antagonist evaluations. This indicates that PR performance was stable over time and not influenced by repeated behavioral testing or factors associated with repeated drug administration.
Ethanol break point was reduced by antagonism of both mGlu1 and mGlu5 receptors. Further analyses of response patterns as measured by time of reinforcer delivery were conducted to determine when the antagonist-induced reductions emerged. Examination of response patterns after vehicle administration for both antagonist assessments showed a dramatic increase in ethanol responding during the first 10 minutes of the session. In fact, by the first 10 minutes of the PR1 test session (vehicle and saline tests pooled), an average of 9.77 reinforcers had been delivered, corresponding to approximately 64.82 total responses. Thus, under this PR schedule, rats showed greater ethanol responding during the first 10 minutes of the test session than during the entire 30-minute training sessions (i.e., compare to ethanol self-administration baseline Fig. 1A). For both antagonists, reductions in the number of reinforcers delivered were evident by the first 5 minutes of the test sessions. This indicates that antagonism of mGluR1 and mGluR5 produced a reduction in ethanol PR responding during a discrete period of peak responding. To further examine response patterns, latency to the first ethanol response (i.e., first ethanol reinforcer delivery) was analyzed. Interestingly, the lowest effective doses of both antagonists (3 mg/kg MPEP and 0.3 JNJ 16259685) did not increase latency to the first ethanol response consistent with previous work in the P-rat (Schroeder et al., 2005). Given this finding, we conclude that the antagonist-induced reductions in ethanol break point were specific to maintenance of ethanol self-administration under increasing response requirements (as produced by the PR schedule), rather than a general reduction in the onset or initiation of ethanol responding. Further, given that antagonist-induced reductions emerged early in the session, when few reinforcers had been delivered, these reductions are probably not the result of a pharmacological interaction between the antagonist and the consumed ethanol. Alone, these analyses suggest that the lowest effective doses of the antagonists produced a specific impact on ethanol self-administration under the PR schedule.
An alternative explanation to an antagonist-induced reduction in motivation to self-administer ethanol is that the antagonist(s) produced a motor impairment. Indeed, a motor impairment would probably produce a reduction in responding which would be reflected as decreased break point. In the present work, locomotor activity was monitored for 10 minutes to correspond to the period of peak ethanol responding during the PR1 tests and to correspond to the emergence of the antagonist-induced reductions during those tests. The lowest effective dose of JNJ 16259685 (0.3 mg/kg) that reduced ethanol break point also produced a motor impairment. In contrast, the lowest effective dose of MPEP (3 mg/kg) that reduced ethanol break point had no effect on locomotor activity. This suggests that the MPEP-induced reduction in ethanol break point was because of a reduction in ethanol reinforcement.
Functional involvement of mGluR5 in modulation of ethanol self-administration under a PR schedule is consistent with the neural distribution of the receptors and brain regional regulation of ethanol’s behavioral effects. MGluR5 is highly expressed in mesocorticolimbic brain regions, such as the cortex, caudate, nucleus accumbens, lateral septum and hippocampus with moderate expression in the ventral tegmentum (VTA) and globus pallidus, and less expression in the cerebellum (Romano et al., 1995; Shigemoto et al., 1993). mGluR1 receptors are expressed in mesocorticolimbic regions but are richly expressed in the cerebellum (Fotuhi et al., 1993; Shigemoto et al., 1992), where they have been shown to regulate motor processes, such as motor activity and coordination (Ichise et al., 2000). This differential neural distribution pattern suggests differential involvement in ethanol self-administration. That is, evidence from site-specific infusion studies shows that ethanol self-administration is modulated by the VTA, nucleus accumbens, and frontal cortex (Hodge et al., 1994, 1996; Rassnick et al., 1992; Samson et al., 1993) where one might predict greater involvement of mGluR5 as compared to mGluR1. Consistent with this hypothesis, MPEP has been shown to selectively reduce ethanol self-administration by P-rats or C57BL/6J mice, but the mGluR1 antagonist, CPCCOEt had no effect (Hodge et al., 2006; Schroeder et al., 2005). In another study, both MPEP and CPCCOEt reduced ethanol self-administration; however, CPCCOEt interacted with ethanol to produce a locomotor reduction (Lominac et al., 2006), suggesting a nonspecific mechanism by which ethanol self-administration may be reduced by an mGluR1 antagonist. Thus, it is plausible that mGluR5 may regulate motivation to self-administer ethanol.
To determine if antagonism of Group I mGluRs produces a general reduction in motivation to self administer a rewarding substance, JNJ 16259685 and MPEP were tested on PR performance with sucrose reinforcement. Results showed that, both JNJ 16259685 (0.3 mg/kg) and MPEP (10 mg/kg) reduced sucrose break point. Further analysis of response patterns showed that antagonist-induced reductions in sucrose responding were present within the first 5 minutes and continued throughout the session. However, the lowest effective dose of each antagonist produced significant reductions in motor activity at a time corresponding to the peak effects on operant performance. Thus, under the conditions of the present experiment, it was not possible to dissociate the motor-impairing effects of the antagonists from any potential effect on motivation to self-administer sucrose.
One interpretation of these results is that mGluR5 may specifically regulate the motivation to self-administer ethanol. This is supported by the observed dissociation of MPEP dose effects on PR performance and locomotor activity under conditions of ethanol but not sucrose reinforcement. However, this conclusion is complicated by differential breakpoints observed under the two reinforcement conditions and merits discussion. The methods employed in this study manipulated sucrose concentration to equate baseline reinforcer history, which was important given the daily (Monday to Friday) exposure to the self-administration situation. Accordingly, we expected breakpoints to be similar for the two reinforcers. However, lower break points were observed under sucrose as compared to ethanol reinforcement conditions. Given that PR tests are considered to be a measure of a drug’s reinforcing efficacy (Markou et al., 1993; Richardson and Roberts, 1996), this suggests that ethanol demonstrated greater reinforcing efficacy than sucrose. Although these results are consistent, the fact that P-rats were selectively bred to prefer alcohol (Li et al., 1979) and maintain preference for ethanol when palatable solutions are presented as an alternative (Lankford et al., 1991) they complicate the use of this rat strain for evaluating ethanol specificity. One way of resolving this issue in future studies might be to increase response rate under the sucrose self-administration condition, which could lead to equivalent breakpoints for the two reinforcers in alcohol preferring P-rats. For instance, similar breakpoints for ethanol and sucrose reinforcement were found using Marchigian Sardinian alcohol-preferring rats when baseline sucrose response rate was approximately 3-fold higher than ethanol (Ciccocioppo et al., 2004). However, this procedural variation would raise differential reinforcer history as a potential confound and also limit conclusions related to ethanol specificity. Alternatively, future studies could utilize rats that are not bred to prefer alcohol but recent evidence indicates that this strategy leads to the opposite result i.e., higher sucrose breakpoints as compared to ethanol (Economidou et al., 2006). Thus, it may be difficult to equate both baseline and PR performance when comparing ethanol and sucrose reinforcement and it remains possible that differential effects of the mGluR5 antagonist found in the present study were a function of differential PR performance.
A relevant concern of the present MPEP results is that sensitivity to the mGluR5 antagonist may have changed with repeated testing and this is especially important given that order of reinforcer history was not counterbalanced. However, in previous work assessing alcohol self-administration after repeated deprivations in the P-rat, the same MPEP dose tested on 3 separate occasions produced similar reductions in ethanol self-administration (Schroeder et al., 2005). Further, in the present work JNJ 16259685 produced the same pattern of effects with ethanol and sucrose reinforcement.
In sum, blockade of mGluR5 reduces ethanol-reinforced responding under a PR schedule of reinforcement in selectively bred alcohol-preferring (P) rats. That is, the mGluR5 antagonist MPEP reduced ethanol break point at a dose that did not alter locomotor activity. By contrast, the lowest dose of the mGluR1 antagonist JNJ 16259685 that reduced ethanol breakpoint also significantly impaired locomotor activity. Both mGluR1 and mGluR5 antagonism produced significant reductions in sucrose-reinforced responding under a PR schedule of reinforcement. However, these reductions were probably because of motor impairments, rather than changes in motivational factors, as these doses produced reductions in motor activity. It is possible that a dose of JNJ 16259685 that does not produce motor impairments may reduce motivation to self-administer ethanol. However, given the relatively high concentration of these receptors in the cerebellum, interaction with locomotor systems will probably remain a major concern in regards to future work with this receptor system, but a lesser concern with the mGlu5 receptor system.
ACKNOWLEDGMENTS
This work was supported by Grants AA016009 to JB and AA014983 and AA011605 to CWH from the National Institute on Alcohol Abuse and Alcoholism and by the Bowles Center for Alcohol Studies. The authors thank Dr. David H. Overstreet for breeding and providing the P-rats.
REFERENCES
- Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, King C, Cosford ND, Varney MA. In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine) Eur J Pharmacol. 2003;473:35–40. doi: 10.1016/s0014-2999(03)01935-6. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Baskys A, Bayazitov I, Fang L, Blaabjerg M, Poulsen FR, Zimmer J. Group I metabotropic glutamate receptors reduce excitotoxic injury and may facilitate neurogenesis. Neuropharmacology. 2005;49(Suppl 1):146–156. doi: 10.1016/j.neuropharm.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Bhave G, Karim F, Carlton SM, Gereau RWt. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4:417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- Bordi F, Ugolini A. Group I metabotropic glutamate receptors: implications for brain diseases. Prog Neurobiol. 1999;59:55–79. doi: 10.1016/s0301-0082(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin / orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 2004;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, Trabace L, Ciccocioppo R. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology (Berl) 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Sharp AH, Glatt CE, Hwang PM, von Krosigk M, Snyder SH, Dawson TM. Differential localization of phosphoinositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. J Neurosci. 1993;13:2001–2012. doi: 10.1523/JNEUROSCI.13-05-02001.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Kuhn R, Pin JP. Allosteric modulators of group I metabotropic glutamate receptors: novel subtype-selective ligands and therapeutic perspectives. Curr Opin Pharmacol. 2002;2:43–49. doi: 10.1016/s1471-4892(01)00119-9. [DOI] [PubMed] [Google Scholar]
- Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Velicelebi G, Kuhn R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM, Samson HH. Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1996;20:1631–1638. doi: 10.1111/j.1530-0277.1996.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL / 6J mice. Psychopharmacology (Berl) 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Tolliver GA, Haraguchi M. Effects of intra-accumbens injections of dopamine agonists and antagonists on sucrose and sucrose-ethanol reinforced responding. Pharmacol Biochem Behav. 1994;48:141–150. doi: 10.1016/0091-3057(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, Katsuki M, Aiba A. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res. 2000;24:278–284. [PubMed] [Google Scholar]
- Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, Koob GF, Skoubis PD, Markou A. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- Lankford MF, Roscoe AK, Pennington SN, Myers RD. Drinking of high concentrations of ethanol versus palatable fluids in alcohol-preferring (P) rats: valid animal model of alcoholism. Alcohol. 1991;8:293–299. doi: 10.1016/0741-8329(91)90417-u. [DOI] [PubMed] [Google Scholar]
- Lavreysen H, Wouters R, Bischoff F, Nobrega Pereira S, Langlois X, Blokland S, Somers M, Dillen L, Lesage AS. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004;47:961–972. doi: 10.1016/j.neuropharm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312:1232–1240. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Waller MB. Progress toward a voluntary oral consumption model of alcoholism. Drug Alcohol Depend. 1979;4:45–60. doi: 10.1016/0376-8716(79)90040-1. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lumeng L, Li TK. The development of metabolic tolerance in the alcohol-preferring P rats: comparison of forced and free-choice drinking of ethanol. Pharmacol Biochem Behav. 1986;25:1013–1020. doi: 10.1016/0091-3057(86)90079-1. [DOI] [PubMed] [Google Scholar]
- Makarewicz D, Duszczyk M, Gadamski R, Danysz W, Lazarewicz JW. Neuroprotective potential of group I metabotropic glutamate receptor antagonists in two ischemic models. Neurochem Int. 2006;48:485–490. doi: 10.1016/j.neuint.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Pickering C, Moreira T, Liljequist S. Delayed access to alcohol accelerates self-administration of alcohol on a progressive ratio schedule. Basic Clin Pharmacol Toxicol. 2007;100:109–114. doi: 10.1111/j.1742-7843.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Rassnick S, D’Amico E, Riley E, Pulvirenti L, Zieglgansberger W, Koob GF. GABA and nucleus accumbens glutamate neurotransmission modulate ethanol self-administration in rats. Ann N Y Acad Sci. 1992;654:502–505. doi: 10.1111/j.1749-6632.1992.tb26013.x. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li TK, McBride WJ. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O’Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevostianova N, Danysz W. Analgesic effects of mGlu1 and mGlu5 receptor antagonists in the rat formalin test. Neuropharmacology. 2006;51:623–630. doi: 10.1016/j.neuropharm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5 in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Vassout A, Neijt HC, Kuhn R, Gasparini F, Roux S, Porsolt RD, Gentsch C. Anxiolytic-like effects of the prototypical metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl) pyridine in rodents. J Pharmacol Exp Ther. 2000;295:1267–1275. [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Steckler T, Lavreysen H, Oliveira AM, Aerts N, Van Craenendonck H, Prickaerts J, Megens A, Lesage AS. Effects of mGlu1 receptor blockade on anxiety-related behaviour in the rat lick suppression test. Psychopharmacology (Berl) 2005a;179:198–206. doi: 10.1007/s00213-004-2056-7. [DOI] [PubMed] [Google Scholar]
- Steckler T, Oliveira AF, Van Dyck C, Van Craenendonck H, Mateus AM, Langlois X, Lesage AS, Prickaerts J. Metabotropic glutamate receptor 1 blockade impairs acquisition and retention in a spatial water maze task. Behav Brain Res. 2005b;164:52–60. doi: 10.1016/j.bbr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]