Abstract
Purpose: Hydroxyapatite/tri-calcium phosphate (HA/TCP) mixture is an osteoconductive material used as a bone graft substitute, and demineralised bone matrix (DBM) is an osteoinductive material. A combination of DBM and HA/TCP mixture would probably create a composite with both osteoconductive and osteoinductive properties. The purpose of this study was to determine the effect of the combination of DBM and HA/TCP mixture on healing of rat radius segmental defects. Methods: Twenty-four adult male Wistar rats were used. Bilateral radial defects were created in each animal. Radial defects were implanted with DBM, HA/TCP mixture and a combination of both substances. Control defects were left unfilled. Ten weeks after implantation, the animals were sacrificed, and the radii were evaluated by radiograhic and histopathological studies. Results: The use of DBM alone demonstrated improved healing on radiographic and histological studies compared to other groups and the control group. There were no differences between the other two groups and the control group. Conclusion: The DBM group showed the best healing response. Combined use of DBM and HA/TCP mixture did not improve bone healing, and the osteoinductive properties of DBM were inhibited by HA/TCP mixture.
Résumé
Le mélange hydroxyapatite/phosphate tri-calcique (HA/TCP) est un matériel d’ostéo conduction utilisé comme greffe ou substitut osseux comme les matrices osseuses déminéralisées (DBM) utilisées en tant que matériel d’ostéo induction. La combinaison de DBM et HA/TCP devrait probablement avoir les deux propriétés d’ostéo induction et d’ostéo conduction. Le propos de cette étude est d’évaluer les effets d’un mélange de DBM et d’HA/TCP sur la consolidation de défauts osseux du radius chez le rat. Vingt quatre rats adultes Wistar ont été utilisés. Chaque animal était préparé avec un defect radial bilatéral. Les radius droits des rats ont été implantés soit avec DBM, soit avec HA/TCP isolée, soit avec une combinaison des deux substances, le radius gauche servant de contrôle. Dix semaines après l’implantation, les animaux ont été sacrifiés et les os ont été évalués sur la plan radiographique et histopathologique. L’utilisation de DBM isolée montre une amélioration de la guérison sur le plan radiographique et histologique. On ne retrouve pas de différence avec les autres groupes. En conclusion, le groupe DBM montre une bonne réponse sur le plan de la consolidation. L’utilisation d’une mixture DBM et HA/TCP n’entraîne pas une amélioration et une guérison, les propriétés d’ostéo induction du DBM étant inhibées par le mélange HA/TCP.
Introduction
An ideal bone graft substitute should have osteoconductive, osteoinductive and osteogenic properties. Autogenous bone graft is the gold standard among the graft materials because it provides all of these properties [7, 10, 18]. Autogenous bone graft has been the implant of choice for most of the orthopaedic procedures. However, autogenous and allogenic bone grafts have several limitations, such as donor-site infection, pain, and disease transfer [2, 4, 8]. Because of these limitations, biosynthetic bone graft substitutes are being investigated. Hydroxyapatite/tri-calcium phosphate (HA/TCP) mixture is an osteoconductive material being used as a bone graft substitute, and demineralized bone matrix (DBM) is an osteoinductive material [7, 10, 18, 23]. A combination of DBM and HA/TCP mixture would probably create a composite with both osteoconductive and osteoinductive properties. The purpose of this study was to test the effect of a combination of DBM and HA/TCP mixture on healing of rat radii segmental defects.
Materials and methods
Animal studies were conducted after the obtaining ethical approval from the Gazi University Research Committee, 24 skeletally mature male Wistar albino rats (aged six months and weighting 250-300 g), all obtained from the same source, were used in this study in order to decrease genetic variability. Bilateral radial osteotomies were performed surgically. Rats were divided into four treatment groups, with 12 radii in each group. Group I was the control group, and the rats in this group received no treatment. The segmental defects in group II were implanted with DBM alone, and group III were implanted with HA/TCP mixture. Group IV were implanted with DBM and HA/TCP mixture combination with equal parts.
Surgical procedures were done after IM administration of ketamine (100 mg/kg; Alfasan International, Woerden, Holland) [25], and an approximately 5-mm bone defect was created in the radius of both forearms [1, 17] This osteotomy site was then irrigated with 0.9% saline, but periosteum around the osteotomy site was preserved and retracted with the overlying muscles. The osteotomy site was then treated according to the treatment protocol for each rat. As only the radius had been osteotomised, no fixation was applied, and the animals used both extremities effectively. The health status of the rats was monitored daily [6, 9, 15, 16, 22] After the procedure, X-rays of the limbs were taken while the rats were still anaesthetized, and the rats were observed until they recovered.
All procedures were in full compliance with Turkish Law 6343/2, Veterinary Medicine Deontology Regulation 6.7.26, and with the Helsinki Declaration of Animal Rights.
Grafton DBM Putty 5 ml (Osteotech Inc., Eatontown, NJ, USA) was used as demineralized bone matrix and Bonemix 5 ml (INOVA Biotechnologies Inc., Izmir, Turkey) as hydroxyapatite/tricalcium phospate mixture—consisting of 100% synthetic hydroxyapatite/β-tricalciumphospate molecules with an average granular diameter of 1–4 mm in this study.
Ten weeks after the surgical procedure, the rats were sacrificed with an intra-peritoneal injection of pentobarbital (200 mg/kg; Tiopental I.E Ulagay Pharmaceuticals Topkapi, Istanbul, Turkey) [3, 25]. After follow-up A/P X-rays, both limbs were placed in a container filled with 10% formalin solution and then stored for histological examination.
For radiographic evaluation, anteroposterior radiographs of the forelimbs were made immediately after surgery and at the time that the animals were sacrificed at ten weeks. In order to quantify the degree of healing, each radiograph was assigned a numerical score using the grading scale described by Cook et al. (Table 1) [5, 13, 17, 21].
Table 1.
Radiographic grading scale for the degree of healing
| No change from immediate postoperative appearance | 0 |
| A slight increase in radiodensity distinguishable from the graft | 1 |
| Recognizable increase in radiodensity, bridging of one cortex with new-bone formation to the graft | 2 |
| Bridging of at least one cortex with material of nonuniform radiodensity, early incorporation of the graft suggested by obscurity of graft borders | 3 |
| Defect bridged on both medial and lateral sides with bone of uniform radiodensity, cut ends of the cortex still visible, graft and new bone not easy to differentiate | 4 |
| Same as grade 3, with at least one of four cortices obscured by new bone | 5 |
| Defect bridged by uniform new bone, cut ends of cortex no longer distinguishable, graft no longer visible | 6 |
For histological examination, the harvested tissues were decalcified with 10% formic acid solution which was changed daily. The samples were embedded in paraffin, and 5 μm-thick sections cut through the long axis and stained with hematoxylin and eosin [11]. During the histological examination, the pathologist was unaware of the group to which each specimen belonged. Specimens were evaluated with a 14-point histological grading scale described by Salkeld (Table 2) to determine the quality of the union, the appearance and quality of the cortical and cancellous bone-remodelling, and the degree of bone-graft incorporation and remodelling [21].
Table 2.
Histological grading scale for the degree of healing
| Quality of union | No sign of fibrous or other union | 0 |
| Fibrous union 1 | 1 | |
| Fibrocartilaginous union or cartilage union 2 | 2 | |
| Mineralizing cartilage and bone union 3 | 3 | |
| Bone union | 4 | |
| Cortex development and remodeling | No cortex formed | 0 |
| Formation of new bone along exterior borders | 1 | |
| Recognizable formation of both the outer cortex border and the medullary space | 2 | |
| Cortices formed but incomplete bridging | 3 | |
| Complete formation of cortices with bridging of defect | 4 | |
| Bone-graft incorporation and new-bone formation | ||
| No new bone, all or most of graft visible | Graft material present, no incorporation, and no new-bone formation | 0 |
| Graft present, some incorporation with new-bone formation, and small amount of new bone | 1 | |
| Graft present, some incorporation with new-bone formation, and moderate amount of new bone | 2 | |
| Decreasing graft, increasing new bone | Graft present, some incorporation with new-bone formation continuous with host bone, and early remodelling changes in new bone | 3 |
| Decreased amount of graft (compared with grade 3), good graft incorporation, and ample new bone | 4 | |
| Less amount of graft still visible (compared with grade 4), good incorporation of graft and new bone with host and ample new bone | 5 | |
| No graft visible, extensive new bone | Difficult to differentiate graft from new bone, excellent incorporation, and advanced remodelling of new bone with graft and host | 6 |
Data obtained from the radiological and histological grading scale was analysed with SPSS 11.0 software for Windows with the paired sample test and the Wilcoxon non-parametric two-related sample test.
Results
Radiographic evaluation results (Table 3)
Table 3.
Radiographical and histological evaluation results
| Radiographical | Control | DBM | HA/TCP | DBM+HA/TCP | |
| Mean | 1.50 | 5.08 | 2.18 | 1.91 | |
| Standard deviation | 1.09 | 1.31 | 0.81 | 0.75 | |
| Range | 3 | 3 | 3 | 3 | |
| Quality of union | Mean | 2.42 | 3.25 | 2.41 | 2.27 |
| Standard deviation | 0.51 | 0.75 | 0.93 | 0.88 | |
| Range | 1.00 | 3.00 | 4.00 | 4.00 | |
| Cortex development and remodelling | Mean | 0.50 | 2.58 | 1.24 | 0.82 |
| Standard deviation | 0.67 | 1.31 | 1.35 | 0.96 | |
| Range | 2.00 | 4.00 | 4.00 | 4.00 | |
| Bone-graft incorporation and new-bone formation | Mean | 0.00 | 4.66 | 1.47 | 1.22 |
| Standard deviation | 0.00 | 1.77 | 1.69 | 1.54 | |
| Range | 0.00 | 6.00 | 5.00 | 5.00 |
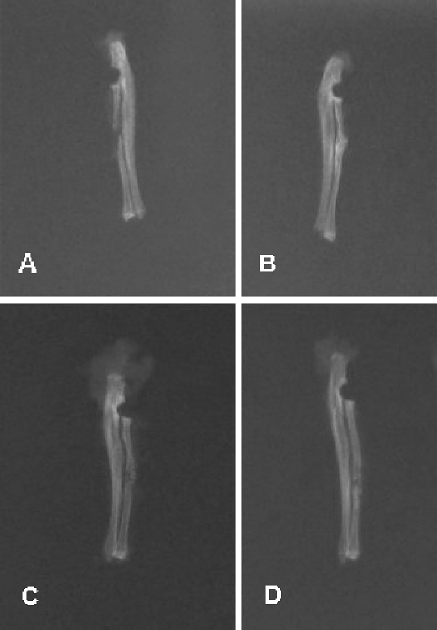
Radiographic evaluation revealed that in the control group there was no significant bone healing in any of the limbs. But a slight increase in radiodensity was seen in four of the forelimbs. In the DBM group nine of 12 radii healed completely, and four of them showed varied degrees of healing. In the HA/TCP group, radiography showed increase in radiodensity and changes, but there were no bridged defects on medial and lateral sides. The DBM+HA/TCP group was similar to the group 3, and there was no evidence of bridged cortex on both sides.
There was a statistically significant difference between the DBM group and the control group (p<0.05). Further analyses showed no significant difference between the HA/TCP group and the control group (p=0.119) and between the HA/TCP+DBM group and the control group (p=0.374). Meanwhile, the DBM group was radiologically evaluated to show better characteristics of fracture healing when compared to both the HA/TCP and the HA/TCP+DBM groups (p<0.05), whereas the HA/TCP and HA/TCP+DBM groups did not show any significant difference when compared to each other(p=0.281). As a result, the DBM group was found to be providing significantly better radiological healing when compared to the other three groups, including the control group. Data also showed that both the HA/TCP and HA/TCP+DBM groups were providing a similar level of radiological healing to the control group (Fig. 1).
Fig. 1.
Radiographs of radii in the control (a), DBM (b), HA/TCP (c), and HA/TCP+DBM (d) groups
Histopathological evaluation results (Table 3)
Histopathological evaluation was performed according to: (1) Quality of the fracture union, (2) Cortex development and remodelling, (3) Bone-graft incorporation and new-bone formation.
When the quality of the fracture union was taken in to account, significantly better results were accomplished in the DBM group (p<0.05) when compared to the other groups. The HA/TCP and control groups (p=0.849) and the HA/TCP+DBM and control groups (p=0.439) turned out to show no significant difference.(Figs. 2, 3, 4, 5)
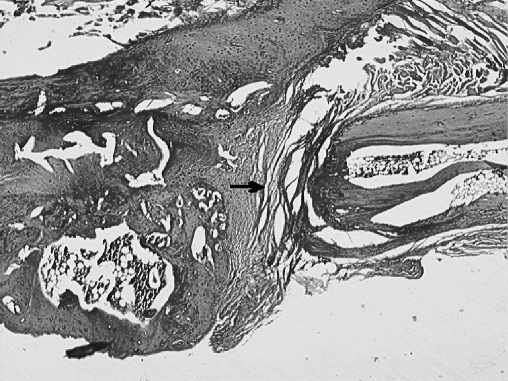
Fig. 2.
The osteotomy site in the control group (black arrow) is filled with fibrous tissue; there is no evidence of union (H.E. original magnification×40)
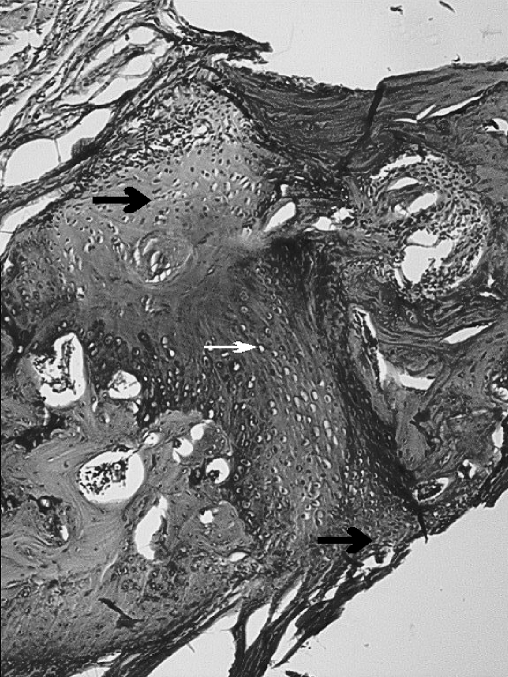
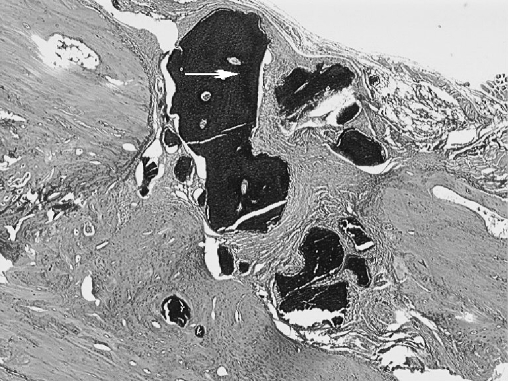
Fig. 3.
The osteotomy site in the DBM group. Periosteal new-bone, bridging and cortex development (black arrow), medulla is being formed (white arrow) (H.E. original magnification×40)
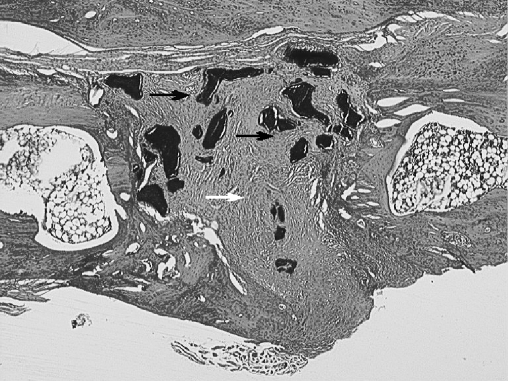
Fig. 4.
The osteotomy site in the HA/TCP group. The osteotomy site is filled with fibrous tissue (white arrow); there is no graft incorporation (black arrow) (H.E. original magnification×40)
Fig. 5.
The osteotomy site in the HA/TCP+DBM group. There is no callus formation, and the graft substitute (white arrow) is surrounded with fibrous tissue (H.E. original magnification×40)
When cortical proliferation and remodelling was studied as the variable, the DBM group showed significant difference when compared to the HA/TCP+DBM group and the control group (p<0.05); however, the DBM group did not show any difference when compared to the HA/TCP group (p=0.114). Analyses revealed a significant difference between the HA/TCP group and the control group, with superior healing findings for the HA/TCP group (p<0.05); on the other hand, no difference was detected between the HA/TCP group and the HA/TCP+DBM group (p=0.130). Finally, there was no difference between the HA/TCP+DBM group and the control group (p=0.312).
When bone-graft incorporation and new-bone formation was considered, DBM group showed a significant difference when compared to the HA/TCP+DBM group and the HA/TCP group (p<0.05). No difference was detected between the HA/TCP group and the HA/TCP+DBM group (p=0.289).
Only one of the rats showed significant foreign-body reaction in the HA/TCP+DBM group. There were no such cases encountered within any of the other groups.
This study proved that the DBM group was superior to the other groups as a provider of union in the segmental radius defects of the rats, by means of both radiological and the histopathological evaluation.
Discussion
Although the use of autogenous graft in segmental fracture healing has advantages, it also has well-known disadvantages, such as the need for the secondary surgical site, donor-site morbidity, infection and quantity-related problems. On the other hand, the use of allografts had been found to be less successful when compared to autografts. Such problems led investigators in search of allografts, which have comparable results to the autografting procedures. As a result, some investigators used mixtures of synthetic scaffolding biomaterials and osteoinductive organic agents to achieve better results [1, 12, 14, 19, 20].
As an osteoinductive agent, DBM has been proved to establish good results for the healing of fractures and bone defects. Taking this into account, delivering this material to the defect site through the use of an scaffolding synthetic material seems logical.
One of the most popular mixtures is the mixture of DBM and HA, which has demonstrated variable results in different studies. Because resorption of HA takes a relatively long time, HA/TCP mixtures were recently introduced. These mixtures are believed to provide rapid fracture healing and resorption [1, 12, 14, 19, 20].
In our study, DBM and the HA/TCP mixture were combined to provide better fracture healing. Although HA and DBM mixtures have been studied extensively, reference to the combination of HA/TCP and DBM use was not discovered in the related literature. Different results were obtained in similar research projects, whilst some of them supported our results. In our study, the best fracture healing was observed in the pure DBM group, and there were no significant differences between the remaining three groups including the control group. Because the HA/TCP and HA/TCP+DBM groups did not reveal any radiological and histopathological difference, we concluded that the use of HA/TCP and DBM mixture does not provide any additional benefits for fracture healing. Our findings were found to be similar to some of the earlier studies. The HA/TCP+DBM group was defective in fracture healing compared to the DBM group. It might be thought that the relative lack of effect of this group was due to the lower dosage of the DBM used in this group compared to the pure DBM group, but the osteoinductive capacity of DBM has been well-documented, and the ability of these preparations to stimulate bone regeneration is largely dependent on the activity of the bone morphogenetic proteins (BMP), and the absolute concentration of BMP within a particular DBM preparation does not necessariliy correlate with its efficacy [24]. We concluded therefore, that the positive effects of DBM were inhibited by the presence of HA/TCP.
Alper and colleagues [1] designed a study to identify the role of hydroxyapatite (HA) and demineralised bone matrix (DBM) combination in fracture healing. After creating 5-mm segmental defects in the radii of the rats, defects were grafted by DBM, HA and DBM/HA mixture. After eight weeks of fracture healing, radii were investigated histologically, and the HA group was found to have worse results when compared to the control group. They stated that DBM alone was an osteogenetic material for the healing in non-union models of the rats, but that using HA in conjunction caused these effects to fade away.
Moore and colleagues grafted wide ulnar defects of dogs with HA/TCP mixtures. They used a half-and-half mixture of autogenous cancellous bone and HA/TCP mixtures for one of the other groups, and pure autogenous cancellous bone for the control group. After six months, all groups were evaluated radiologically, mechanically and histhologically, and it was found that six dogs which were only grafted with the HA/TCP mixture showed fibrous non-union, whereas other groups displayed union. As a result, they suggested that HA/TCP should be used as a mixture with autogenous graft [14].
Hopp and colleagues found similar results. They designed an experiment in which the ulnar defects of rats were grafted by HA, DBM, HA/DBM, autogenous bone graft and allogenic bone graft in five different groups. At the sixth week, they found that the plain HA group scored worse than the control group, whereas all other groups displayed better scores [12].
Challenging the data provided by the authors above, there are some experiments that are promoting the usage of a combination of HA with other materials. In an experiment by Ragni and Lindholm in which they aimed to create lumbar intervertebral fusion in rats, they used HA and DBM in combination, and compared the outcomes with three other groups which consisted of HA, DBM and autogenous grafting. At the end of the second month, the HA/DBM group provided better fusion than the plain HA and plain DBM groups. The histological studies of the DBM/HA group at the end of sixth month seemed to be providing better fusion and resorbing faster than the plain HA group [20].
Ragni, Ala-Mononen and Lindholm used HA, DBM, and autogenous bone marrow for the vertebral fusion model in rabits. The animals were divided into two groups with different physical forms of HA; one was porous and the other was in block form. The groups were further divided into subgroups which consisted of plain HA, HA+DBM, HA+bone marrow, and HA+DBM+bone-marrow grafting. One other group was left ungrafted, and another was grafted with autogenous bone, and these two groups were used as control groups for the study. Two months after the operation, it was found that the groups treated with HA blocks displayed results comparable with the autogenous bone grafting group, and showed better healing than the HA+DBM and HA+bone marrow groups. However, it was found that the porous HA groups did not display similar results. Histological evidence showed that porous HA granules were wrapped with fibrous reaction tissue and weak bone formation [19].
As a result, we consider that DBM is a good choice for the healing of segmental bone defects, and provides rapid graft incorporation without causing an inflammatory reaction. Secondly, HA/TCP mixture is not as effective as DBM for the healing of segmental defects of tubular bones in rats. We also concluded that combining HA/TCP with DBM does not seem to be a good combination, because the positive effects of DBM appeared to be inhibited by the negative effects of HA/TCP.
References
- 1.Alper G, Bernick S, Yazdi M, Nimni ME (1989) Osteogenesis in bone defects in rats: the effects of hydroxyapatite and demineralized bone matrix. Am J Med Sci 298(6):371–376 [DOI] [PubMed]
- 2.Bauer TW, Muschler GF (2000) Bone graft materials. An overview of the basic science. Clin Orthop 371:10–27 [DOI] [PubMed]
- 3.Bingel SA (1999) Euthanasia and necropsy. In: An YH, Friedman RJ (eds) Animal models in orthopaedic research. CRC Press, Boca Raton, pp 71–81
- 4.Burchardt H (1987) Biology of bone transplantation. Orthop Clin North Am 18:187–196 [PubMed]
- 5.Cook SD, Baffes GC, Wolfe MW, Sampath TK, Rueger DC (1994) Recombinant human bone morphogenetic protein-7 induces healing in a canine long-bone segmental defect model. Clin Orthop 301:302–312 [PubMed]
- 6.Farso-Nielsen F, Karring T, Gogolewski S (1992) Biodegradable guide for bone regeneration. Polyurethane membranes tested in rabbit radius defects. Acta Orthop Scand 63:66–69 [DOI] [PubMed]
- 7.Finkemeier CG (2002) Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 84(3):454–464 [DOI] [PubMed]
- 8.Fleming JE, Cornell CN, Muschler GF (2000) Bone cells and matrices in orthopedic tissue engineering. Orthop Clin North Am 31:357–374 [DOI] [PubMed]
- 9.Gepstein R, Weiss RE, Hallel T (1987) Bridging large defects in bone by demineralized bone matrix in the form of a powder. A radiographic, histological, and radioisotope-uptake study in rats. J Bone Joint Surg Am 69:984–992 [PubMed]
- 10.Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN (2001) American Academy of Orthopaedic Surgeons. The Committee on Biological Implants. Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg Am 83 Suppl 2 Pt 2:98–103 [DOI] [PubMed]
- 11.Gruber HE, Stasky AA (1999) Histologic study in orthopaedic animal research. In: An YH, Friedman RJ (eds) Animal models in orthopaedic research. CRC Press, Boca Raton, pp 115–137
- 12.Hopp SG, Dahners LE, Gilbert JA (1989) A study of the mechanical strength of long bone defects treated with various bone autograft substitutes: an experimental investigation in the rabbit. J Orthop Res 7(4):579–584 [DOI] [PubMed]
- 13.Lane JM, Sandhu HS (1987) Current approaches to experimental bone grafting. Orthop Clin North Am 18:213–225 [PubMed]
- 14.Moore DC, Chapman MW, Manske D (1987) The evaluation of a biphasic calcium phosphate ceramic for use in grafting long-bone diaphyseal defects. J Orthop Res 5(3):356–365 [DOI] [PubMed]
- 15.Nunamaker DM (1998) Experimental models of fracture repair. Clin Orthop 355(Suppl):S56–S65 [DOI] [PubMed]
- 16.Nyman R, Magnusson M, Sennerby L, Nyman S, Lundgren D (1995) Membrane-guided bone regeneration. Segmental radius defects studied in the rabbit. Acta Orthop Scand 66:169–173 [DOI] [PubMed]
- 17.Ozturk AM, Cila E, Kanatli U, Isik I, Senkoylu A, Uzunok D, Piskin E (2005) Treatment of segmental bone defects in rats by the stimulation of bone marrow osteo-progenitor cells with prostaglandin E(2). Int Orthop 29(2):73–77 [DOI] [PMC free article] [PubMed]
- 18.Parikh SN (2002) Bone graft substitutes in modern orthopedics. Orthopedics 25(11):1301–1309 [DOI] [PubMed]
- 19.Ragni P, Ala-Mononen P, Lindholm TS (1993) Spinal fusion induced by porous hydroxyapatite blocks (HA). Experimental comparative study with HA, demineralized bone matrix and autogenous bone marrow. Ital J Orthop Traumatol 19(1):133–144 [PubMed]
- 20.Ragni P, Lindholm TS (1991) Interaction of allogeneic demineralized bone matrix and porous hydroxyapatite bioceramics in lumbar interbody fusion in rabbits. Clin Orthop 272:292–299 [PubMed]
- 21.Salkeld SL, Patron LP, Barrack RL, Cook SD (2001) The effect of osteogenic protein-1 n the healing of segmental one defects treated with utograft or allograft bone. J Bone Joint Surg Am 83(6):803–816 [DOI] [PubMed]
- 22.Solheim E, Pinholt EM, Andersen R, Bang G, Sudmann E (1992) The effect of a composite of polyorthoester and demineralized bone on the healing of large segmental defects of the radius in rats. J Bone Joint Surg Am 74:1456–1463 [PubMed]
- 23.Thalgott JS, Giuffre JM, Klezl Z, Timlin M (2002) Anterior lumbar interbody fusion with titanium mesh cages, coralline hydroxyapatite, and demineralized bone matrix as part of a circumferential fusion. Spine 2(1):63–69 [DOI] [PubMed]
- 24.Whang PH, Wang JC (2003) Bone graft substitutes for spinal fusion. Spine 3(2):155–165 [DOI] [PubMed]
- 25.Wixson SK, Smiler KL (1998) Anesthesia and analgesia in rodents. In: Kohn DF, Wixson SK, White WJ, Benson GJ (eds) Anesthesia and analgesia in laboratory animals. Academic, San Diego, pp 173–184