Abstract
The use of autologous osteochondral grafts (mosaicplasty) to repair articular cartilage defects is a well-established technique. Between 1998 and 2003, 19 patients with grade IV cartilage defects in the knee joint were treated by mosaicplasty. The average age of these 13 men (68%) and six women (32%) was 33.1 years (20–46). The mean follow-up was 32.4 months (84–24). The mean preoperative and postoperative Lysholm score was 45.8 (21–60) and 87.5 (74–100), respectively (p<0.001). Postoperative evaluation showed significant improvement. The results at the last follow-up were excellent in seven patients (27%), good in 11 patients (58%) and fair in one patient (15%). Preoperative complaints of pain, crepitation and locking disappeared in all patients. Magnetic resonance imaging (MRI) examination at the last follow-up visit revealed that congruency was restored in 16 (84.2%) without any signs of fissuring or delamination but in three patients (15.8%) a 1-mm difference between graft and recipient surface was detected. No complications were observed in the patients. Mosaicplasty is a really effective method of treatment for grade IV cartilage lesions in the knee joint.
Résumé
La technique de la mosaïc–plastie utilisant des greffes ostéocartilagineuses autologues pour réparer les dommages articulaires est une technique actuellement bien établie. Entre 1998 et 2003, 19 patients avec des lésions cartilagineuse de grade IV au niveau de l’articulation du genou, ont été traités par mosaïc–plastie. L’âge moyen de cette population (13 hommes 68% et 6 femmes 32%) était de 33,1 ans (20–46), le suivi moyen a été de 32,4 mois (84–24). Le score de Lysholm pré–op et post–op a été de 45,8 (21–60) et 87,5 (74–100) (p<0,001). L’évaluation post–opératoire a montré une amélioration significative, avec un résultat excellent chez 7 patients (27%), bon chez 11 patients (58%) et médiocre chez 1 patient (15%). La symptomatologie préopératoire (douleurs, craquements et blocages) a disparu chez tous les patients. L’examen IRM au dernier contrôle a montré que la cicatrisation était bien restaurée chez 16 patients (84.2%), sans signe de fissuration ni de délamination mais avec, chez 3 patients (15,8%) une différence d’un millimètre entre la greffe et le site receveur. Aucune complication n’a été observée chez ces patients. La mosaïc–plastie est une technique sûre et efficace du traitement des lésions cartilagineuses de grade IV au niveau du genou.
Introduction
Articular cartilage lesions are a common problem and 63% of knees have these lesions at arthroscopy. Five percent are in patients less than 40 years old and in the form of full–thickness cartilage defects [1]. Since articular cartilage has a low capacity to regenerate, treatment of chondral lesions is very difficult especially full-thickness defects. Different open or arthroscopic treatment options such as abrasion arthroplasty, drilling and microfracture have been described [2–6]. However, these procedures result in the formation of fibrocartilage instead of normal hyaline cartilage [7, 8]. Consequently, interest has focused on hyaline cartilage transplantation and grafting techniques including osteochondral allografts, autologous chondrocyte transplantation and osteochondral autografts (mosaicplasty) [9–11].
Mosaicplasty has been developed over recent years and involves multiple cylindrical osteochondral grafts harvested from a weight-bearing area of less importance in the knee joint and inserted into the drilled holes at the defect site [11–14]. Successful results have been reported from animal [12] and clinical studies [11, 13–15], but experience with mosaicplasty in published data is limited.
In this study, we aimed to evaluate the medium-term clinical and radiological results of patients undergoing mosaicplasty for full-thickness cartilage lesions of the knee joint.
Materials and methods
Mosaicplasty was performed in 19 knees of 19 patients with grade IV cartilage defects of the knee joint between 1998 and 2003.
Patients with full-thickness cartilage or osteochondral defects diagnosed by magnetic resonance imaging (MRI) preoperatively, confirmed at arthroscopy, with defects between 1–2.5 cm in diameter located in the femoral condyles and with radiologically closed physes and closed physes radiographically were included in the study. Patients with associated tibial or patellar articular cartilage defects, generalized osteoarthritic changes, osteophyte formation in the intercondylar notch, mechanical axis malalignment, collagen disease and skeletal immaturity were excluded.
Defects located on the lateral condyle of the femur were grafted with a mini-open technique while those on the medial condyle of the femur were grafted arthroscopically. The COR-System (Mitek Products, Westwood, MA, USA) was used in this study. The system comprises graft harvester, drill bit, delivery guide and a plunger for the purposes of harvesting and implantation. Standard diameter and length of the grafts were 6 and 15 mm, respectively. The lateral condyle was used as donor site. The graft harvester was positioned at a right angle to the donor site and with a small mallet it was driven into the bone to a depth of 15 mm. Then, by rotating the T-handle in a clockwise direction, the graft could be removed. The procedure was repeated if additional grafts were needed.
The recipient site was prepared by removing loose fragments with a shaver or a curette to visualise the edges of the defect. The subchondral bone was drilled at right angles to the recipient site to a depth of 15 mm.
To implant the graft, a delivery guide which must be rotated to a suitable position before the final tapping to ensure that the graft’s cartilage contour fits recipient surface. Finally, the graft is gently tapped into place. A proper press fit is essential. The knee is put through a full range of movement to check stability of the graft. The joint was drained and a compression bandage applied.
Postoperatively, patients remained non-weight bearing for three weeks, while active knee motion was unrestricted. During the next six weeks partial weight bearing was allowed. After that full weight bearing was allowed if no contraindication existed.
The patients were evaluated clinically using the Lysholm knee score [16] grading the patients as excellent, good, fair and poor.
Radiological evaluation was done with weight-bearing anteroposterior, lateral radiographs and MRI scan according to the International Cartilage Repair Society (ICRS) [17] at the last follow-up visit. Radiographs were evaluated using the Fairbank’s criteria [18] including ridge and osteophyte formation, flattening or squaring of the femoral condyle and joint space narrowing.
Statistical evaluation was done with Student’s t-test and the significance was set at p<0.05.
Results
We included 19 patients in the study. The average age of these 13 men (68%) and six women (32%) was 33.1 years (20–46) with a mean follow-up of 32.4 months (84–24). The age distribution was as follows: three patients aged 20–25 years, three patients aged 26–30 years, five patients aged 31–35 years, six patients aged 36–40 years and two patients aged 40–46 years. Mosaicplasty was performed in 11 right and eight left knees. The aetiology was trauma in nine (47.4%) and osteochondritis dissecans in two patients (10.5%). The location of lesions was medial femoral condyle in 15 (79%) and lateral femoral condyle in four (21%) knees.
Preoperatively all patients had activity-related pain in their knees. Ten (52.6%) patients had repeated swelling, three patients (15.8%) described a locking sensation and 12 patients (63.2%) had crepitation. Thirteen (68.4%) loose bodies were removed. Twelve (63.2%) partial meniscectomies and three (15.8%) anterior cruciate ligament (ACL) reconstructions were performed at the same time. The average size of the defects was 15 mm (10–23) in diameter. On average, 2.3 grafts (1–3) were used for defect reconstruction.
The mean preoperative and postoperative Lysholm score was 45.8 (21–60) and 87.5 (74–100) (p<0.001). Postoperative evaluation showed significant improvement. The results at the last follow-up were excellent in seven patients (27%), good in 11 patients (58%) and fair in one patient (15%).
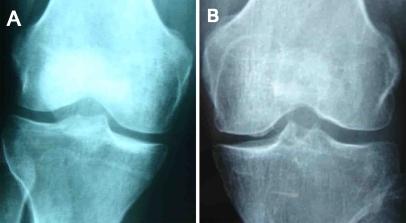
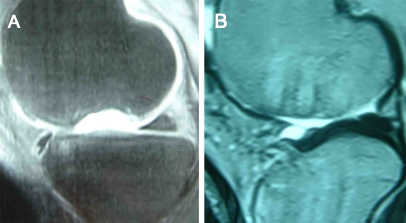
One patient was found to have minor degenerative changes accoding to Fairbank’s criteria (Fig. 1). Preoperative complaints of pain, crepitation and locking disappeared in all patients. MRI examination at the follow-up visit revealed that congruency was restored in 16 (84.2%) without any signs of fissuring or delamination but in three patients (15.8%) a 1-mm gap between graft and recipient surface was detected (Fig. 2) (Table 1).
Fig. 1.
Anteroposterior radiographs of the knee joint in a 34–year–old woman. a Preoperative radiograph. b Seven years postoperatively showing a 1-mm surface incongruency of the osteochondral autograft on the medial femoral condyle and minor degenerative changes according to Fairbank’s criteria
Fig. 2.
Sagittal MRI of the knee joint in a 35–year–old man. a Preoperative MRI showing grade IV cartilage defect on the medial femoral condyle. b Postoperative MRI after 28 months showing good osteointegration of the graft, filling of the chondral defect and restoration of surface congruency
Table 1.
Clinical and MRI findings of patients at the last control
| Case | Age/gender | Side (R/L) | Preoperative complaint | Postoperative evaluation | MRI (congruency) |
|---|---|---|---|---|---|
| 1 | 22/M | R | Pain, swelling, crepitation | Excellent | Congruent |
| 2 | 36/M | R | Pain | Good | Congruent |
| 3 | 20/F | L | Pain, crepitation, locking sensation | Excellent | Congruent |
| 4 | 41/M | R | Pain | Excellent | 1-mm difference |
| 5 | 26/F | L | Pain, swelling, crepitation | Good | Congruent |
| 6 | 37/M | L | Pain, swelling, crepitation | Good | Congruent |
| 7 | 32/M | L | Pain, locking sensation | Excellent | Congruent |
| 8 | 30/M | R | Pain, crepitation | Good | Congruent |
| 9 | 38/F | R | Pain, swelling, crepitation | Excellent | Congruent |
| 10 | 34/F | R | Pain, swelling, crepitation, locking sensation | Fair | 1-mm difference |
| 11 | 25/M | L | Pain, swelling, crepitation | Good | Congruent |
| 12 | 46/F | R | Pain | Good | Congruent |
| 13 | 35/M | R | Pain, swelling, crepitation, locking sensation | Good | Congruent |
| 14 | 27/M | L | Pain | Excellent | Congruent |
| 15 | 40/M | R | Pain, swelling, crepitation | Good | Congruent |
| 16 | 31/F | L | Pain | Good | 1-mm difference |
| 17 | 37/M | R | Pain, swelling, crepitation | Good | Congruent |
| 18 | 33/M | L | Pain, swelling, crepitation | Excellent | Congruent |
| 19 | 38/M | R | Pain | Good | Congruent |
No intraoperative or postoperative complications occurred.
Discussion
Full-thickness cartilage lesions cause problems such as pain, swelling and locking and if left untreated give rise to early development of osteoarthritis. The aim in the treatment of these full-thickness cartilage lesions is to relieve pain, increase function of the knee joint and through this prevent or delay total knee replacement.
Different treatment options have been described including abrasion arthroplasty, drilling and microfracture [2–6]. However, these techniques result in the development of fibrocartilage having poor biomechanical characteristics and the clinical results deteriorate over time [7, 8]. Subsequent studies have focused on producing hyaline articular cartilage by autologous osteochondral grafting, osteochondral allografts or autologous chondrocyte transplantation [9–11]. Transplantation of autologous chondrocytes is an exciting but a two–stage, laboratory-dependent and high-cost procedure [10]. Osteochondral allograft is another treatment option. Although it has an advantage of grafting large defects, the disadvantages include disease transmission and adverse effects of preservation techniques on chondrocyte viability, while cost and tissue availability limit the use of this technique [9].
Autologous osteochondral grafting (mosaicplasty) first popularised by Hangody et al. [19] is being applied in an effort to reconstruct the affected articular surface with properties similar to those of hyaline cartilage [12–15, 20, 22, 23]. Initial animal experiments [19] and subsequent clinical studies [12, 13, 21] by Hangody et al. [12] reported 91% excellent and good outcome in 57 patients younger than 45 years with more than three years follow-up for osteochondral lesions of 1–8.5 cm2 in size. Kish et al. [13] treated 52 athletes with the same technique and after a minimum one year follow-up stated that 63% of the patients had returned to full sport participation. Chow et al. [20], in a study with 30 patients, carried out arthroscopic mosaicplasty for osteochondral lesions sized 1–2.5 cm2 and with a minimum two year follow-up found 83.3% excellent and good outcome. Marcacci et al. [15], in a study of 37 patients, concluded that mosaicplasty was a safe and satisfactory technique at the short- and medium-term follow-up. Jakob et al. [22], in a study with 52 patients and an average follow-up of 37 months, found that there was improved function of the knees in 92% of the patients at the latest follow-up. Hangody and Füles [23] evaluated 831 patients undergoing mosaicplasty and found good to excellent results in 92% of the patients treated with femoral condylar implantations. In this study, we performed mosaicplasty for chondral lesions up to 2.5 cm2 in 19 patients with the oldest patient aged 46 years and after a mean follow-up of 32.4 months found 11 excellent and seven good results, which are similar to those in the literature.
Autologous osteochondral grafting (mosaicplasty) does not depend on a laboratory for procedures of chondrocyte proliferation, matrix implantation or mesenchymal cell proliferation. Furthermore, the hyaline cartilage is harvested from the donor site of the same knee joint. Thus, mosaicplasty is a safe, one–stage and low-cost procedure having no risk of disease transmission or immunological reaction to the graft. This technique is minimally invasive applied arthroscopically or a mini-incision arthrotomy. This makes it easier to handle comorbidities such as ACL tears. Chow et al. [20] simultaneously performed two ACL reconstructions in 30 patients with mosaicplasties. Marcacci et al. [15] performed mosaicplasty in association with 12 ACL reconstructions during the same operation. Jakob et al. [22] carried out supplementary surgical procedures along with mosaicplasty such as correction of femoropatellar and femorotibial malalignment, ACL reconstructions, partial medial meniscectomy, reconstruction of the lateral collateral ligament, suture of meniscal tear and total synovectomy. In this study, we performed three ACL reconstructions and 12 partial meniscectomies in conjunction with the mosaicplasty.
It is debatable whether the patient’s age affects the clinical results of mosaicplasty. Kish et al. [13] and Marracci et al. [15] reported better results in younger patients. Chow et al. [14], however, said that patient age is not a limiting factor for the procedure and found that older patients with an isolated chondral defect and a stable joint without osteoarthritic changes, seem to benefit from mosaicplasty. In this study, we had too few patients older than 40 years to be able to evaluate age as a variable for the results, but two patients older than 40 years had a good outcome.
The management of joint congruency is a challenging procedure in treating chondral lesions. Chow et al. [14] mentioned graft harvesting and insertion should be perpendicular to the articular surface and a wrong angle would compromise the end result. Marcacci et al. [15] also supported this finding and said that in their three failed cases they had problems with osteointegration and surface matching. Jakob et al. [22] also stressed the importance of surface congruity of the recipient. They pointed out the challenge for the lesions of greater than 4 cm2 for which they mentioned certain angle alterations in order to ensure that the graft conformed to the local radius of the joint’s curvature. Hangody and Füles [23] also supported the importance of surface match between the graft and recipient site. We attribute our satisfactory results to the proper alignment of the osteochondral grafts.
Second-look arthroscopy is the most helpful and reliable method for cartilage repair assessment. Chow et al. [20], in their study of 30 knees, performed second-look arthroscopy in eight patients without symptoms and found a correlation between the clinical result and second-look arthroscopy. Marcacci et al. [15] did second-look arthroscopy in five patients which showed good coverage and integration at the short-term follow-up. Jakob et al. [22], in a study of 52 patients, did second-look arthroscopy for persistence of symptoms in five patients, a second surgery and found no problems with the grafts. However, asymptomatic patients are unwilling to undergo second-look arthroscopy. For that reason and because it is expensive, we did not perform second-look arthroscopy in patients with minimal symptoms.
MRI is a noninvasive method for the assessment of articular cartilage abnormalities [24, 25]. It can be used to examine both the degree to which the defect is filled with repair tissue and the integration of repair tissue with adjacent tissues [26, 27]. Additionally, MRI can be used to evaluate the subchondral bone plate and marrow beneath the repair site [26, 27]. Integration of the cartilage repair tissue must be considered in conjuction with the incorporation of the osseous portion of the graft into the bone. An interface that consists of fluid-like signal intensity suggests that integration between the repair tissue and the native cartilage is incomplete and a fissure may exist. Chow et al. [20], in their study of 30 patients, used MRI to examine 12 patients and found that congruency was restored in 11 (91.7%) patients without signs of fissuring or delamination, but they found an abnormal marrow signal in the subchondral bone beneath the region of cartilage repair, even four years after the procedure. Jakob et al. [22] evaluated 52 patients with MRI after mosaicplasty and noted bony integration of osteochondral plugs and continuity of joint surface curvature, but they did not mention the MRI results of all patients. We performed an MRI examination at the last follow-up visit for cartilage repair assessment and found that joint congruency was restored without signs of fissuring or delamination in 16 patients.
In conclusion, our results suggest that mosaicplasty is an acceptable and reliable option for treating full-thickness chondral defects of small size and prevention of the development of early arthritis in young patients. The technique is a minimally invasive, one-stage procedure with a low rate of complications and low cost.
References
- 1.Curl WW, Krome J, Gordon S, Rushing J, Paterson-Smith B, Poehling GG (1997) Cartilage injuries: a review of 31516 knee arthroscopies. Arthroscopy 13:456–460 [DOI] [PubMed]
- 2.Kim HW, Moran ME, Salter RS (1991) The potential for regeneration of articular cartilage in defects created by chondral shaving and subchondral abrasion. An experimental investigation in rabbits. J Bone Joint Surg Am 73:1301–1315 [PubMed]
- 3.Bert JM (1997) Abrasion arthroplasty. Oper Tech Orthop 4:294–299 [DOI]
- 4.Pirdie AH (1959) The method of resurfacing osteoarthritic knee joints. J Bone Joint Surg Br 41:613–618
- 5.Mitchell N, Shepard N (1976) Resurfacing of adult rabbit articular cartilage by multiple perforations of the subchondral bone. J Bone Joint Surg Am 58:230–233 [PubMed]
- 6.Steadman JR, Rodkey WG, Singleton SB, Briggs KK (1997) Microfracture technique for full thickness chondral defects. Oper Tech Orthop 7:300–307 [DOI]
- 7.Mankin HJ, Mow VC, Buckwalter JA, Iannotti J, Ratcliffe A (1994) Form and function of articular cartilage. In: Simon SR (ed) Orthopaedic basic science. American Academy ofOrthopaedic Surgeons, Rosemont, IL, pp 3–41
- 8.Newman AP (1998) Articular cartilage repair. Current concepts. Am J Sports Med 26:309–324 [DOI] [PubMed]
- 9.Garrett JC (1998) Osteochondral allografts for reconstruction of articular defects of the knee. Instr Course Lect 47:517–522 [PubMed]
- 10.Brittberg M, Lindhal A, Nilsson A (1996) Rabbit articular cartilage defects treated by autologous cultured chondrocytes. Clin Orthop 326:270–283 [DOI] [PubMed]
- 11.Bobic V (1996) Arthroscopic osteochondral autograft transplantation in anterior cruciate ligament reconstruction: a preliminary clinical study. Knee Surg Sports Traumatol Arthrosc 3:262–264 [DOI] [PubMed]
- 12.Hangody L, Kish G, Karpati Z, Szerb I, Udvarhelyi I (1997) Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Traumatol Arthrosc 5:262–267 [DOI] [PubMed]
- 13.Kish G, Modis L, Hangody L (1999) Osteochondral mosaicplasty for the treatment of focal chondral and osteochondral lesions of the knee and talus in an athlete. Clin Sports Med 18:45–66 [DOI] [PubMed]
- 14.Chow J, Barber A (1998) Osteochondral transplantation: preliminary results and technique analysis. Presented at the 17th Annual Meeting of Proceedings of the Arthroscopy Association of North America, Orlando, FL, April 1998
- 15.Marcacci M, Kon E, Zaffagnini S, Iacono F, Neri MP, Vascellari A, Visani A, Russo A (2005) Multiple osteochondral arthroscopic grafting (mosaicplasty) for cartilage defects of the knee: prospective study results at 2–year follow-up. Arthroscopy 21:462–470 [DOI] [PubMed]
- 16.Tegner Y, Lysholm J (1985) Rating system in the evaluation of knee ligament injuries. Clin Orthop 198:43–49 [PubMed]
- 17.Bobic V. ICRS Articular Cartilage Imaging Committee. ICRS MR Imaging protocol knee articular cartilage, 2000. http://www.cartilage.org/files/publication00–1.pdf. Accessed August 6, 2002
- 18.Fairbank TJ (1948) Knee joint changes after meniscectomy. J Bone Joint Surg Br 30:664–670 [PubMed]
- 19.Hangody L, Kish G, Karpati Z, Szerb I (1997) Autogenous osteochondral graft technique for replacing knee cartilage defects in dogs. Ortop Tod Int 175–181
- 20.Chow JCY, Hantes ME, Houle JB, Zalavras CG (2004) Arthroscopic autogenous osteochondral transplantation for treating knee cartilage defects: a 2– to 5–year follow-up study. Arthroscopy 20:681–690 [DOI] [PubMed]
- 21.Hangody L, Kish G, Karpati Z, Udvarheyli I, Szigeti I, Bely M (1998) Mosaicplasty for the treatment of articular cartilage defects. Application in clinical practice. Orthopaedics 21:751–756 [DOI] [PubMed]
- 22.Jakob RP, Franz T, Gautier E, Mainil-Varlet P (2002) Autologous osteochondral grafting in the knee: indication, results, and reflections. Clin Orthop 401:170–184 [DOI] [PubMed]
- 23.Hangody L, Füles P (2003) Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints. J Bone Joint Surg Am 85:25–32 [DOI] [PubMed]
- 24.Recht MP, Kramer J, Marcelis S, Pathria MN, Trudell D, Haghighi P, Sartoris DJ, Resnick D (1993) Abnormalities of articular cartilage in the knee: analysis of available MR techniques. Radiology 187:473–478 [DOI] [PubMed]
- 25.Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB (1998) Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am 80:1276–1284 [DOI] [PubMed]
- 26.Alparslan L, Winalski CS, Boutin RD, Minas T (2001) Postoperative magnetic resonance imaging of articular cartilage repair. Semin Musculoskeletal Radiol 5:345–363 [DOI] [PubMed]
- 27.Winalski CS, Minas T (2000) Evaluation of chondral injuries by magnetic resonance imaging: repair assessments. Oper Tech Sports Med 8:108–119 [DOI]