Abstract
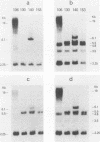
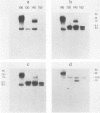
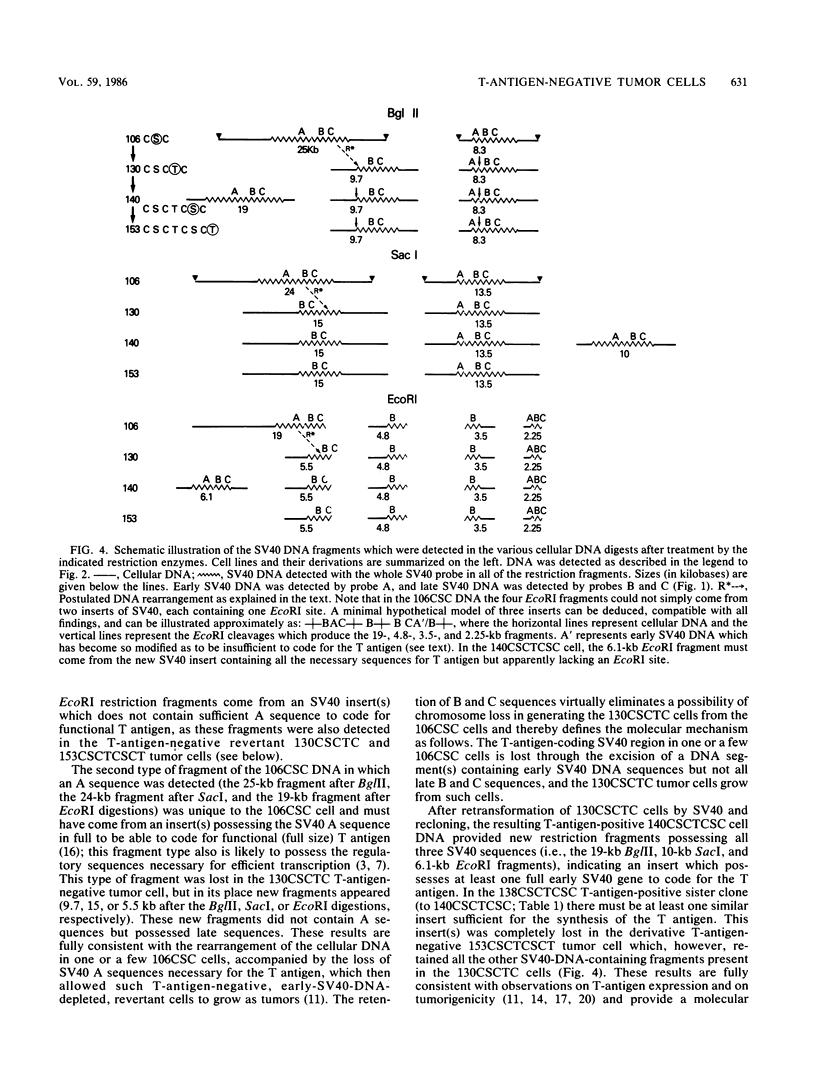
A clonal line of highly oncogenic spontaneously transformed mouse cells (104C) was transformed in tissue culture by simian virus 40 (SV40) and subsequently recloned (106CSC). This 106CSC cell line expressed T antigen and transplantation antigen but was about 100 times less tumorigenic than the 104C parent. When 10(5) 106CSC cells were injected into immunocompetent syngeneic mice, tumors were produced. From such tumors, cell lines were established in culture, all of which were consistently negative for T antigen. We found previously by solution DNA hybridization methods that the tumor cells were depleted in the early region of SV40 DNA which codes for the T antigen. We postulated that this loss occurs through a DNA rearrangement of unknown mechanism in one or a few 106CSC cells and that the tumors are then produced from such a cell or cells, whereas all the T-antigen-positive 106CSC cells are rejected by immunologic means. In this investigation we showed by the DNA transfer method using appropriately selected SV40 DNA probes that indeed the tumor cell clone (130CSCT) we selected to investigate came from one 106CSC cell in which the T-antigen-coding SV40 DNA sequences (but not all the early SV40 DNA sequences) were lost by an excision and recombination mechanism. We also showed that the 130CSCT cells, which are highly tumorigenic, could again be transformed by SV40 and that the resulting T-antigen-positive cloned derivative cells became much less tumorigenic (approximately 10(5)-fold), apparently again because of immunologic recognition and rejection. Indeed, when 10(7) T-antigen-positive cloned cells were injected, all the T-antigen-positive cells were rejected and the tumor was produced again from one or more T-antigen-negative cells. Thus, a one-step in vivo transplantation experiment allowed a selection (for tumorigenicity and against the SV40 T antigen) of a mutant mammalian cell with a DNA deletion at a definable site.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramczuk J., Pan S., Maul G., Knowles B. B. Tumor induction by simian virus 40 in mice is controlled by long-term persistence of the viral genome and the immune response of the host. J Virol. 1984 Feb;49(2):540–548. doi: 10.1128/jvi.49.2.540-548.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Chang C., Martin R. G., Livingston D. M., Luborsky S. W., Hu C. P., Mora P. T. Relationship between T-antigen and tumor-specific transplantation antigen in simian virus 40-transformed cells. J Virol. 1979 Jan;29(1):69–75. doi: 10.1128/jvi.29.1.69-75.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. E., Murray B. M. The Soviet Union's Zond 5: Is It Also a Planetary Spacecraft? Science. 1968 Oct 11;162(3850):245–246. doi: 10.1126/science.162.3850.245. [DOI] [PubMed] [Google Scholar]
- Flyer D. C., Pretell J., Campbell A. E., Liao W. S., Tevethia M. J., Taylor J. M., Tevethia S. S. Biology of simian virus 40 (SV40) transplantation antigen (TrAg). X. Tumorigenic potential of mouse cells transformed by SV40 in high responder C57BL/6 mice and correlation with the persistence of SV40 TrAg, early proteins and sequences. Virology. 1983 Nov;131(1):207–220. doi: 10.1016/0042-6822(83)90546-9. [DOI] [PubMed] [Google Scholar]
- Gruss P., Dhar R., Khoury G. Simian virus 40 tandem repeated sequences as an element of the early promoter. Proc Natl Acad Sci U S A. 1981 Feb;78(2):943–947. doi: 10.1073/pnas.78.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M. A., Vanderryn D. F., Meltzer M. L., Martin M. A. Characterization of polyoma viral DNA sequences in polyoma-induced hamster tumor cell lines. J Biol Chem. 1980 Apr 25;255(8):3798–3805. [PubMed] [Google Scholar]
- Kit S., Kurimura T., Dubbs D. R. Transplantable mouse tumor line induced by injection of SV40-transformed mouse kidney cells. Int J Cancer. 1969 Jul 15;4(4):384–392. doi: 10.1002/ijc.2910040403. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Koncar M., Pfizenmaier K., Solter D., Aden D. P., Trinchieri G. Genetic control of the cytotoxic T cell response to SV40 tumor-associated specific antigen. J Immunol. 1979 May;122(5):1798–1806. [PubMed] [Google Scholar]
- Kuster J. M., Mora P. T., Brown M., Khoury G. Immunologic selection against simian virus-40 transformed cells: concomitnat loss of viral antigens and early viral gene sequences. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4796–4800. doi: 10.1073/pnas.74.11.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland V. W., Mora P. T., Schultz A., Pancake S. Cell properties after repeated transplantation of spontaneously and of SV40 virus transformed mouse cell lines. I. Growth in culture. J Cell Physiol. 1975 Feb;85(1):101–111. doi: 10.1002/jcp.1040850111. [DOI] [PubMed] [Google Scholar]
- Mora P. T., Brady R. O., Bradley R. M., McFarland V. W. Gangliosides in DNA virus-transformed and spontaneously transformed tumorigenic mouse cell lines. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1290–1296. doi: 10.1073/pnas.63.4.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora P. T., Chandrasekaran K., Hoffman J. C., McFarland V. W. Quantitation of a 55K cellular protein: similar amount and instability in normal and malignant mouse cells. Mol Cell Biol. 1982 Jul;2(7):763–771. doi: 10.1128/mcb.2.7.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora P. T., Chang C., Couvillion L., Kuster J. M., McFarland V. W. Immunological selection of tumour cells which have lost SV40 antigen expression. Nature. 1977 Sep 1;269(5623):36–40. doi: 10.1038/269036a0. [DOI] [PubMed] [Google Scholar]
- Mora P. T. The immunopathology of SV40-induced transformation. Springer Semin Immunopathol. 1982;5(1):7–32. doi: 10.1007/BF00201954. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Seif R., Seif I., Wantyghem J. Rat cells transformed by simian virus 40 give rise to tumor cells which contain no viral proteins and often no viral DNA. Mol Cell Biol. 1983 Jun;3(6):1138–1145. doi: 10.1128/mcb.3.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. W., Morganroth J., Mora P. T. SV40 virus-induced tumour specific transplantation antigen in cultured mouse cells. Nature. 1970 Jul 11;227(5254):141–145. doi: 10.1038/227141a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., Flyer D. C., Tjian R. Biology of simian virus 40 (SV40) transplantation antigen (TrAg). VI. Mechanism of induction of SV40 transplantation immunity in mice by purified SV40 T antigen (D2 protein). Virology. 1980 Nov;107(1):13–23. doi: 10.1016/0042-6822(80)90268-8. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., Tevethia M. J., Lewis A. J., Reddy V. B., Weissman S. M. Biology of simian virus 40 (SV40) transplantation antigen (TrAg). IX. Analysis of TrAg in mouse cells synthesizing truncated SV40 large T antigen. Virology. 1983 Jul 30;128(2):319–330. doi: 10.1016/0042-6822(83)90259-3. [DOI] [PubMed] [Google Scholar]
- Wesslén T. SV40-tumorigenesis in mouse. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(4):479–487. doi: 10.1111/j.1699-0463.1970.tb04331.x. [DOI] [PubMed] [Google Scholar]
- Winberg G., Hammarskjöld M. L. Isolation of DNA from agarose gels using DEAE-paper. Application to restriction site mapping of adenovirus type 16 DNA. Nucleic Acids Res. 1980 Jan 25;8(2):253–264. doi: 10.1093/nar/8.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourne D. J., Mora P. T. Altered metabolism of heparan sulfate in simian virus 40 transformed cloned mouse cells. J Biol Chem. 1978 Jul 25;253(14):5109–5120. [PubMed] [Google Scholar]
- de Lapeyriere O., Hayot B., Imbert J., Courcoul M., Arnaud D., Birg F. Cell lines derived from tumors induced in syngeneic rats by FR 3T3 SV40 transformants no longer synthesize the early viral proteins. Virology. 1984 May;135(1):74–86. doi: 10.1016/0042-6822(84)90118-1. [DOI] [PubMed] [Google Scholar]