Abstract
Cierny-Mader (C-M) Type III osteomyelitis is defined as a localised lesion with both medullary and cortical involvement that is stable mechanically after debridement. The treatment of C-M Type III osteomyelitisis is difficult and requires a precise protocol to achieve a disease-free long-term follow-up. We report here the results of our study on 26 patients (19 men and 7 women; average age: 34.7 years) with C-M Type III osteomylelitis who were treated with radical debridement, irrigation, vancomycin-impregnated custom-made beads and culture-specific systemic antibiotics. Those patients with metaphyseal involvement were treated with deroofing of the cortex and debridement by means of a “trough” (16 patients); those with diaphyseal involvement were treated with both intramedullary reaming and debridement from a trough (ten patients). Antibiotic cement rods were used as an additional therapy in five patients with diaphyseal involvement. Recurrence developed in three patients and was attributed to inadequate debridement; all three patients were treated again in the same manner with success. The mean follow-up is currently 3.6 years (range: 2–6 years). All of the patients have normal clinical, radiographic and laboratory parameters, and all are ambulatory and have returned to their pretreatment level of activity or better. We conclude that C-M Type III chronic osteomyelitis can be safely treated with this protocol.
Résumé
L’ostéomyélite de type III Cierny-Mader (C-M) peut se définir comme une localisation de lésions localisées à la fois sur la médullaire et la corticale et qui reste stable sur le plan mécanique après mise à plat. Son traitement est difficile et demande un protocole précis. Pour cette étude, nous avons suivi les résultats de 26 patients (19 hommes et 7 femmes) dont l’âge moyen était de 34.7 ans, traités par mise à plat, irrigation, et billes de vancomycine. Dans les lésions métaphysaires, la mise à plat est réalisée à travers une fenêtre (16 patients), dans les lésions diaphysaires on réalise à la fois un alésage des lésions et une mise à plat par la fenêtre (10 patients). En addition aux billes de vancomycine, un ciment aux antibiotiques a été utilisé chez 5 patients avec lésions diaphysaires. Trois patients ont présenté une récidive secondaire à une mise à plat insuffisante. Tous ces patients ont été à nouveau traités de la même manière et avec succès. Le suivi moyen a été de 3.6 ans (2 à 6 ans) et aucun patient n’a présenté d’anomalie post-opératoire des différents paramètres clinique, radiographique et biologique. Les patients peuvent marcher et ont retrouvé leur activité d’avant le traitement. L’ostéomyélite chronique de type III de CM peut être traitée avec succès selon ce protocole.
Introduction
The treatment of chronic osteomyelitis is a common problem facing many clinicians. Radical debridement, copious irrigation and systemic and local antibiotic therapy are essential to avoid recurrent infection [3, 4, 12, 14, 19, 20, 22], with the quality of the surgical debridement being the most important parameter for long-term success in its management. Dead space management by means of antibiotic beads has lowered the failure rates and also enables a staged reconstruction, if needed [7, 8, 11, 21, 23].
The Cierny-Mader (C-M) classification provides prognostic data and assists in the planning of the treatment strategy. The four anatomical types are numerically ordered according to the complexity of the disease and/or its treatment: Type I, medullary; Type II, superficial; Type III, localised; Type IV, diffuse. Type III chronic osteomyelitis is the most difficult to treat and is characterised by full-thickness cortical sequestration and/or cavitation. This type may evolve from either medullary or superficial osteomyelitis and usually shows combined features of either type [4]. In this type of osteomyelitis, an excision through the viable tissue margin can be performed without compromising the integrity of the bone segment. Type III osteomyelitis is second most difficult type to treat. In most of the case series reported in the literature, the study cohorts have consisted of patients with different types of osteomyelitis who were treated with gentamicin- or tobramycin-impregnated beads. To our knowledge, our study is one of the largest type-specific series to be reported to date in which the patients were treated with vancomycin beads. Our objective was to investigate the treatment results of C-M Type III osteomyelitic patients treated with radical debridement, irrigation, vancomycin beads and culture-specific systemic antibiotics.
Material and methods
Between January 2001 and December 2004, 53 consecutive patients were treated for chronic osteomyelitis in our clinic. All patients were evaluated systemically for local, systemic or combined comorbid conditions that could lead to treatment failure or complications. The patients were classified physiologically according to the C-M criteria as A, B or C hosts [4]. The extent of the osteomyelitis was also classified anatomically as Types I through to IV. The anatomical classification was then be combined with the physiological class to designate the clinical stage of the patient [4].
On the basis of the above criteria, we identified 26 C-M Type III chronic osteomyelitic patients [19 men and seven women; average age: 34.7 years (range: 5–7 years)]. All of these patients had an active infection, and 16 had a draining sinus on admission. Most of the patients were in pain and experiencing functional limitation of the affected extremity. The average duration of the symptoms upon admission was 29.5 months (range: 1 month to 8 years). Fifteen of the patients had an average of 1.6 surgical procedures (range: 1–4) before presenting to our clinic. Nine patients had post-traumatic osteomyelitis, 15 had a history of haematogenous osteomyelitis and two developed osteomyelitis following elective procedures. The affected bones and demographic data of the patients are presented in Table 1. The motion of the adjacent joints, associated deformities, limb length discrepancy, the neurovascular status, quality of the soft tissues and previous scars were evaluated and documented. Fourteen of the patients were defined as A hosts, two as B hosts with local compromise, eight as B hosts with systemic compromise and two were B hosts with both local and systemic compromise.
Table 1.
Demographic data of patients
| Patient no. | Age (years) | Sex | Symptoms (months)a | Siteb | Intraoperative culturec | Beadd | Systemic antibioticsd | Bead removal | Follow-up (years) | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 47 | M | 12 | Hum P, S | MRSA | VANCO beads + rods | Teikoplanin | 2 months | 6 | Twice, re-debrid |
| 2 | 48 | M | 24 | TIib D | Staphylococcus aureus | VANCO | Fuscidic A + CIPRO | 2 months | 4.5 | – |
| 3 | 15 | M | 2 | Fem S | MRSA | VANCO | Teikoplanin | 2 months | 5 | – |
| 4 | 18 | F | 2.5 | Fem S | Coag (−) Staphylococcus | VANCO | Fuscidic A + CIPRO | 2 months | 3.5 | – |
| 5 | 35 | M | 2 | Rad P | S. aureus | VANCO | Fuscidic A + CIPRO | 2 months | 2.5 | – |
| 6 | 13 | M | 1 | Fem P, septic art. | S. aureus | VANCO | Teikoplanin + CIPRO | 2 months | 4.5 | – |
| 7 | 40 | F | 36 | Tib P,D | S. aureus, Klebsiella | VANCO + GENTA | Teikoplanin + CIPRO | 2 months | 4.5 | Once, re-debrid |
| 8 | 74 | F | 9 | Rad P | S. aureus | VANCO | Teikoplanin + CIPRO | 2 months | 3 | – |
| 9 | 63 | M | 4 | Both Fem P/Hip | MRSA | VANCO | Teikoplanin + CIPRO | 2 months | 3 | – |
| 10 | 15 | M | 60 | Fem S | S. aureus | VANCO | TMP/SMX | 1 month | 5 | – |
| 11 | 35 | F | 4 | Fem S | S. aureus | VANCO | Fuscidic A + CIPRO | 2 months | 2 | – |
| 12 | 36 | M | 42 | Hum S, D | S. aureus | VANCO beads + rods | Teikoplanin | 3 months | 4 | – |
| 13 | 35 | F | 48 | Tib S | S. aureus | VANCO beads + rods | Teikoplanin | 2 months | 3 | – |
| 14 | 50 | M | 8 | Fem P | S. aureus | VANCO | VANCO | 2 months | 3 | – |
| 15 | 25 | M | 96 | Fem S, D | S. aureus | VANCO beads + rods | Teikoplanin + FuscidicA | 2 months | 4 | – |
| 16 | 16 | M | 12 | Fem P | S. aureus | VANCO | Fuscidic A + CIPRO | 2 months | 3 | – |
| 17 | 73 | F | 96 | Fem D | S. aureus | VANCO | Imipenem + CIPRO | 2 months | 2 | – |
| 18 | 50 | M | 12 | Midfoot | S. aureus | No beads | Teikoplanin + CIPRO | 2 months | 4 | – |
| 19 | 20 | M | 3 | Tib D | Pseudomonas aeruginosa | VANCO beads + rods | CIPRO + Cefuroxime | 2 months | 4 | – |
| 20 | 56 | F | 72 | Fem D | S. aureus | VANCO | Fuscidic A + CIPRO | 2 months | 2 | – |
| 21 | 12 | M | 96 | Fem S,D | S. aureus | VANCO | VANCO | 2 months | 4 | – |
| 22 | 20 | M | 12 | Fem S | Enterobacter | VANCO | Teikoplanin | 2 months | 5 | Once, re-debrid |
| 23 | 50 | M | 12 | Fem P/Hip | MRSA | VANCO | Teikoplanin + TMP/SMX | 2 months | 4 | – |
| 24 | 39 | M | 96 | Tib D | S. aureus | VANCO | Teikoplanin | 3 weeks | 5 | – |
| 25 | 5 | M | 3 | Tib P | Enteroccoci | VANCO | Ceftriaxone | 2 months | 2 | – |
| 26 | 13 | M | 3 | Hum P | S. aureus | VANCO | Fuscidic A + CIPRO | 2 months | 2 | – |
aSymptoms: The duration of syptoms upon admission
bHum, Humerus; Rad, radius; Fem, femur; Tib, tibia; P, proximal; S, shaft; D, distal
cMRSA, methicillin-resistant Staphylococcus aureus
dVANCO, vancomycin; GENT, gentamicin; CIPRO, ciprofloxacin; TMP/SMX, trimethoprim-sulfamethoxazole
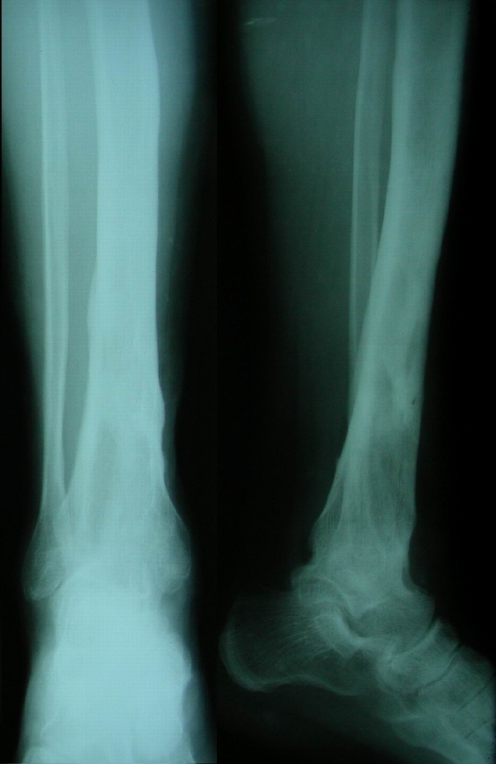
All patients were evaluated by direct radiographs and magnetic resonance imaging (MRI) prior to preoperative planning in order to determine the likely extent of bone debridement (Figs. 1, 2). The anatomical pattern of involvement, skip lesions and soft tissue and medullary extension were revealed [9].
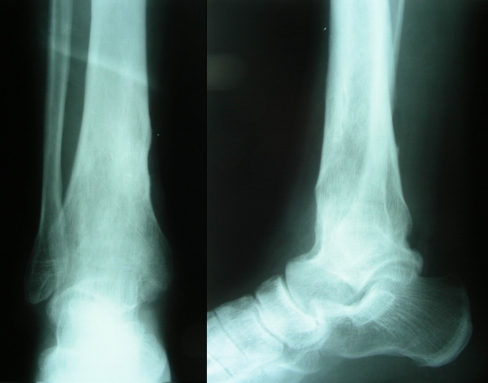
Fig. 1.
Anteroposterior (left) and lateral (right) views of patient no. 24 with right distal tibial osteomyelitis. This male patient symptomatic for 8 years. The ankle joint had been ankylosed as the result of infection
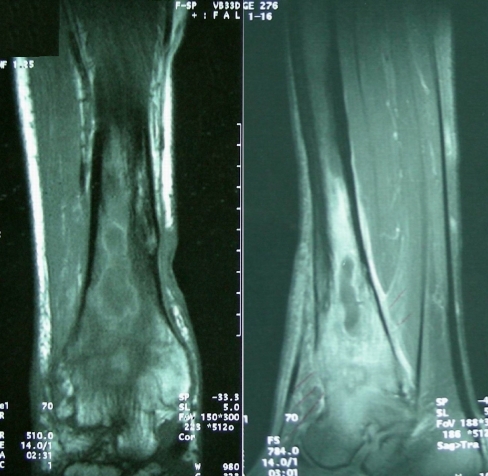
Fig. 2.
Magnetic resonance image showing pus collection in the distal tibial medulla, oedema and anteroinferior cortical destruction
Combined three-phase Tc and indium-labeled leukocyte scintigraphy were carried out in most patients for academic purposes. The laboratory examinations included a complete blood count (CBC) and differential erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), hepatic and renal function tests, blood glucose, electrolytes, bleeding profile and infectious disease markers. Cultures were taken from all draining sinuses preoperatively.
Surgical technique
In the two patients previously operated upon, the existing incisions and approaches were followed as much as possible in order to minimise ischemic wound necrosis. The fibrotic scar tissue surrounding the bone was also excised. The thickened and fibrotic periosteum was removed, but periosteal stripping was limited to the area planned to be resected in order to preserve cortical viability. Multiple biopsy specimens and cultures were taken during the surgical procedure and also during the second operation for bead removal. In patients with combined diaphyseal and metaphyseal involvement, both intramedullary reaming and deroofing of the cortex in an oval fashion and debridement were carried out. Deroofing was accomplished by means of a “trough” that was 7–10 mm in width and an average of 100 mm in length [22]. In cases that required longer troughs, we decreased the width and made two separate troughs, leaving an intact cortex between them for stress distribution. In metaphyseal involvement, only troughs were used for debridement (Fig. 3). After sequestrectomy – if present – a new and sharp high-speed burr was used for bony debridement. Saline irrigation was carried out to prevent thermal necrosis of the bone. Punctate haversian bleeding, the so-called “Paprika sign” was used to determine the extent of debridement to the living bone [4, 22]. In our protocol, more than 30% of cortical bone removal for debridement necessitates prophylactic external fixation with a monolateral system to prevent iatrogenic fracture risk. In neither of these patients was prophylactic fixation carried out. Following debridement, a minimum of 12 L of saline were used to irrigate, and, in some cases, jet lavage was used. In order to achieve a high level of local antibiotic concentration and also for dead space management, antibiotic-impregnated bone cement beads were used (Figs. 3, 4). Most of the patients’ preoperative cultures revealed vancomycin-sensitive bacteria, mainly Staphylococcus aureus. Four grams of vancomycin was mixed with 40 g of dry powdered polymethylmethacrylate to make oval-shaped custom-made beads 5–8 mm in diameter. The beads were applied into the bone and also in the soft tissue defect after debridement. A large bore drain was then placed and tension-free wound closure completed the surgical procedure. The drain was used for overflow purposes and not used in suction mode in order not to decrease the local antibiotic concentration. At 48 h postoperative, the drain was removed. CBC, ESR and CRP values were evaluated during the postoperative period at 3-day intervals until discharge of the patient. The antibiotic regimen was planned with the help of the Department of Infectious Diseases and was based on Gram’s stain and the results from intraoperative cultures. Antibiotics were given for 6 weeks following the surgery. After 6–8 weeks, the beads were removed at a second operation, multiple cultures and a bone biopsy were carried out, then a repeat debridement and irrigation was done (Fig. 5). In some patients, bone grafting was necessary to obliterate dead space.
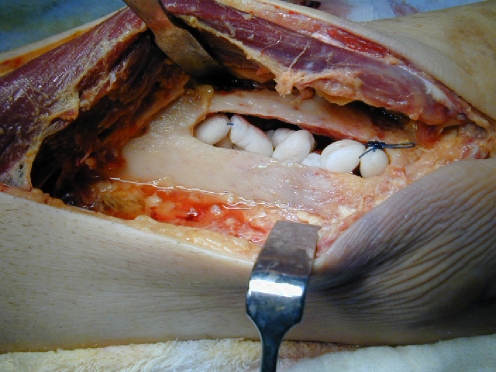
Fig. 3.
Intraoperative view after debridement by means of a trough, irrigation and vancomycin-impregnated bead application
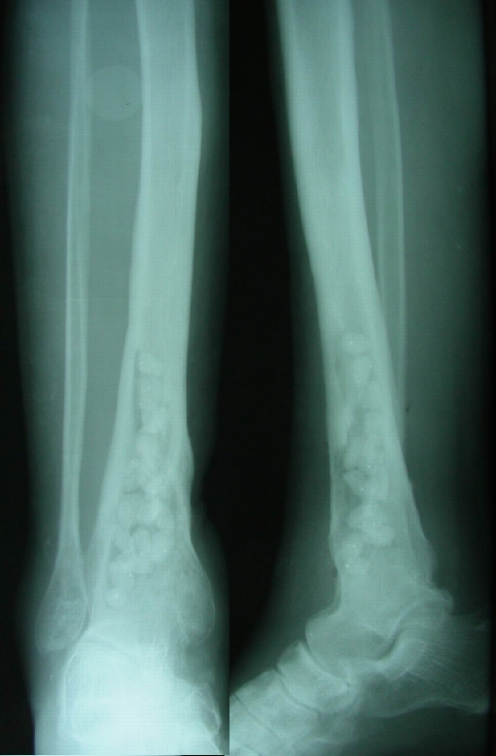
Fig. 4.
Early postoperative radiograms. Antibiotic beads were placed in the post-debridement 8–9 cm from the joint level
Fig. 5.
Follow-up radiograms after bead removal. The patient was asymptomatic and had normal radiographic and laboratory findings
Results
All of the operations and follow-ups were carried out by the senior surgeon. In all but four patients, a one-step simultaneous debridement, irrigation and bead placement was done. Of the four exceptions, a two-stage debridement was carried out on two patients, and three debridements were carried out on one patient, with the beads being replaced at the final debridement. In the fourth patient, patient no. 18, after a one-step debridement and irrigation, autogenous iliac crest bone grafting was performed and no beads were placed. This is the only patient in the series without bead placement. In 16 patients with metaphyseal involvement, unroofing of the cortex and debridement were accomplished by means of a “trough”; in ten patients with diaphyseal or meta-diaphyseal involvement, both intramedullary reaming and debridement from a trough was carried out. In five patients, antibiotic-impregnated cement rods were used in combination with beads for diaphyseal osteomyelitis [17]. The most commonly isolated microorganism was Staphylococcus aureus, followed by methicillin-resistant Staphylococcus aureus (MRSA) and Gram microorganisms. In one patient, gentamicin was combined with vancomycin in the beads. As neither gentamicin nor tobramycin is available in powder form in Turkey, we were unable to combine an aminoglycoside with the vancomycin in all of the patients. For certain patients, aminoglycosides were obtained from neighbouring countries if the preoperative cultures revealed the necessity for these drugs. The beads were removed after an average of 2 months (range: 3 weeks to 3 months). Following bead removal, bone grafting was carried out in 12 patients, mainly those with diaphyseal involvement. All patients received systemic antibiotics in accordance with the advice of a specialised infectious disease consultant. An average of 7 weeks of systemic therapy was given (range: 4–12 weeks). In parenteral therapy, teicoplenin was primarily used since there is an outpatient therapy program available with this drug. In oral therapy, fuscidic acid, ciprofloxacin and trimethoprim-sulfamethoxazole were used in most of the patients.
A satisfactory result was defined as no drainage or open wound and no clinical, radiographic or laboratory evidence of infection. The follow-up ranged from 2 to 6 years (average: 3.6 years). Recurrence occurred during the first year in three patients. In patient number 1, a second recurrence occurred 5 months after the first. Patients 1 and 22 were type IIIBS patients and patient 7 was of the type IIIL,S. The reason for these failures is believed to be inadequate debridement, although all of the patients were compromised systemically. These patients were treated with the same protocol successfully. All have had more than 4 years of follow-up after the recurrence and are currently asymptomatic.
At the latest follow-up, the results were satisfactory in all patients. All of the patients are ambulatory and have returned to their pretreatment activity level or better. They have no drainage, no pain and no signs of inflammation, and all of the radiographic and laboratory parameters are normal, except in one patient: proximal humeral osteomyelitis was detected this patient (patient no.1) on the opposite side and he is currently under treatment.
Discussion
Cierny and Mader describe the hallmark of Type III lesions to be full-thickness cortical sequestration and/or cavitation [4]. This lesion has the combined features of intramedullary and superficial osteomyelitis but does not need stabilisation. In this type of lesion, an excision through the viable tissue margin can be performed without compromising the integrity of the bone segment. Complete removal of all devitalised tissues and preferably the implants by an atraumatic approach is essential.
Siegel et al. reported the results of post-traumatic 46 patients with a minimum 18 months follow-up. Their study group included nine patients with C-M Type III osteomyelitis. Treatment of the patients consisted of extensive debridement, autogenous bone grafting and soft tissue reconstruction. These researchers reported that 71% of all failures in their series were smokers and that all patients older than 45 years were treated unsuccessfully [19]. In our group all failures were systemically compromised and staged as B hosts. One of these was both locally and systemically compromised. The ages of the failed patients were 47, 40 and 20 years, respectively, and all were non-smokers. We attributed the recurrences to inadequate debridement, and these patients all healed after redebridements.
Eckardt et al. reported the results of 13 patients with an average follow-up of 5 years. The group consisted of C-M Type III and IV osteomyelitic patients who were treated with serial debridements (average four debridements) and subsequent bone grafting or soft tissue procedures. These authors reported no recurrence in the follow-up [5].
Beals and Bryant treated 30 consecutive patients, including nine patients with CM Type III osteomyelitis [2], either of which were Type IIIB. Five of the patients were treated with one local debridement only, two were treated with debridement and local muscle flap coverage and one was treated by serial debridements and posterolateral bone grafting. At an average follow-up of 6 years, these authors reported no recurrence and good functional results.
Emara used hemi-corticotomy and partial bone segment transfer in the management of chronic osteomyelitis following debridement and the removal of the anterior half of the tibial cortex. Of the 20 patients in his series, eight were staged as type IIIA osteomyelitis. He reported one failure out of the 20 patients treated and attributed this one failure to inadequate debridement; the patient was treated with further debridement successfully [6].
Without adequate debridement, the failure rate is very high regardless of the duration of the systemic antibiotic therapy or the presence of antibiotic beads. Fitzgerald showed that locally delivered high-dose gentamicin in the absence of debridement was not effective in the treatment of canine tibial osteomyelitis [8]. However, even after adequate debridement, the remaining bed of tissue should be considered to be contaminated. Therefore, antibiotic therapy is essential. Unfortunately, once bacteria attach to bone or implants, their metabolism is decreased and they cover themselves with a biofilm (glycocalyx) that protects them from antimicrobials, opsonisation and phagocytosis [13, 15]. To kill these bacteria in the biofilm, antibiotic concentrations should be 10- to 100-fold the usual bactericidal concentration. This can not be safely achieved by systemic antibiotic therapy [16]. Antibiotic-impregnated cement beads can achieve this concentration without any systemic side effects. High local levels of antibiotics provide sufficient antibiotic levels in avascular areas by diffusion, and in many circumstances the organisms that are resistant to drug concentrations achieved by systemic antibiotics are susceptible to the extremely high local drug concentrations provided by local antibiotic delivery [10]. Most of the data reported in the literature supports the safe usage of antibiotic-impregnated beads [4, 7, 10, 11, 15, 18, 20, 22]. This system is superior in terms of antibiotic concentration to suction-antibiotic irrigation systems, more convenient to the patient and not associated with the risks of superinfection and blood loss by drains [1].
The treatment of chronic osteomyelitis with sequential debridement and bead application followed by autogenous bone grafting was reported by Patzakis et al. [18]. There was no recurrence in 12 patients treated with septopal beads and 5 days of systemic antibiotic therapy.
In 63 patients with more than 2 years follow-up, Cierny et al. [4] reported one recurrence among 17 patients with Stage III osteomyelitis. These authors also explained the cause of recurrence as inadequate debridement. For dead space management, they used beads impregnated with cefazolin, moxalactam, cefotaxime, tobramycin, vancomycin or ticarcillin. The beads were removed at 2 to 4 weeks and replaced with cancellous bone grafts.
Staphylococcus aureus has been reported to be the most common pathogen in chronic osteomyelitis (65–70%), followed by Pseudomonas aeruginosa (20–37%). In our group of patients, S. aureus and MRSA was detected in 84% of all intraoperative wound cultures. Vancomycin and aminoglycosides are the most commonly used antibiotics for treating these because of their wide spectrum, heat stability, availability in powder form and low allergenicity [23]. Hanssen reported that vancomycin has fewer adverse effects on bone regeneration than cefazolin, aminoglycosides, rifampicin and quinolons [10]. Since gentamicin-impregnated commercial beads are not available in our country, vancomycin was used in all of the beads and rods in our study, and the results are not different from those reported elsewhere. We believe that a thorough debridement with high-speed burrs, based on the MR findings and Paprika sign, copious irrigation and vancomycin-loaded cement beads combined with culture-specific systemic antibiotherapy are the mainstays of treatment for C-M Type III chronic osteomyelitis. Vancomycin-impregnated beads can safely be used in most cases instead of tobramycin or gentamicin. This treatment protocol is likely to result in good long-term functional results and a lower recurrence rate.
References
- 1.Bansal VP, Harmit S (1988) Management of chronic osteomyelitis using an irrigation suction technique. Int Orthop 12:265–268 [DOI] [PubMed]
- 2.Beals RK, Bryant RE (2005) The treatment of chronic open osteomyelitis of the tibia in adults. Clin Orthop Relat Res 433:212–217 [DOI] [PubMed]
- 3.Cierny G 3rd (1999) Infected tibial non-unions (1981–1995). The evolution of change. Clin Orthop Relat Res 360:97–105 [DOI] [PubMed]
- 4.Cierny G 3rd, Mader JT, Penninck JJ (2003) A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res 414:7–24 [DOI] [PubMed]
- 5.Eckardt JJ, Wirganowicz PZ, Mar T (1994) An aggressive approach to the management of chronic osteomyelitis. Clin Orthop Relat Res 298:229–239 [PubMed]
- 6.Emara KM (2002) Hemi-corticotomy in the management of chronic osteomyelitis of the tibia. Int Orthop 26:310–313 [DOI] [PMC free article] [PubMed]
- 7.Evans RP, Nelson CL (1993) gentamicin-impregnated polymethylmethacrylate beads compared with systemic antibiotic therapy in the treatment of chronic osteomyelitis. Clin Orthop Relat Res 295:37–42 [PubMed]
- 8.Fitzgerald RH Jr (1983) Experimental osteomyelitis: description of a canine model and the role of depot administration of antibiotics in the prevention and treatment of sepsis. J Bone Joint Surg Am 67:371–380 [PubMed]
- 9.Gross T, Kaim AH, Regazzoni P, Widmer AF (2002) Current concepts in posttraumatic osteomyelitis: a diagnostic challenge with new imaging options. J Trauma 52:1210–1219 [DOI] [PubMed]
- 10.Hanssen AD (2005) Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin Orthop Relat Res 437:91–96 [DOI] [PubMed]
- 11.Klemm KW (1993) Antibiotic bead chains. Clin Orthop Relat Res 295:63–76 [PubMed]
- 12.Lazzarini L, Mader JT, Calhoun JH (2004) Osteomyelitis in long bones. J Bone Joint Surg Am 86-A:2305–2318 [DOI] [PubMed]
- 13.Lazzarini L, Lipsky BA, Mader JT (2005) Antibiotic treatment of osteomyelitis: what have we learned from 30 years of clinical trials? Int J Infect Dis 9:127–138 [DOI] [PubMed]
- 14.Mader JT, Cripps MW, Calhoun JH (1999) Adult posttraumatic osteomyelitis of the tibia. Clin Orthop Relat Res 360:14–21 [DOI] [PubMed]
- 15.Mader JT, Shirtliff ME, Bergquist SC, Calhoun J (1999) Antimicrobial treatment of chronic osteomyelitis. Clin Orthop Relat Res 360:47–65 [DOI] [PubMed]
- 16.Nelson CL (2004) The current status of material used for depot delivery of drugs. Clin Orthop Relat Res 427:72–78 [DOI] [PubMed]
- 17.Paley D, Herzenberg JE (2002) Intramedullary infections treated with antibiotic cement rods: preliminary results in nine cases. J Orthop Trauma 16:723–729 [DOI] [PubMed]
- 18.Patzakis MJ, Mazur K, Wilkins J, Sherman R, Holtom P (1993) Septopal beads and autogenous bone grafting for bone defects in patients with chronic osteomyelitis. Clin Orthop Relat Res 295:112–118 [PubMed]
- 19.Siegel HJ, Patzakis MJ, Holtom PD, Sherman R, Shepherd L (2000) Limb salvage for chronic tibial osteomyelitis: an outcomes study. J Trauma 48:484–489 [DOI] [PubMed]
- 20.Simpson AH, Dakin M, Latham JM (2001) The effect of the extent of surgical resection on infection-free survival. J Bone Joint Surg Br 83:403–407 [DOI] [PubMed]
- 21.Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ (2004) Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res 427:47–51 [DOI] [PubMed]
- 22.Tetsworth K, Cierny G 3rd (1999) Osteomyelitis debridement techniques. Clin Orthop Relat Res 360:87–96 [DOI] [PubMed]
- 23.Zalavras CG, Patzakis MJ, Holtom P (2004) Local Antibiotic Therapy in the Treatment of Open Fractures and Osteomyelitis. Clin Orthop Relat Res 427:86–93 [DOI] [PubMed]