Abstract
Venous thromboembolism (VTE) is an important complication of major orthopaedic surgery of the lower limbs. Fondaparinux, a synthetic pentasaccharide and highly selective inhibitor of activated Factor Xa, is the first in a new class of antithrombotic agents. To determine the optimal dose in Japanese patients, double-blind, placebo-controlled, dose-ranging studies of fondaparinux were conducted in patients undergoing total knee replacement (TKR) or total hip replacement (THR) surgery. Patients were randomly assigned to receive a once-daily subcutaneous injection of fondaparinux (0.75, 1.5, 2.5, or 3.0 mg) or placebo in Study 1 (TKR) and Study 2 (THR). In Study 1, the incidence of VTE was 65.3% in the placebo group and was 34.2%, 21.3%, 16.2%, and 9.5% in the groups receiving 0.75, 1.5, 2.5, and 3.0 mg fondaparinux respectively. In Study 2, the incidence of VTE was 33.8% in the placebo group and was 24.2%, 4.6%, 7.4%, and 14.4% in the 0.75, 1.5, 2.5, and 3.0 mg fondaparinux groups respectively. Dose–response effects were observed in both studies; however, no statistically significant differences in major bleeding events were found among any groups. Fondaparinux proved to be a potent anticoagulant with a favourable benefit-to-risk ratio in the prevention of VTE in these study patients.
Résumé
Les complications thromboemboliques sont nombreuses dans la plupart des interventions de chirurgie orthopédique au niveau des membres inférieurs. Le fondaparinux (pentas saccharide synthétique) est un élément important parmi tous les agents anti-thrombotiques. De façon à déterminer la dose optimale de ce produit, une étude en double aveugle avec placebo a été conduite chez des patients devant bénéficier d’une prothèse totale du genou ou d’une prothèse totale de hanche. Les patients ont été randomisés de façon à recevoir une fois par jour une injection sous cutanée de fondaparinux (0.75, 1.5, 2.5, ou 3 mg) ou de placebo. L’incidence de la thrombose veineuse a été de 65.3% dans le groupe placebo et de 34.2%, 21.3%, 16.2% et 9.5% dans les groupes recevant respectivement 0.75, 1.5, 2.5 et 3 mg de fondaparinux, pour le groupe prothèse du genou. Pour le groupe prothèse de hanche l’incidence des complications thromboemboliques a été de 33.8% dans le groupe placebo et a été respectivement de 24.2%, 4.6%, 7.4% et 14.4% dans les groupes ayant reçu 0.75, 1.5, 2.5 et 3 mg de fondaparinux. Il n’y a pas de différence significatives en terme de saignement, dans chaque groupe. le fondaparinux est un anti-coagulant actif avec un bénéfice/risque important dans la prévention des thromboses veineuses et des accidents thromboemboliques dans cette étude de patients.
Introduction
Fondaparinux is the first synthetic, selective Factor Xa inhibitor. Factor Xa is an important coagulation factor located at the junction of the extrinsic and intrinsic coagulation pathways [11]. Consequently, inhibition of Factor Xa results in effective inhibition of the coagulation cascade and inhibition of thrombin generation. Unlike unfractionated heparin (UFH) and low molecular weight heparin (LMWH), fondaparinux is a single chemical entity (1728 Dalton) comprising five saccharides designed specifically to bind to antithrombin [8]. In experimental animal models, fondaparinux was associated with less bleeding than was UFH, at an equivalent antithrombotic concentration [8]. In humans, a therapeutic dose of fondaparinux did not prolong bleeding time [2].
A dose-ranging study in total hip replacement (THR) [16] demonstrated a statistically significant dose-response for the prevention of venous thromboembolism (VTE) in the range from 0.75 mg to 8.0 mg. Moreover, the results suggested that fondaparinux had the potential to improve significantly the risk-benefit ratio for VTE prophylaxis compared with LMWH. Based on the results, a 2.5 mg, once-daily dosage of fondaparinux was selected for the following four phase 3 studies. And these studies [1, 3, 10, 17] demonstrated that a once-daily fondaparinux 2.5 mg significantly improved the risk–benefit ratio for VTE prophylaxis in major orthopaedic surgery of the lower limbs. Eriksson BI et al. [4] reported that 4-week fondaparinux treatment was superior to 1-week fondaparinux in VTE prophylaxis for the patients with hip fracture surgery.
In the United States and Europe, a once-daily subcutaneous dose of 2.5 mg fondaparinux is indicated and used as VTE prophylaxis in fracture surgery, hip/knee replacement surgery and abdominal surgery [12, 15]. In the Seventh American College of Chest Physicians (ACCP) Guidelines on Prevention of VTE [7], fondaparinux, along with LMWH and vitamin K antagonists, was recommended with a Grade 1A rating for VTE prophylaxis in TKR and THR. Fondaparinux were the only anticoagulant recommended with a Grade 1A rating for hip-fracture surgery.
In Japan, UFH and warfarin are indicated for VTE prophylaxis, but there is no randomised clinical trial (RCT) in Japanese patients. LMWH has no indication for VTE prophylaxis. Therefore, no established active control is available in Japan.
These studies was conducted to compare the efficacy and safety of fondaparinux with a placebo, and to evaluate the dose-response relationship between 0.75 mg, 1.5 mg, 2.5 mg, and 3.0 mg of fondaparinux and the incidence of VTE, in TKR or THR surgery.
Methods
Patients
Patients of either gender were eligible if their age was 20 years or greater, and they were scheduled for TKR or THR surgery or revision surgery for TKR or THR. Exclusion criteria were: (a) active, clinically significant bleeding, (b) bleeding tendency/disorder (e.g., ulcer of the digestive tract, diverticulitis of the digestive tract, colitis, acute bacterial endocarditis, severe hypertension, or severe diabetes), (c) severe hepatic disorder, (d) hypersensitivity to UFH or LMWH, (e) requirement of an indwelling intrathecal or epidural catheter during the treatment period (after the first dose of test drug, until the completion of venography), or (f) brain, spine, or ophthalmologic surgery within the 3 months preceding enrollment. Patients with: (g) a body weight less than 40 kg (88 lb), or (h) severe renal disorder (serum creatinine concentration >2.0 mg/dL [180 μmol/L]) were also excluded.
The use of UFH, LMWH, heparinoids, antithrombin agents (argatroban), oral anticoagulants (warfarin), fibrinolytic agents and dextrans was prohibited, beginning 1 week before the first dose of study drug and study period. Nonsteroidal anti-inflammatory drugs (NSAIDs) and antiplatelet medications were also strongly discouraged during the treatment period, but were allowed, if necessary, in a condition of unchanged regimen. During the study, the use of intermittent pneumatic compression or a venous foot pump was prohibited during surgery, and continuous spinal and epidural anaesthesia (intrathecal or epidural catheterisation) were prohibited, beginning 2 hours before the first dose of study drug and study period.
Study design
There are two studies described in this paper, Study 1 for TKR and Study 2 for THR, both of which are multicentre, randomised, double-blind, placebo-controlled, parallel-group, dose–response studies of subcutaneous fondaparinux.
The studies were conducted according to the provisions of the revised Declaration of Helsinki and the guidelines for Good Clinical Practice. The study protocols were approved by the institutional review board (IRB) at each centre. Written informed consent was obtained from each patient before enrollment in the trial. A Central Independent Adjudication Committee for Efficacy (CIACE) evaluated diagnostic images in a blind manner for the incidence of VTE. A Central Independent Adjudication Committee for Safety (CIACS) evaluated all reported bleeding events and adverse events (AEs), also in a blind manner.
Outcome measures
The primary efficacy outcome was assessed by the rate of VTE [defined as deep vein thrombosis (DVT), pulmonary embolism (PE), or both] up to day 11. Patients were examined for deep-vein thrombosis by systematic bilateral ascending venography of the legs between day 11 and day 17, but no more than 2 days after the last injection of study drug, or earlier if thrombosis was clinically suspected. Symptomatic PE was confirmed by a lung scan indicating a high probability of PE, pulmonary angiography, or helical computed tomography, or at autopsy.
The primary safety outcome was the incidence of major bleeding, which included fatal bleeding; bleeding that was retroperitoneal, intracranial, or intraspinal or that involved any other critical organ; bleeding leading to reoperation; and overt bleeding with a bleeding index of 2 or more. The bleeding index was calculated as the number of units of packed red cells or whole blood transfused plus the haemoglobin values before the bleeding episode minus the haemoglobin values after the episode (in grams per decilitre).
Treatment and regimen
Patients were assigned to receive a once-daily subcutaneous injection of fondaparinux (0.75 mg, 1.5 mg, 2.5 mg, or 3.0 mg) or placebo. Treatment was scheduled from Day 2 to Days 11–15 (at least 10 days, with the day of surgery defined as Day 1).
The first dose of study drug was administered at 24 ± 2 h after surgery, before 11:00 pm on Day 2; subsequent doses were administered at 9:00 am ± 2 h, from Days 3 to 15. The first dose on Day 2 and the second dose on Day 3 were at least 12 hours apart.
Disposable pre-filled syringes containing 0.75 mg, 1.5 mg, 2.5 mg, or 3.0 mg of fondaparinux or placebo were supplied by Sanofi-Winthrop Industrie (Notre Dame de Bondeville, France). Fondaparinux and placebo were provided as isotonic solutions in 0.25 ml, and placebo was isotonic sodium chloride. All pre-filled syringes were indistinguishable from one another.
Results
Disposition of patients
Study 1
Study 1 was conducted from October 2001 to August 2003 at 56 centres in Japan. A total of 432 patients were enrolled and randomised. Six of the 432 patients did not receive any study drug and were excluded from further analyses, with 426 patients remaining in the “all treated patients” (ATP) population (339 in the fondaparinux groups and 87 in the placebo group). A total of 29 (6.8%) withdrew. There were no statistically significant differences in values for demographic variables (Table 1) among the five treatment groups. The physical prophylaxis during the study is summarised in Table 2.
Table 1.
Summary of demographic characteristics: all-treated-patients population
| n (%) | ||||||
|---|---|---|---|---|---|---|
| Study 1 (TKR) | ||||||
| Parameter | Placebo | Fondaparinux | ||||
| 0.75 mg | 1.5 mg | 2.5 mg | 3.0 mg | Total | ||
| n = 87 | n = 86 | n = 85 | n = 84 | n = 84 | n = 426 | |
| Gender | ||||||
| Female | 72 (82.8) | 71 (82.6) | 67 (78.8) | 67 (79.8) | 74 (88.1) | 351 (82.4) |
| Age, y ± (SD) | ||||||
| 70.4 ± 7.9 | 71.4 ± 8.7 | 70.5 ± 8.0 | 71.2 ± 7.8 | 71.5 ± 7.6 | 71.0 ± 8.0 | |
| Weight, kg ± (SD) | ||||||
| 58.94 ± 9.80 | 57.87 ± 10.71 | 59.99 ± 10.16 | 59.14 ± 9.88 | 59.30 ± 8.43 | 59.05 ± 9.81 | |
| Height, cm (SD) | ||||||
| 150.51 ± 7.59 | 150.91 ± 7.55 | 151.45 ± 6.96 | 150.15 ± 6.85 | 150.04 ± 6.45 | 150.61 ± 7.09 | |
| BMI, kg/m2 | ||||||
| <30 | 79 (90.8) | 73 (84.9) | 70 (82.4) | 69 (82.1) | 71 (84.5) | 362 (85.0) |
| >=30 | 8 (9.2) | 13 (15.1) | 15 (17.6) | 15 (17.9) | 13 (15.5) | 64 (15.0) |
| Baseline creatinine clearance, mL/min | ||||||
| <30 | 2 (2.3) | 1 (1.2) | 1 (1.2) | 1 (1.2) | 0 | 5 (1.2) |
| 30 – 50 | 7 (8.0) | 10 (11.6) | 12 (14.1) | 12 (14.3) | 10 (12.0) | 51 (12.0) |
| 50 – 80 | 44 (50.6) | 44 (51.2) | 43 (50.6) | 37(44.0) | 41 (49.4) | 209 (49.2) |
| >=80 | 34 (39.1) | 31 (36.0) | 29 (34.1) | 34 (40.5) | 32 (38.6) | 160 (37.6) |
| Missing | 0 | 0 | 0 | 0 | 1 | 1 |
| Type of surgery | ||||||
| Primary | 82 (94.3) | 85 (98.8) | 84 (98.8) | 84 (100) | 80 (95.2) | 415 (97.4) |
| Revision | 5 (5.7) | 1 (1.2) | 1 (1.2) | 0 | 4 (4.8) | 11 (2.6) |
| Study 2 (THR) | ||||||
| Parameter | Placebo | Fondaparinux | ||||
| 0.75 mg | 1.5 mg | 2.5 mg | 3.0 mg | Total | ||
| n = 82 | n = 80 | n = 80 | n = 81 | n = 83 | n = 406 | |
| Gender | ||||||
| Female | 64 (78.0) | 69 (86.3) | 60 (75.0) | 74 (91.4) | 66 (79.5) | 333 (82.0) |
| Age, y ± (SD) | ||||||
| 62.3 ± 12.4 | 60.8 ± 9.8 | 60.9 ± 10.1 | 61.5 ± 10.8 | 62.7 ± 11.4 | 61.6 ± 10.9 | |
| Weight, kg ± (SD) | ||||||
| 56.31 ± 9.40 | 55.81 ± 9.61 | 60.21 ± 9.73 | 54.19 ± 8.61 | 56.20 ± 11.28 | 56.54 ± 9.92 | |
| Height, cm (SD) | ||||||
| 153.20 ± 8.24 | 152.38 ± 7.55 | 154.58 ± 7.92 | 150.96 ± 6.88 | 152.35 ± 7.00 | 152.69 ± 7.59 | |
| BMI, kg/m2 | ||||||
| <30 | 77 (93.9) | 73 (91.3) | 73 (91.3) | 79 (97.5) | 78 (94.0) | 380 (93.6) |
| >=30 | 5 (6.1) | 7 (8.8) | 7 (8.8) | 2 (2.5) | 5 (6.0) | 26 (6.4) |
| Baseline creatinine clearance, mL/min | ||||||
| 30 – 50 | 7 (8.9) | 6 (7.6) | 1 (1.3) | 5 (6.2) | 5 (6.0) | 24 (6.0) |
| 50 – 80 | 30 (38.0) | 28 (35.4) | 27 (34.6) | 36 (44.4) | 31 (37.3) | 152 (38.0) |
| >=80 | 42 (53.2) | 45 (57.0) | 50 (64.1) | 40 (49.4) | 47 (56.6) | 224 (56.0) |
| Missing | 3 | 1 | 2 | 0 | 0 | 6 |
| Type of surgery | ||||||
| Primary | 76 (92.7) | 72 (90.0) | 76 (95.0) | 74 (91.4) | 79 (95.2) | 377 (92.9) |
| Revision | 6 (7.3) | 8 (10.0) | 4 (5.0) | 7 (8.6) | 4 (4.8) | 29 (7.1) |
BMI: body mass index; THR: total hip replacement; TKR: total knee replacement
Table 2.
Summary of patients who used physical methods for DVT prophylaxis from surgery to end of treatment: all-treated-patients population
| n (%) | ||||||
|---|---|---|---|---|---|---|
| Study 1 (TKR) | ||||||
| Parameter | Placebo | Fondaparinux | ||||
| 0.75 mg | 1.5 mg | 2.5 mg | 3.0 mg | Total | ||
| n = 87 | n = 86 | n = 85 | n = 84 | n = 84 | n = 426 | |
| Elastic stocking/bandage (%) | ||||||
| No elastic stocking/bandage | 26 (29.9) | 28 (32.6) | 33 (38.8) | 26 (31.0) | 31 (36.9) | 144 (33.8) |
| Used elastic stocking/bandage 1–10 days | 33 (37.9) | 36 (41.9) | 31 (36.5) | 35 (41.7) | 34 (40.5) | 169 (39.7) |
| Used elastic stocking/bandage 11 days or more | 28 (32.2) | 22 (25.6) | 21 (24.7) | 23 (27.4) | 19 (22.6) | 113 (26.5) |
| Study 2 (THR) | ||||||
| Parameter | Placebo | Fondaparinux | ||||
| 0.75 mg | 1.5 mg | 2.5 mg | 3.0 mg | Total | ||
| n = 82 | n = 80 | n = 80 | n = 81 | n = 83 | n = 406 | |
| Elastic stocking/bandage (%) | ||||||
| No elastic stocking/bandage | 40 (48.8) | 40 (50.0) | 40 (50.0) | 41 (50.6) | 38 (45.8) | 199 (49.0) |
| Used elastic stocking/bandage 1–10 days | 20 (24.4) | 24 (30.0) | 21 (26.3) | 25 (30.9) | 29 (34.9) | 119 (29.3) |
| Used elastic stocking/bandage 11 days or more | 22 (26.8) | 16 (20.0) | 19 (23.8) | 15 (18.5) | 16 (19.3) | 88 (21.7) |
DVT: deep vein thrombosis; THR: total hip replacement; TKR: total knee replacement
Study 2
Study 2 was conducted from October 2001 to June 2003 at 57 centers in Japan. A total of 411 patients were enrolled and randomised. Five out of 411 patients did not receive any study drug and were excluded from further analyses, with 406 patients remaining in the ATP population (324 in the fondaparinux groups and 82 in the placebo group). A total of 25 (6.2%) withdrew. There were no statistically significant differences in the values for demographic variables (Table 1) among the five treatment groups. The use of physical prophylaxis is summarised in Table 2.
Efficacy
Study 1 (TKR)
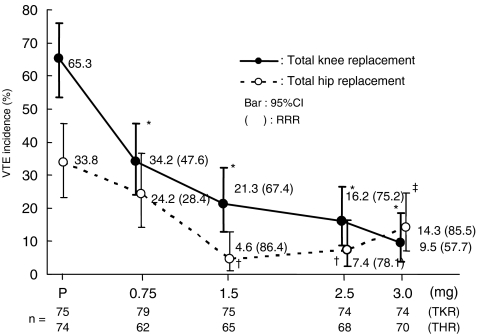
In the intent to treat (ITT) population, 65.3%, 34.2%, 21.3%, 16.2% and 9.5% of the patients showed VTE in the groups given placebo, 0.75 mg, 1.5 mg, 2.5 mg, and 3.0 mg of fondaparinux respectively. The Cochran-Armitage trend test demonstrated a statistically significant difference (P < 0.001) in VTE incidence by fondaparinux, compared with placebo (Fig. 1). VTE incidence in all groups receiving fondaparinux was significantly lower (P < 0.001) than in the group receiving placebo, by Fisher’s exact probability tests. The calculated relative risk reductions (RRR) of VTE with 0.75 mg, 1.5 mg, 2.5 mg and 3.0 mg of fondaparinux, compared with placebo, were 47.6%, 67.4%, 75.2%, and 85.5% respectively.
Fig. 1.
Venous thromboembolism: incidence in all groups. VTE: venous thromboembolism; TKR: total knee replacement; THR: total hip replacement; RRR: relative risk reduction * P < 0.001, †P < 0.01, ‡P = 0.007 (Fisher’s exact probability test)
Study 2 (THR)
In the ITT population, 33.8%, 24.2%, 4.6%, 7.4% and 14.3% of the patients showed VTE in the groups receiving placebo, 0.75 mg, 1.5 mg, 2.5 mg, and 3.0 mg of fondaparinux respectively. The Cochran-Armitage trend test demonstrated a statistically significant reduction (P < 0.001) in VTE incidence by fondaparinux, compared with placebo (Fig. 1). The groups receiving 1.5 mg, 2.5 mg, or 3.0 mg fondaparinux were significantly lower (P < 0.01, P < 0.01 and P = 0.007 respectively) from the placebo group by Fisher’s exact probability tests. RRR of VTE with 0.75 mg, 1.5 mg, 2.5 mg and 3.0 mg of fondaparinux were 28.4%, 86.4%, 78.1%, and 57.7% respectively, compared with placebo.
Safety evaluation
The incidences of major and minor bleeding are presented Table 3. In the studies, major bleeding during the treatment period was the primary safety endpoint.
Table 3.
The proportion of patients with bleeding by treatment group: all-treated-patients population, Study 1 (TKR)
| Study 1 (TKR) | |||||
|---|---|---|---|---|---|
| Types of bleeding (%) | Placebo | Fondaparinux | |||
| 0.75 mg | 1.5 mg | 2.5 mg | 3.0 mg | ||
| n = 87 | n = 86 | n = 85 | n = 84 | n = 84 | |
| Major bleeding | 1 (1.1) | 0 | 0 | 1 (1.2) | 1 (1.2) |
| [0.0 – 6.2] | [0.0 – 4.2] | [0.0 – 4.2] | [0.0 – 6.5] | [0.0 – 6.5] | |
| Minor bleeding only | 3 (3.4) | 0 | 5 (5.9) | 2 (2.4) | 3 (3.6) |
| [0.7 – 9.7] | [0.0 – 4.2] | [1.9 – 13.2] | [0.3 – 8.3] | [0.7 – 10.1] | |
| Any bleeding | 4 (4.6) | 0 | 5 (5.9) | 3 (3.6) | 4 (4.8) |
| [1.3 – 11.4] | [0.0 – 4.2] | [1.9 – 13.2] | [0.7 – 10.1] | [1.3 – 11.7] | |
| Any bleeding Cochran-Armitage (P)a | 0.57 | ||||
| Study 2(THR) | |||||
| Types of bleeding (%) | Placebo | Fondaparinux | |||
| 0.75 mg | 1.5 mg | 2.5 mg | 3.0 mg | ||
| n = 82 | n = 80 | n = 80 | n = 81 | n = 83 | |
| Major Bleeding | 0 | 1 (1.3) | 0 | 2 (2.5) | 0 |
| [0.0 – 4.4] | [0.0 – 6.8] | [0.0 – 4.5] | [0.3 – 8.6] | [0.0 – 4.3] | |
| Minor bleeding only | 0 | 3 (3.8) | 2 (2.5) | 4 (4.9) | 0 |
| [0.0 – 4.4] | [0.8 – 10.6] | [0.3 – 8.7] | [1.4 – 12.2] | [0.0 – 4.3] | |
| Any bleeding | 0 | 4 (5.0) | 2 (2.5) | 6 (7.4)* | 0 |
| [0.0 – 4.4] | [1.4 12.3] | [0.3 – 8.7] | [2.8 – 15.4] | [0.0 – 4.3] | |
| Any bleeding Cochran-Armitage (P)a | 0.54 | ||||
n (%), [95% CI]
A significant dose-response relationship in bleeding was not observed with the 0.75 mg to 3.0 mg fondaparinux dose range in either of the studies.
a Comparisons across all 5 treatment populations, using the values of the doses as score (0, 0.75, 1.5, 2.5, and 3.0)
* P = 0.013 vs placebo group
THR: total hip replacement; TKR: total knee replacement
In Study 1 (TKR), the incidence of major bleeding was 1.1% with placebo and 0%, 0%, 1.2%, and 1.2% with 0.75 mg, 1.5 mg, 2.5 mg, and 3.0 mg of fondaparinux respectively. The incidences of major or minor bleeding among the treatment groups were not statistically significant; there was no fatal bleeding, bleeding in a critical organ, or bleeding leading to re-operation. All of the patients who experienced major bleeding received >2 units of blood. Two patients treated with fondaparinux had bleeding at the surgical site. One patient in the placebo group had major bleeding in the gastrointestinal tract.
There were no deaths during the study; three severe AEs were reported in two patients. One patient (receiving fondaparinux 3.0 mg) experienced skin necrosis that was not considered by the investigator to be related to the study drug; another patient (receiving placebo) developed a gastric ulcer and had a gastrointestinal hemorrhage. Both patients recovered without sequelae from these events.
There was no statistically significant difference in drug-related AEs among treatment groups.
In Study 2 (THR), there were no statistically significant differences in major or minor bleeding events between the fondaparinux groups and the placebo group. The incidences of major and minor bleeding events by fondaparinux were not dose-dependent. Three cases of major bleeding included a reduction in haemoglobin of >2 g/dL in one patient (receiving fondaparinux 2.5 mg) and transfusion of more than two units of blood in two patients (receiving fondaparinux 0.75 mg, 2.5 mg). No fatal bleeding occurred. Furthermore, although clinically abnormal blood loss occurred in more patients in the 2.5 mg fondaparinux group, all abnormal blood loss in this group was considered to be associated with surgery and not related to fondaparinux treatment.
There were no deaths during the study; however, three severe AEs were reported in two patients. One patient (0.75 mg fondaparinux) had hepatic dysfunction on Day 4 that was not related to test drug. The second patient (0.75 mg fondaparinux) experienced a cerebral infarction and supraventricular tachycardia on Day 5 but recovered; both events were considered possibly related to the test drug.
Discussion
In both studies, fondaparinux groups demonstrated significant dose-dependent effect in VTE incidence. 1.5 mg and 2.5 mg fondaparinux respectively reduced risk of VTE by 67.4% and 75.2% in the THR study, and that of VTE by 86.4% and 78.1% in the TKR study, compared with placebo. Fondaparinux also showed a good safety profile, in terms of bleeding complication, and the incidences of bleeding events by fondaparinux were not dose-dependent in both the TKR and THR studies.
In the United States, VTE is recognised as a silent, life-threatening disease and VTE prophylaxis is considered critical in a variety of medical settings, not only in postsurgical patients but also in acutely ill medical patients [7]. It is estimated that there are approximately 2 million cases of DVT and 600,000 cases of PE—including 60,000 fatal cases—per year in the United States [9]. In contrast, in Japan, 3,492 PE cases were estimated in 1996, based on surveillance by the Japanese Government [13, 14]. Because of the reported lower incidence of VTE in Japan, the importance of VTE prophylaxis has not been well-recognised by Japanese physicians.
Recently, Fujita et al. reported that, similar to Western data, VTE incidences of 48.6% and 22.6% followed TKR and THR surgery respectively, in Japanese patients [5].
According to the Sixth ACCP Guidelines [6], overall RRR of VTE following TKR surgery was 33% with low-dose UFH (two studies) and 52% with LMWH (13 studies), and the overall RRR of VTE following THR surgery was 45% with low dose UFH (11 studies) and 70% with LMWH (30 studies).
In Study 2 for THR, the incidence of VTE in both the 2.5 mg and 3.0 mg fondaparinux groups was slightly higher than that of the 1.5 mg group. However, there were no statistically significant differences among these groups; therefore, these differences could be observed by chance. There were no differences in demographic or VTE risk factors among the groups; however, there were more patients with ischaemic heart disease or diabetes in the 3.0 mg group, and it is speculated that these disorders may affect the efficacy of anticoagulant therapy. The incidences of VTE with 1.5 mg to 2.5 mg of fondaparinux in Study 2 for THR are similar to those in other published reports [1, 3, 10, 17].
The efficacies of these studies were completely equal to that in the United States and Europe. Major bleeding associated with fondaparinux was reported in 2.1% (11/517) of patients undergoing TKR [1], and 1.8% [17] and 4.1% [10] in THR; however, fatal bleeding or critical organ bleeding was not reported in Western studies. In the present studies, major bleeding occurred in one patient (0.6%) in the placebo group, one patient (0.6%) at 0.75 mg, 3 (1.8%) at 2.5 mg, and one patient (0.6%) at 3.0 mg of fondaparinux. The incidences of major bleeding in Japanese studies were somewhat lower than in the United States and Europe. It is considered that lower incidence of major bleeding could be due to the initial administration fondaparinux 24 hours after operation. Compared with overseas data, the efficacy and safety findings in this study support a once-daily dose of 2.5 mg fondaparinux is favorable for VTE prophylaxis in Japanese patients undergoing TKR or THR.
Finally, our study demonstrated that fondaparinux effectively prevents VTE without increasing the risk of bleeding or other AEs in patients undergoing TKR or THR. Fondaparinux could be a promising option for the prevention of VTE major orthopedic surgery of the lower limbs.
Conclusion
The incidence of VTE in Japanese TKR and THR patients are similar to Western data.
Once-daily, subcutaneous doses of 1.5 mg to 2.5 mg fondaparinux have a favourable risk (bleeding and other AEs) to benefit (VTE prevention) ratio in these patients.
Fondaparinux, the first in a new class of anticoagulants, could be one of the best options for managing the risk of VTE in patients at major orthopaedic surgery of the lower limbs.
In order to define optimal daily dose of fondaparinux for Japanese patients, further clinical study is needed.
Acknowledgements
These studies were supported by a grant from GlaxoSmithKline, Sanofi-synthelabo and NV Organon.
Members of Steering Committee
Takahiro Ochi (Chairman), National Sagamihara Hospital, Sagamihara
Takeo Matsuno, Orthopedic Surgery, Asahikawa Medical College, Asahikawa
Kozo Nakamura, Orthopedic Surgery, Tokyo University, School of Medicine, Tokyo
Tomihisa Koshino, International University of Health and Welfare, Tokyo
Hisashi Iwata, Center for Rheumatic Diseases and Artificial Joint, Nagoya Kyoritsu Hospital, Nagoya
Hideki Yoshikawa, Orthopedic Surgery, Osaka University, School of Medicine, Osaka
Takeshi Fuji, Orthopedic Surgery, Osaka Koseinenkin Hospital, Osaka
Toru Sato, Orthopedic Surgery, Okayama University, School of Medicine, Okayama
Sumiki Yamamoto, Centre for Rheumatic Diseases, Matsuyama Red Cross Hospital, Matsuyama
Takehiko Torisu, Orthopedic Surgery, Ohita Medical University, School of Medicine, Ohita
The members of the Central Independent Adjudication Committee for Efficacy (CIACE) of Study 1 & 2:
Satoru Fujita, Orthopaedic Surgery, Dai-ichi Hospital Medical Corporation of Showakai, Takarazuka
Hironobu Nakamura, Osaka University, Graduate School of Medicine Faculty of Medicine, Course of Advanced Medicine; Medical Robotics and Image Science, Osaka
Saki Nakata, Osaka University, Graduate School of Medicine Faculty of Medicine, Course of Advanced Medicine; Medical Robotics and Image Science, Osaka
Kenji Nakamura, Department of Radiology, Osaka City University Medical School, Osaka
The members of the central independent adjudication committee for safety (CIACS) of Study 1 & 2:
Hisaichi Fujii, Department of Transfusion and Cell Processing, Tokyo Women’s Medical University, School of Medicine, Tokyo
Ikuro Maruyama, Department of Laboratory Medicine; Faculty of Medicine, Kagoshima University, Kagoshima
Mitsuyoshi Nakashima, Hamamatsu University School of Medicine, Hamamatsu
Taisuke Tomatsu, Department of Rheumatology; Tokyo Women’s Medical University, School of Medicine, Tokyo
The principal investigators for Study 1 (TKR):
Yukiyoshi Oishi, Toyohashi Municipal Hospital, Toyohashi
Tatsunori Maeda, Asahikawa Medical College Hospital, Asahikawa
Hiroshi Tanaka, Yamaguchi University Hospital, Ube
Fujio Higuchi, Kurume University Medical Center, Kurume
Toshihisa Kanamono, Nagano Red Cross Hospital, Nagano
Takaharu Nabeshima, Toneyama National Hospital, Toyonaka
Masaaki Kakiuchi, Osaka Police Hospital, Osaka
Takeshi Fuji, Osaka Koseinenkin Hospital, Osaka
Yoshiaki Yanase, Kitano Hospital, Osaka
Takashi Soejima and Takahiro Ohkawa, Kurume University Hospital, Kurume
Hideto Machida, Kanto Rosai Hospital, Kawasaki
Hideji Kura, Sapporo Medical University Hospital, Sapporo
Hiromi Oda, The University of Tokyo Hospital, Tokyo
Masafumi Ishizuki, Tsuchiura Kyodo General Hospital, Tsuchiura
Makoto Kawakubo, Tokyo Dental College Ichikawa General Hospital, Ichikawa
Shoji Kumaki, Hokushin General Hospital, Nakano
Naoki Kodama, Gifu Prefectural Gero-Onsen Hospital, Gero
Masashi Kataoka, Oita Medical University Hospital, Hasama
Toshihiko Hara, Kyushu-Rosai Hospital, Kitakyushu
Shin-ichi Katsuo, Fukui General Hospital, Fukui
Naoto Mitsuki and Renzo Okamoto, Yokohama City University Medical Center, Yokohama
Tomoyuki Saito, Yokohama City University Hospital, Yokohama
Shigeru Harada, Tsukuba Rokujinkai Foundation Tsukuba Gakuen Hospital, Tsukuba
Masanori Shimode, Kanto Medical Center NTT EC, Tokyo
Yoshiki Okuda, Shakaihoken Kobe Central Hospital, Kobe
Hirofumi Kuroki, International Medical Center of Japan, Tokyo
Makoto Takasu, Aizu Chuo Hospital, Aizuwakamatsu
Tadashi Tanaka, Kimitsu Chuo Hospital, Kisarazu
Takahisa Yasoda and Naoto Mitsuki, Fujisawa Municipal Hospital, Fujisawa
Yukio Yoshida, Higashi Municipal Hospital of Nagoya, Nagoya
Kazuhiro Yamaguchi and Shinichiro Hara, Nagasaki Rosai Hospital, Sasebo
Toshihito Mori, National Sagamihara Hospital, Sagamihara
Kazuo Kaneko, Juntendo University Juntendo Izunagaoka Hospital, Izunagaoka
Sampei Nakata and Sumiki Yamamoto, Matsuyama Red Cross Hospital, Matsuyama
Toshikazu Tanaka, Tsukuba Memorial Hospital, Tsukuba
Taiki Kanno, Eniwa Hospital, Eniwa
Katsumi Chiba, Medical Corporation Fukushima Kouseikai Fukushima Daiichi Hospital, Fukushima
Shoichi Kushitani, Rinku General Medical Center, Izumisano
Masayoshi Ohga, Hiroshima Red Cross Hospital & Atomic-Bomb Survivors Hospital, Hiroshima
Toshiyuki Tsurumoto, Nagasaki University Hospital, Nagasaki
Etsuo Chosa, Miyazaki Medical College Hospital, Kiyotake
Chiaki Tanaka, Kyoto Municipal Hospital, Kyoto
Sen-eki Kobayashi, Shinshu University Hospital, Matsumoto
Shigeo Sano, Sanraku Hospital, Tokyo
Takashi Ohya, Obihiro Kosei Hospital, Obihiro
Kazunori Ohno, Teine Keijinkai Hospital, Sapporo
Katsuhiro Shimada, National Murayama Hospital, Musashi-murayama
Yoji Mikami, Japan Labour Health and Welfare Organization Yokohama Rosai Hospital, Yokohama
Akira Arakaki, Tomishiro Chuo Hospital, Tomigusuku
Yoshimitsu Hoshikawa, St Luke’s International Hospital, Tokyo
Shuji Okinaga, Tokyo Teishin Hospital, Tokyo
Syojiro Kato and Makoto Kurimura, Jinseisha Edogawa Hospital, Tokyo
Koji Suzuki, Hokkaido Orthopedic Memorial Hospital, Sapporo
Osamu Sugawara, Kitami Red Cross Hospital, Kitam
Kazuyoshi Hirose, Nagoya Kyoritsu Hospital, Nagoya-shi
Keiju Fujiwara, Osaka Prefectural General Hospital, Osaka
Takehiro Takebayashi, Sapporo Insurance General Hospital, Sapporo
Kazumasa Terada, National Kyushu Medical Center, Fukuoka
Hideya Kawamura, Kyushu Kousei Nenkin Hospital, Kitakyushu
The principal investigators for Study 2 (THR):
Yukiyoshi Oishi, Toyohashi Municipal Hospital, Toyohashi
Toru Sato, Okayama University Hospital, Okayama
Tadashi Teranishi, Asahikawa Medical College Hospital, Asahikawa
Hiroshi Tanaka, Yamaguchi University Hospital, Ube
Fujio Higuchi, Kurume University Medical Center, Kurume
Toshihisa Kanamono, Nagano Red Cross Hospital, Nagano
Takaharu Nabeshima, Toneyama National Hospital, Toyonaka
Masaaki Kakiuchi, Osaka Police Hospital, Osaka
Takeshi Fuji, Osaka Koseinenkin Hospital, Osaka
Yoshiaki Yanase, Kitano Hospital, Osaka
Takahiro Ohkawa and Masaru Kumagai, Kurume University Hospital, Kurume
Hideto Machida, Japan Labour Health and Welfare Organization Kanto Rosai Hospital, Kawasaki
Satoshi Nagoya, Sapporo Medical University Hospital, Sapporo
Masafumi Ishizuki, Tsuchiura Kyodo General Hospital, Tsuchiura
Masaaki Matsubara and Tetsuya Jinno, Tokyo Medical and Dental University Medical Hospital, Tokyo
Shoji Kumaki, Hokushin General Hospital, Nagano
Naoki Kodama, Gifu Prefectural Gero-Onsen Hospital, Gero
Toshihiko Hara, Kyushu-Rosai Hospital, Kitakyusyu
Shin-ichi Katsuo, Fukui General Hospital, Fukui
Kazuhiro Mizutani, Toho University Ohashi Hospital, Tokyo
Renzo Okamoto and Naoto Mitsuki, Yokohama City University Medical Center, Yokohama
Tomoyuki Saito, Yokohama City University Hospital, Yokohama
Shigeru Harada, Tsukuba Rokujinkai Foundation, Tsukuba Gakuen Hospital, Tsukuba
Masanori Shimode, Kanto Medical Center NTT EC, Tokyo
Yoshiki Okuda, Shakaihoken Kobe Central Hospital, Kobe
Hirofumi Kuroki, International Medical Center of Japan, Tokyo
Makoto Takasu, Aizu Chuo Hospital, Aizuwakamatsu
Tadashi Tanaka, Kimitsu Chuo Hospital, Kisarazu
Haruo Ito, Tokyo Koseinenkin Hospital, Tokyo
Naoto Mitsuki and Takahisa Yasoda, Fujisawa Municipal Hospital, Fujisawa
Yukio Yoshida, Higashi Municipal Hospital of Nagoya, Nagoya
Shinichiro Hara and Kazuhiro Yamaguchi, Nagasaki Rosai Hospital, Sasebo
Toshihito Mori, National Sagamihara Hospital, Fujisawa
Kazuo Kaneko, Juntendo University Juntendo Izunagaoka Hospital, Izunagaoka
Sumiki Yamamoto and Sampei Nakata, Matsuyama Red Cross Hospital, Matsuyama
Toshikazu Tanaka, Tsukuba Memorial Hospital, Tsukuba
Motoyuki Shundo, Eniwa Hospital, Eniwa
Katsumi Chiba, Medical Corporation Fukushima Kouseikai Fukushima Daiichi Hospital, Fukushima
Shoichi Kushitani, Rinku General Medical Center, Izumisano
Masayoshi Ohga, Hiroshima Red Cross Hospital & Atomic-bomb Survivors Hospital, Hiroshima
Hirosi Enomoto, Nagasaki University Hospital, Nagasaki
Hiroshi Usui, National Tokyo Medical Center, Tokyo
Etsuo Chosa, Miyazaki Medical College Hospital, Kiyotake
Chiaki Tanaka, Kyoto Municipal Hospital, Kyoto
Sen-eki Kobayashi, Shinshu University Hospital, Matsumoto
Shigeo Sano, Sanraku Hospital, Tokyo
Takashi Ohya, Obihiro Kosei Hospital, Obihiro
Katsuhiro Shimada, National Murayama Hospital, Musashi-murayama
Yoshimitsu Hoshikawa, St Luke’s International Hospital, Tokyo
Shuji Okinaga, Tokyo Teishin Hospital, Tokyo
Naoyuki Katayama, Hokkaido Orthopedic Memorial Hospital, Sapporo
Osamu Sugawara, Kitami Red Cross Hospital, Kitami
Kazuyoshi Hirose, Nagoya Kyoritsu Hospital, Nagoya
Keiju Fujiwara, Osaka Prefectural General Hospital, Osaka
Takehiro Takebayashi, Sapporo Insurance General Hospital, Sapporo
Hisaaki Miyahara, National Kyushu Medical Center, Fukuoka
Masanobu Saito, Osaka Minami National Hospital, Kawachinagano
Footnotes
For the Steering Committee of the Japan Fondaparinux Study in Arthroplasty.
References
- 1.Bauer KA, Eriksson BI, Lassen MR, Turpie AG (2001) The steering committee of the pentasaccharide in major knee surgery study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med 345(18):1305–1310 [DOI] [PubMed]
- 2.Boneu B, Necciari J, Cariou R et al (1995) Pharmacokinetics and tolerance of the natural pentasaccharide (SR90107/ORG31540) with high affinity to antithrombin III in man. Thromb Haemost 74(6):1468–1473 [PubMed]
- 3.Eriksson BI, Bauer KA, Lassen MR, Turpie AG (2001) The Steering committee of the Pentasaccharide in Hip-fracture surgery study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective hip-fracture surgery. N Engl J Med 345(18):1298–1304 [DOI] [PubMed]
- 4.Eriksson BI, Lassen MR, PENTasaccharide in HIp-FRActure Surgery Plus Investigators (2003) Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med 163(11):1337–1342 [DOI] [PubMed]
- 5.Fujita S, Hirota S, Oda T, Kato Y, Tsukamoto Y, Fuji T (2000) Deep venous thrombosis after total hip or total knee arthroplasty in patients in Japan. Clin Orthop 375:168–174 [DOI] [PubMed]
- 6.Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA Jr, Wheeler HB (2001) Prevention of venous thromboembolism. Chest 119(1 Suppl):132S–175S [DOI] [PubMed]
- 7.Geerts WH, Pineo GF, Heit JA et al (2004) Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic therapy. Chest 126(3 Suppl.):S338–S400 [DOI] [PubMed]
- 8.Herbert JM, Petitou M, Lormeau JC, Cariou R, Necciari J, Magnani HN et al (1997) SR 90107A/Org 31540, a novel anti-factor Xa antithrombic agent. Cardiovasc Drug Rev 15(1):1–26 [DOI]
- 9.Hirsh J, Hoak J (1996) Management of deep vein thrombosis and pulmonary embolism: a statement for healthcare professionals. From the council on thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation 93(12):2212–2245 [DOI] [PubMed]
- 10.Lassen MR, Bauer KA, Eriksson BI, Turpie AG (2002) The European Pentasaccharide Hip Elective Surgery Study (EPHESUS) Steering Committee. Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip-replacement surgery: a randomised double-blind comparison. Lancet 359(9319):1715–1720 [DOI] [PubMed]
- 11.Mann KG (1999) Biochemistry and physiology of blood coagulation. Thromb Haemost 82(2):165–74 [PubMed]
- 12.Prescribing Information for Arixtra (fondaparinux). GlaxoSmithKline web site at http://us.gsk.com/products/assets/us_arixtra.pdf
- 13.Statistics and Information Department, Ministry of Health and Welfare: Patient Survey (1993) Tokyo, Japan (Japanese)
- 14.Statistics and Information Department, Ministry of Health and Welfare: Vital Statistics of Japan [Data compiled from 1964 to 1994] Tokyo, Japan. (Japanese)
- 15.Summary of Product Characteristics of fondaparinux. European Medicines Agency web site at http://www.emea.eu.int/humandocs/PDFs/EPAR/arixtra/H-403-PI-en.pdf#search=’arixtra’
- 16.Turpie AG, Gallus AS, Hoek JA (2001) Pentasaccharide Investigators. A synthetic pentasaccharide for the prevention of deep-vein thrombosis after total hip replacement. N Engl J Med 344(9):619–625 [DOI] [PubMed]
- 17.Turpie AG, Bauer KA, Eriksson BI, Lassen MR (2002) The PENTATHLON 2000 Study Steering Committee. Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: a randomised double-blind trial. Lancet 359(9319):1721–1726 [DOI] [PubMed]