Abstract
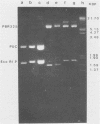
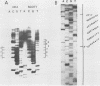
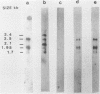
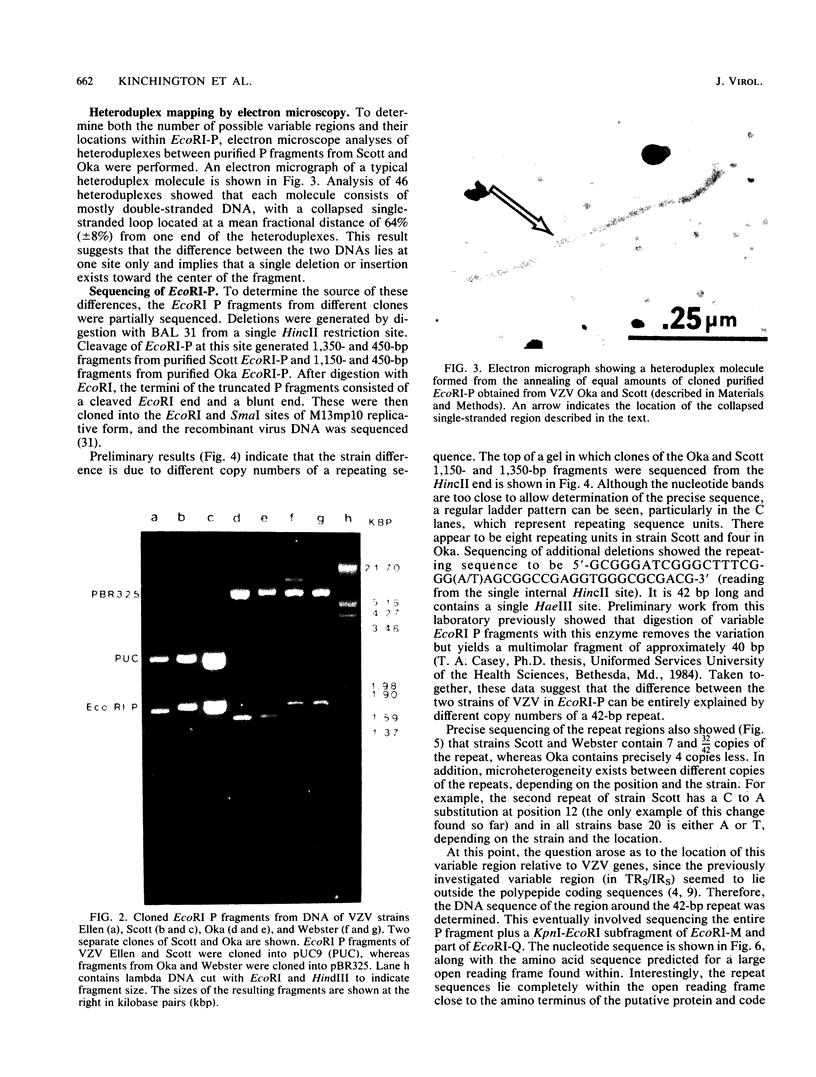
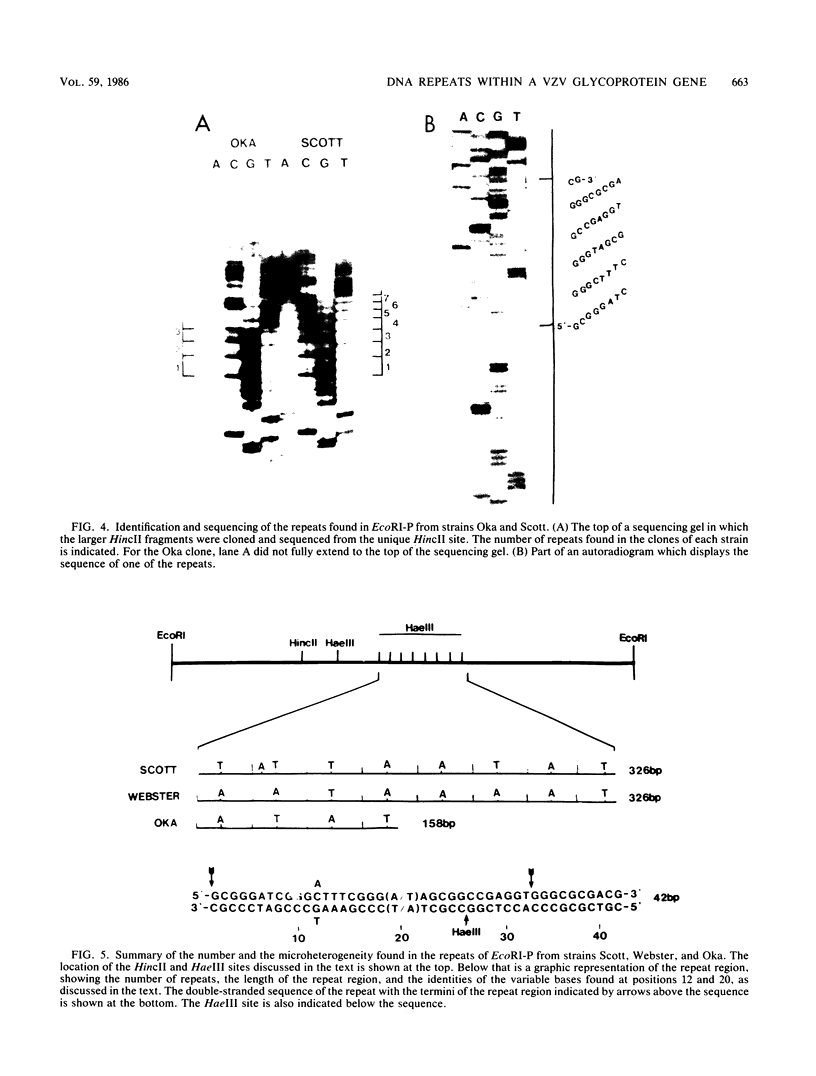
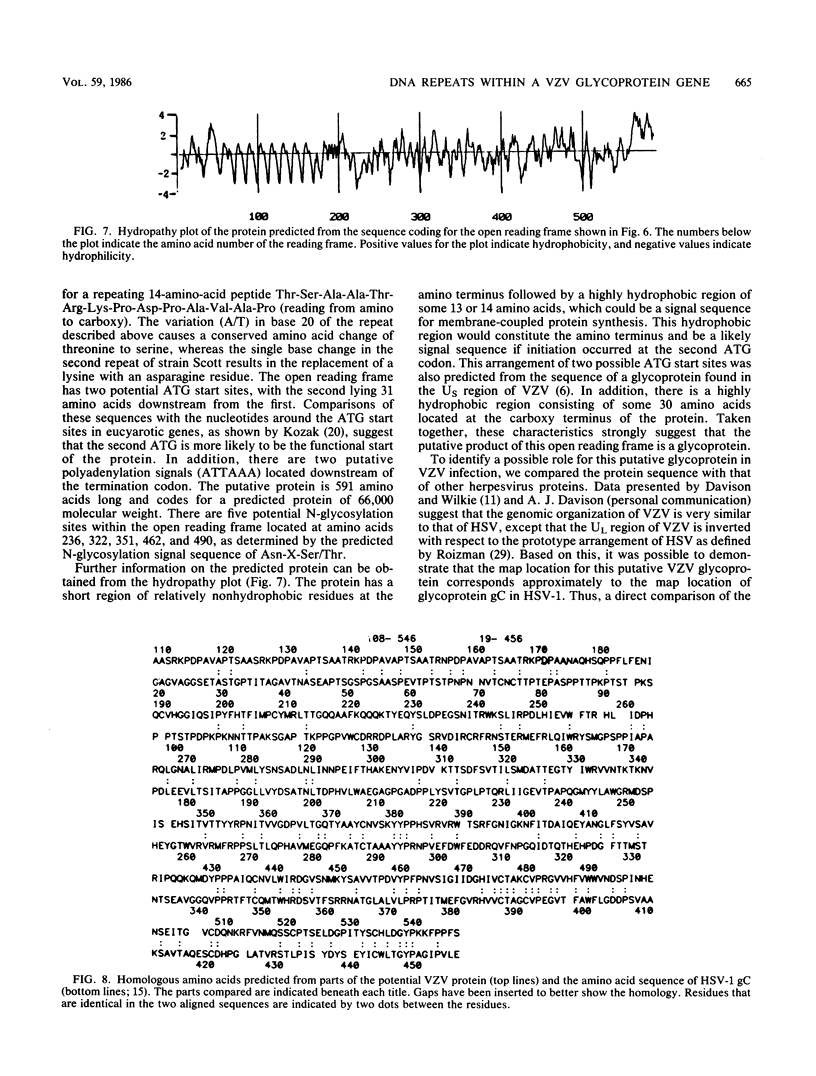
A strain variation of varicella-zoster virus that maps to the UL region of the genome was found to be due to different copy numbers of a high GC 42-base-pair repeat. DNA sequence analysis of this variable region showed the sequence to be 5-GCGGGATCGGGCTTTCGGG(A/T)AGCGGCCGAGGTGGGCGCGACG-3. Strains Scott and Webster both contain 7 and 32/42 copies of the repeat, whereas strain Oka has exactly 4 copies less. Microheterogeneity exists within the repeated sequences, depending on the strain and the repeat number. Sequencing of the entire EcoRI P fragment (which contains the repeated sequences) and part of the adjacent EcoRI M and EcoRI Q fragments from strain Scott showed that the repeats are part of a large open reading frame that could code for a polypeptide core with a molecular weight of 66,000. Several potential TATA boxes exist upstream and two polyadenylation signals are found downstream of the open reading frame. The predicted protein bears several characteristics of a glycoprotein. The region is transcriptionally active in varicella-zoster virus-infected cells, specifying at least three RNA species of 1.7, 1.95, and 2.5 kilobases, which are transcribed from the same DNA strand. Part of the predicted protein has a high degree of homology to the herpes simplex virus type 1 glycoprotein gC.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. P., Yeargan M. R., Bryans J. T. Alterations in the equine herpesvirus 1 genome after in vitro and in vivo virus passage. Infect Immun. 1983 Apr;40(1):436–439. doi: 10.1128/iai.40.1.436-439.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porat T., Deatly A., Veach R. A., Blankenship M. L. Equalization of the inverted repeat sequences of the pseudorabies virus genome by intermolecular recombination. Virology. 1984 Jan 30;132(2):303–314. doi: 10.1016/0042-6822(84)90037-0. [DOI] [PubMed] [Google Scholar]
- Buchman T. G., Roizman B., Adams G., Stover B. H. Restriction endonuclease fingerprinting of herpes simplex virus DNA: a novel epidemiological tool applied to a nosocomial outbreak. J Infect Dis. 1978 Oct;138(4):488–498. doi: 10.1093/infdis/138.4.488. [DOI] [PubMed] [Google Scholar]
- Casey T. A., Ruyechan W. T., Flora M. N., Reinhold W., Straus S. E., Hay J. Fine mapping and sequencing of a variable segment in the inverted repeat region of varicella-zoster virus DNA. J Virol. 1985 May;54(2):639–642. doi: 10.1128/jvi.54.2.639-642.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassai E., Manservigi R., Corallini A., Terni M. Plaque dissociation of herpes simplex viruses: biochemical and biological characters of the viral variants. Intervirology. 1975;6(4-5):212–223. doi: 10.1159/000149476. [DOI] [PubMed] [Google Scholar]
- Davison A. J. DNA sequence of the US component of the varicella-zoster virus genome. EMBO J. 1983;2(12):2203–2209. doi: 10.1002/j.1460-2075.1983.tb01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. DNA sequence of the major inverted repeat in the varicella-zoster virus genome. J Gen Virol. 1985 Feb;66(Pt 2):207–220. doi: 10.1099/0022-1317-66-2-207. [DOI] [PubMed] [Google Scholar]
- Davison A. J. Structure of the genome termini of varicella-zoster virus. J Gen Virol. 1984 Nov;65(Pt 11):1969–1977. doi: 10.1099/0022-1317-65-11-1969. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Location and orientation of homologous sequences in the genomes of five herpesviruses. J Gen Virol. 1983 Sep;64(Pt 9):1927–1942. doi: 10.1099/0022-1317-64-9-1927. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Nucleotide sequences of the joint between the L and S segments of herpes simplex virus types 1 and 2. J Gen Virol. 1981 Aug;55(Pt 2):315–331. doi: 10.1099/0022-1317-55-2-315. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Falk L. A. Herpesvirus saimiri strain variability. J Virol. 1982 Jul;43(1):352–356. doi: 10.1128/jvi.43.1.352-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas A. M., Geelen J. L., Weststrate M. W., Wertheim P., van der Noordaa J. XbaI, PstI, and BglII restriction enzyme maps of the two orientations of the varicella-zoster virus genome. J Virol. 1981 Aug;39(2):390–400. doi: 10.1128/jvi.39.2.390-400.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frink R. J., Eisenberg R., Cohen G., Wagner E. K. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J Virol. 1983 Feb;45(2):634–647. doi: 10.1128/jvi.45.2.634-647.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad W. B., Kanehisa M. I. Pattern recognition in nucleic acid sequences. I. A general method for finding local homologies and symmetries. Nucleic Acids Res. 1982 Jan 11;10(1):247–263. doi: 10.1093/nar/10.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliburton I. W., Morse L. S., Roizman B., Quinn K. E. Mapping of the thymidine kinase genes of type 1 and type 2 herpes simplex viruses using intertypic recombinants. J Gen Virol. 1980 Aug;49(2):235–253. doi: 10.1099/0022-1317-49-2-235. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchington P. R., Reinhold W. C., Casey T. A., Straus S. E., Hay J., Ruyechan W. T. Inversion and circularization of the varicella-zoster virus genome. J Virol. 1985 Oct;56(1):194–200. doi: 10.1128/jvi.56.1.194-200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Murchie M. J., McGeoch D. J. DNA sequence analysis of an immediate-early gene region of the herpes simplex virus type 1 genome (map coordinates 0.950 to 0.978). J Gen Virol. 1982 Sep;62(Pt 1):1–15. doi: 10.1099/0022-1317-62-1-1. [DOI] [PubMed] [Google Scholar]
- Ostrove J. M., Reinhold W., Fan C. M., Zorn S., Hay J., Straus S. E. Transcription mapping of the varicella-zoster virus genome. J Virol. 1985 Nov;56(2):600–606. doi: 10.1128/jvi.56.2.600-606.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Traub N., Pritchett R., Kieff E. DNA of Epstein-Barr virus. III. Identification of restriction enzyme fragments that contain DNA sequences which differ among strains of Epstein-Barr virus. J Virol. 1978 Aug;27(2):388–398. doi: 10.1128/jvi.27.2.388-398.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon F. J., McGeoch D. J. A 3' co-terminal family of mRNAs from the herpes simplex virus type 1 short region: two overlapping reading frames encode unrelated polypeptide one of which has highly reiterated amino acid sequence. Nucleic Acids Res. 1984 Mar 12;12(5):2473–2487. doi: 10.1093/nar/12.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B. The structure and isomerization of herpes simplex virus genomes. Cell. 1979 Mar;16(3):481–494. doi: 10.1016/0092-8674(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Casey T. A., Reinhold W., Weir A. C., Wellman M., Straus S. E., Hay J. Distribution of G + C-rich regions in varicella-zoster virus DNA. J Gen Virol. 1985 Jan;66(Pt 1):43–54. doi: 10.1099/0022-1317-66-1-43. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schoulaker-Schwarz R., Engelberg-Kulka H. Effect of an Escherichia coli traD (ts) mutation on MS2 RNA replication. J Gen Virol. 1983 Jan;64(Pt 1):207–210. doi: 10.1099/0022-1317-64-1-207. [DOI] [PubMed] [Google Scholar]
- Skare J., Summers W. P., Summers W. C. Structure and function of herpesvirus genomes. I. comparison of five HSV-1 and two HSV-2 strains by cleavage their DNA with eco R I restriction endonuclease. J Virol. 1975 Apr;15(4):726–732. doi: 10.1128/jvi.15.4.726-732.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Aulakh H. S., Ruyechan W. T., Hay J., Casey T. A., Vande Woude G. F., Owens J., Smith H. A. Structure of varicella-zoster virus DNA. J Virol. 1981 Nov;40(2):516–525. doi: 10.1128/jvi.40.2.516-525.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Hay J., Smith H., Owens J. Genome differences among varicella-zoster virus isolates. J Gen Virol. 1983 May;64(Pt 5):1031–1041. doi: 10.1099/0022-1317-64-5-1031. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Owens J., Ruyechan W. T., Takiff H. E., Casey T. A., Vande Woude G. F., Hay J. Molecular cloning and physical mapping of varicella-zoster virus DNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):993–997. doi: 10.1073/pnas.79.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Owens J., Ruyechan W. T., Takiff H. E., Casey T. A., Vande Woude G. F., Hay J. Molecular cloning and physical mapping of varicella-zoster virus DNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):993–997. doi: 10.1073/pnas.79.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Reinhold W., Smith H. A., Ruyechan W. T., Henderson D. K., Blaese R. M., Hay J. Endonuclease analysis of viral DNA from varicella and subsequent zoster infections in the same patient. N Engl J Med. 1984 Nov 22;311(21):1362–1364. doi: 10.1056/NEJM198411223112107. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Okuno Y., Otsuka T., Osame J., Takamizawa A. Development of a live attenuated varicella vaccine. Biken J. 1975 Mar;18(1):25–33. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vlazny D. A., Hyman R. W. Errant processing and structural alterations of genomes present in a varicella-zoster virus vaccine. J Virol. 1985 Oct;56(1):92–101. doi: 10.1128/jvi.56.1.92-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezulak K. M., Spear P. G. Mapping of the structural gene for the herpes simplex virus type 2 counterpart of herpes simplex virus type 1 glycoprotein C and identification of a type 2 mutant which does not express this glycoprotein. J Virol. 1984 Mar;49(3):741–747. doi: 10.1128/jvi.49.3.741-747.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink H. J., Morton D. H., Stanton L. W., Neff B. J. Restriction endonuclease analysis of the DNA from varicella-zoster virus: stability of the DNA after passage in vitro. J Gen Virol. 1981 Jul;55(Pt 1):207–211. doi: 10.1099/0022-1317-55-1-207. [DOI] [PubMed] [Google Scholar]