Abstract
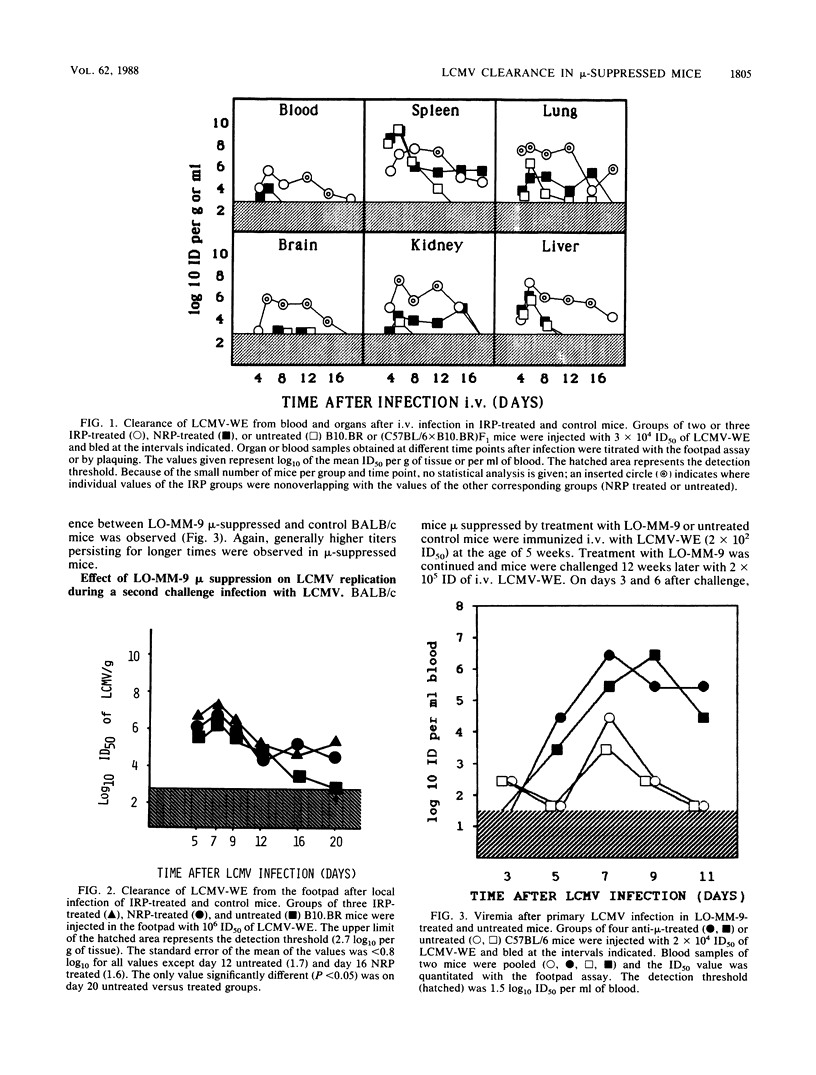
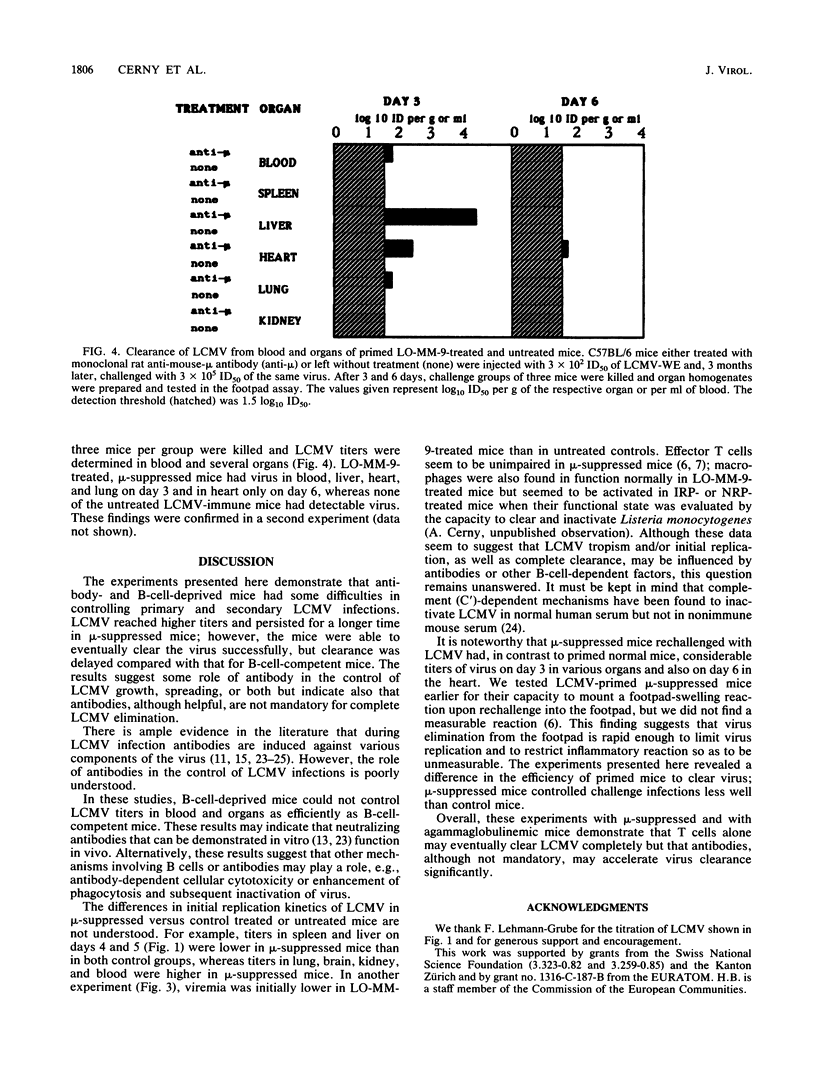
The role of antibody in immune recovery from infection with lymphocytic choriomeningitis virus (LCMV) strain WE was evaluated in B-cell-depleted mice. Mice were treated from birth with either affinity-purified rabbit anti-mouse immunoglobulin M (IgM), normal rabbit immunoglobulin, or, alternatively, an affinity-purified monoclonal rat anti-mouse IgM antibody (LO-MM-9); untreated mice served as controls. B-cell depletion was considered complete in specifically treated mice according to the following criteria: absence of a significant response to the B-cell mitogen lipopolysaccharide, absence of B cells expressing immunoglobulin on their surfaces, absence of detectable IgM or IgG in serum, and presence in the serum of free anti-IgM antibodies. In organs of mu-suppressed BALB/c mice, LCMV-WE replicated, dependent upon organ, at the same rate or more rapidly and, in general, to higher titers than in normal rabbit immunoglobulin-treated mice; untreated mice eliminated the virus most rapidly and showed lower virus titers. In addition, LCMV-primed control mice cleared a second LCMV challenge very rapidly and contained no virus by day 3, whereas mu-suppressed mice had virus in their blood and organs (except the spleen) up to days 3 to 6. The observed effects of anti-mu treatment may reflect the action of neutralizing antibodies (which so far have been difficult to demonstrate in vivo) or other antibody-dependent antiviral mechanisms which, together with T cells, efficiently control LCMV clearance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assmann-Wischer U., Simon M. M., Lehmann-Grube F. Mechanism of recovery from acute virus infection. III. Subclass of T lymphocytes mediating clearance of lymphocytic choriomeningitis virus from the spleens of mice. Med Microbiol Immunol. 1985;174(5):249–256. doi: 10.1007/BF02124809. [DOI] [PubMed] [Google Scholar]
- Bazin H., Cormont F., De Clercq L. Rat monoclonal antibodies. II. A rapid and efficient method of purification from ascitic fluid or serum. J Immunol Methods. 1984 Jun 8;71(1):9–16. doi: 10.1016/0022-1759(84)90200-x. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Byrne J. A., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984 Sep;51(3):682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny A., Heusser C., Sutter S., Huegin A. W., Bazin H., Hengartner H., Zinkernagel R. M. Generation of agammaglobulinaemic mice by prenatal and postnatal exposure to polyclonal or monoclonal anti-IgM antibodies. Scand J Immunol. 1986 Oct;24(4):437–445. doi: 10.1111/j.1365-3083.1986.tb02132.x. [DOI] [PubMed] [Google Scholar]
- Cerny A., Huegin A. W., Sutter S., Bazin H., Hengartner H. H., Zinkernagel R. M. Immunity to lymphocytic choriomeningitis virus in B cell-depleted mice: evidence for B cell and antibody-independent protection by memory T cells. Eur J Immunol. 1986 Aug;16(8):913–917. doi: 10.1002/eji.1830160807. [DOI] [PubMed] [Google Scholar]
- Cerny A., Hügin A. W., Sutter S., Heusser C. H., Bos N., Izui S., Hengartner H., Zinkernagel R. M. Suppression of B cell development and antibody responses in mice with polyclonal rabbit and monoclonal rat anti-IgM antibodies. I. Characterization of the suppressed state. Exp Cell Biol. 1985;53(6):301–313. doi: 10.1159/000163327. [DOI] [PubMed] [Google Scholar]
- Cormont F., Manouvriez P., De Clercq L., Bazin H. The use of rat monoclonal antibodies to characterize, quantify, and purify polyclonal or monoclonal mouse IgM. Methods Enzymol. 1986;121:622–631. doi: 10.1016/0076-6879(86)21061-7. [DOI] [PubMed] [Google Scholar]
- Cowan K. M. Antibody response to viral antigens. Adv Immunol. 1973;17:195–253. doi: 10.1016/s0065-2776(08)60733-6. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1974;19(0):89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Johnson E. D., Monjan A. A., Morse H. C., 3rd Lack of B-cell participation in acute lymphocyte choriomeningitis disease of the central nervous system. Cell Immunol. 1978 Mar 1;36(1):143–150. doi: 10.1016/0008-8749(78)90257-5. [DOI] [PubMed] [Google Scholar]
- Kimmig B., Lehmann-Grube F. The immune response of the mouse to lymphocytic choriomeningitis virus. I. Circulating antibodies. J Gen Virol. 1979 Dec;45(3):703–710. doi: 10.1099/0022-1317-45-3-703. [DOI] [PubMed] [Google Scholar]
- LEHMANN-GRUBE F. LYMPHOCYTIC CHORIOMENINGITIS IN THE MOUSE. I. GROWTH IN THE BRAIN. Arch Gesamte Virusforsch. 1964;14:344–350. doi: 10.1007/BF01555827. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehmann-Grube F., Ambrassat J. A new method to detect lymphocytic choriomeningitis virus-specific antibody in human sera. J Gen Virol. 1977 Oct;37(1):85–92. doi: 10.1099/0022-1317-37-1-85. [DOI] [PubMed] [Google Scholar]
- Lehmann-Grube F., Assmann U., Löliger C., Moskophidis D., Löhler J. Mechanism of recovery from acute virus infection. I. Role of T lymphocytes in the clearance of lymphocytic choriomeningitis virus from spleens of mice. J Immunol. 1985 Jan;134(1):608–615. [PubMed] [Google Scholar]
- Moskophidis D., Lehmann-Grube F. The immune response of the mouse to lymphocytic choriomeningitis virus. III. Differences of numbers of cytotoxic T lymphocytes in spleens of mice of different strains. Cell Immunol. 1983 Apr 15;77(2):279–289. doi: 10.1016/0008-8749(83)90028-x. [DOI] [PubMed] [Google Scholar]
- TRAUB E. Demonstration, properties and significance of neutralizing antibodies in mature mice immune to lymphocytic choriomeningitis (LCM). Arch Gesamte Virusforsch. 1960;10:289–302. doi: 10.1007/BF01250676. [DOI] [PubMed] [Google Scholar]
- Welsh R. M. Host cell modification of lymphocytic choriomeningitis virus and Newcastle disease virus altering viral inactivation by human complement. J Immunol. 1977 Jan;118(1):348–354. [PubMed] [Google Scholar]
- Zinkernagel R. M., Haenseler E., Leist T., Cerny A., Hengartner H., Althage A. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J Exp Med. 1986 Oct 1;164(4):1075–1092. doi: 10.1084/jem.164.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Hengartner H., Stitz L. On the role of viruses in the evolution of immune responses. Br Med Bull. 1985 Jan;41(1):92–97. doi: 10.1093/oxfordjournals.bmb.a072033. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Welsh R. M. H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J Immunol. 1976 Nov;117(5 Pt 1):1495–1502. [PubMed] [Google Scholar]



