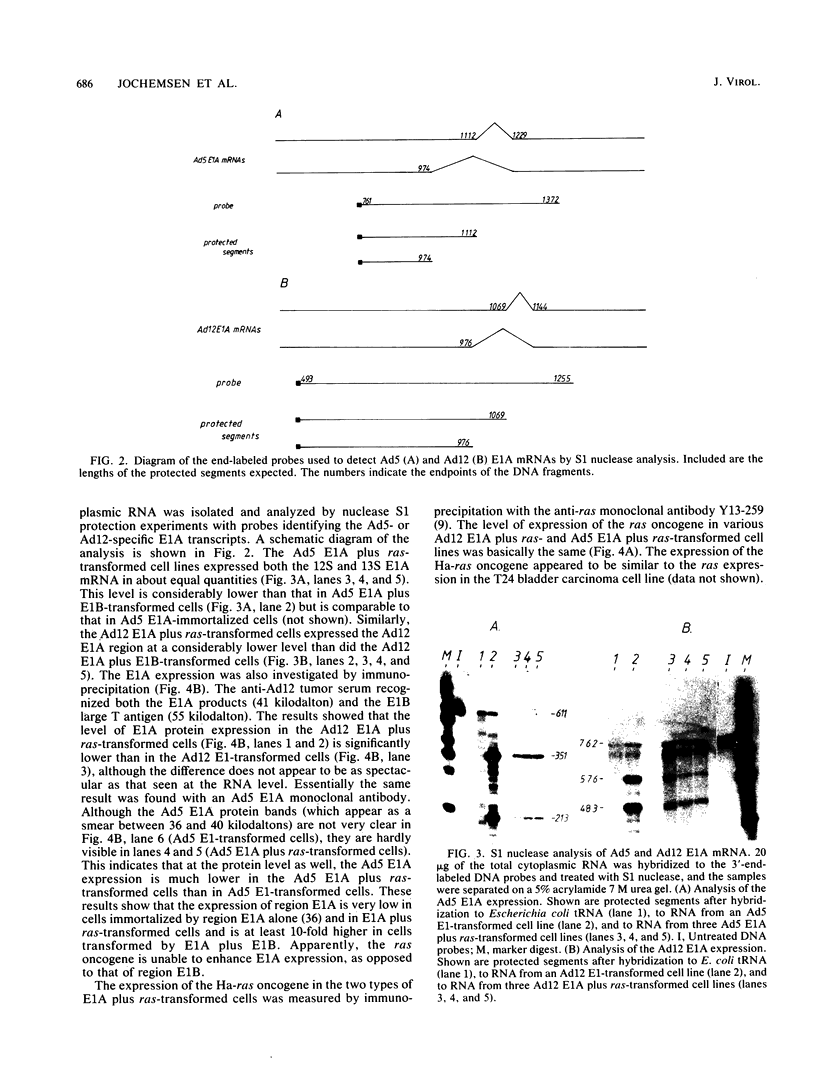
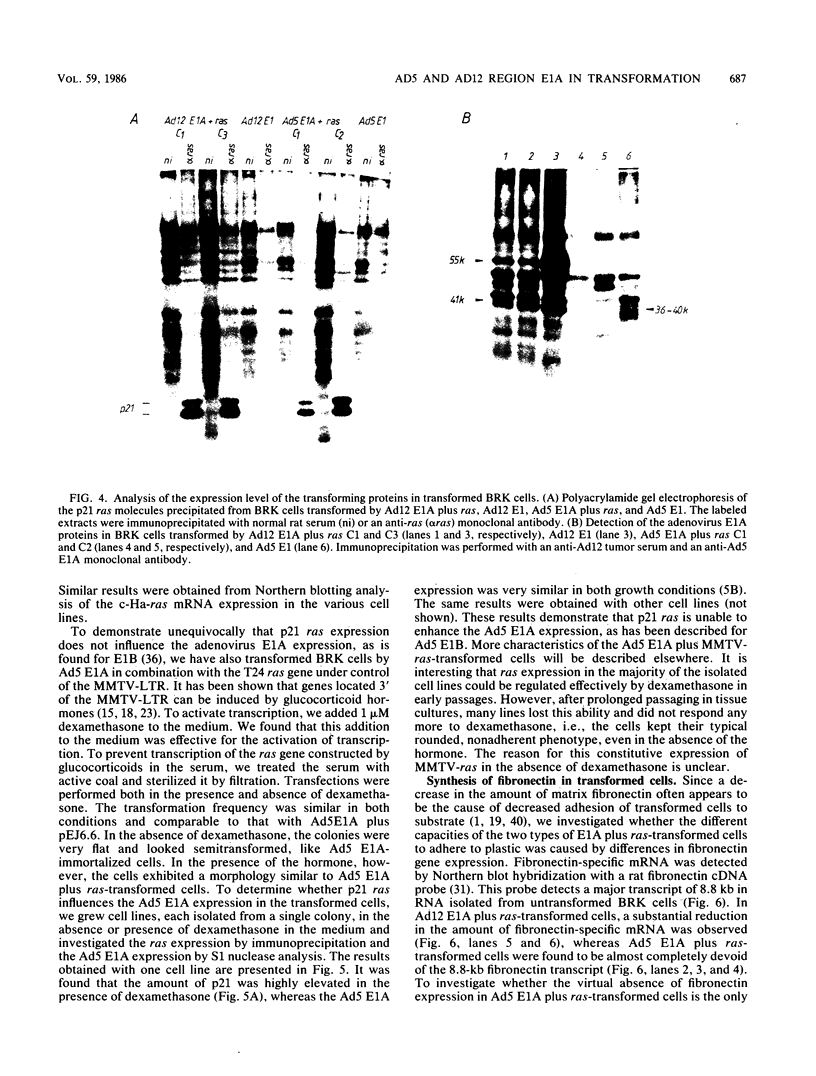
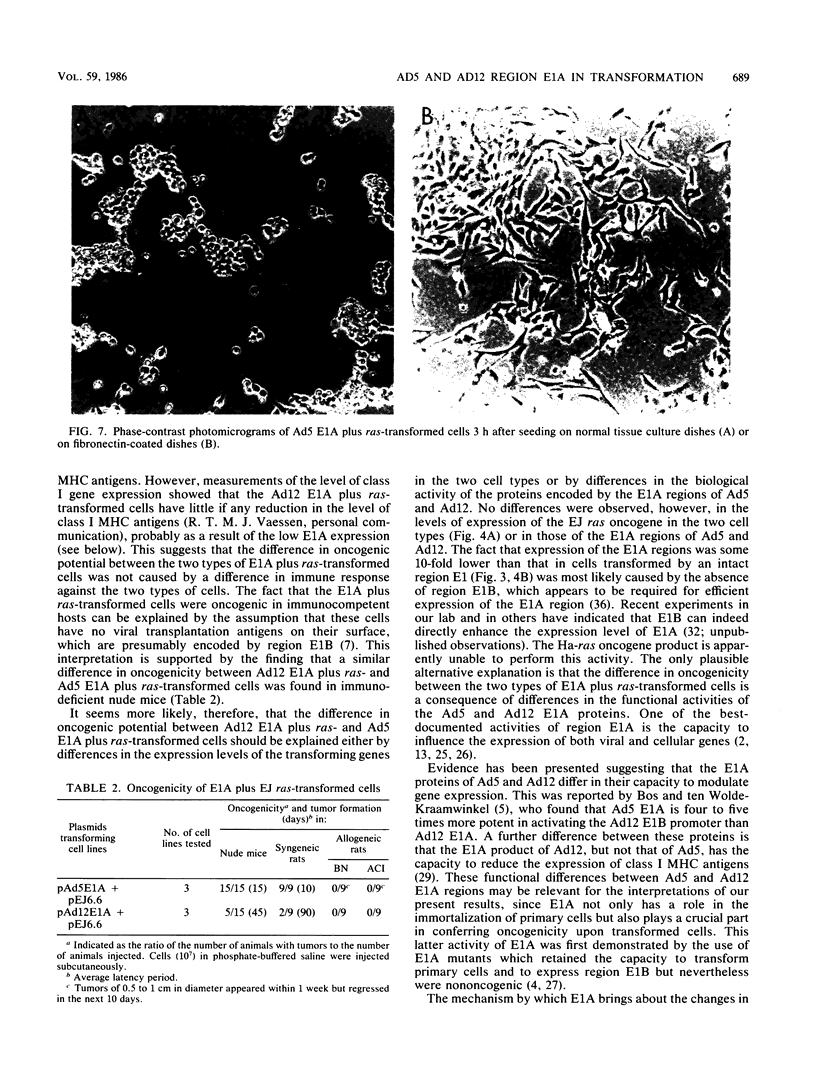
Abstract
We have compared the capacities of the E1A regions of nononcogenic adenovirus type 5 (Ad5) and highly oncogenic Ad12 to cooperate with the EJ bladder carcinoma Ha-ras-1 oncogene in the transformation of primary baby rat kidney cells. Both E1A regions, when cotransfected with the Ha-ras oncogene, transformed the primary cells with a low frequency. Ad5 E1A plus Ha-ras-transformed cells differed in phenotype from cells transformed by Ad12 E1A plus Ha-ras. The cells expressing Ad5 E1A appeared highly transformed and practically failed to adhere to plastic. This phenotype may be due to the virtually complete absence of fibronectin gene expression in these cells. In contrast, the cells expressing Ad12 E1A were flatter and adhered to plastic, whereas fibronectin gene expression was reduced but not absent. The oncogenic potential of the two types of E1A plus ras-transformed cells was tested by their injection into both athymic nude mice and weanling syngeneic rats. The Ad5 E1A plus ras-transformed cells were found to be highly oncogenic in both animal species, whereas the Ad12 E1A plus ras-transformed cells were only weakly oncogenic in both syngeneic rats and nude mice. The difference in oncogenic potential of the Ad5 E1A plus ras- and the Ad12 E1A plus ras-transformed cells is discussed in terms of the different capacities of the Ad5 and Ad12 E1A-encoded proteins to modulate cellular gene expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Mautner V., Lanza R., Hynes R. O. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977 May;11(1):115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Bernards R., Houweling A., Schrier P. I., Bos J. L., Van der Eb A. J. Characterization of cells transformed by Ad5/Ad12 hybrid early region I plasmids. Virology. 1982 Jul 30;120(2):422–432. doi: 10.1016/0042-6822(82)90042-3. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Jochemsen A. G., Bernards R., Schrier P. I., van Ormondt H., van der Eb A. J. Deletion mutants of region E1a of AD12 E1 plasmids: effect on oncogenic transformation. Virology. 1983 Sep;129(2):393–400. doi: 10.1016/0042-6822(83)90178-2. [DOI] [PubMed] [Google Scholar]
- Bos J. L., ten Wolde-Kraamwinkel H. C. The E1b promoter of Ad12 in mouse L tk- cells is activated by adenovirus region E1a. EMBO J. 1983;2(1):73–76. doi: 10.1002/j.1460-2075.1983.tb01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Black P. H., Vanderpool E. A., Henry P. H., Austin J. B., Huebner R. J. Transformation of primary rat embryo cells by adenovirus type 2. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1205–1212. doi: 10.1073/pnas.58.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Föhring B., Gallimore P. H., Mellow G. H., Raska K., Jr Adenovirus type 12 specific cell surface antigen in transformed cells is a product of the E1b early region. Virology. 1983 Dec;131(2):463–472. doi: 10.1016/0042-6822(83)90512-3. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H., Paraskeva C. A study to determine the reasons for differences in the tumorigenicity of rat cell lines transformed by adenovirus 2 and adenovirus 12. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):703–713. doi: 10.1101/sqb.1980.044.01.075. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Berk A. J. Cis-acting induction of adenovirus transcription. Cell. 1983 Jul;33(3):683–693. doi: 10.1016/0092-8674(83)90011-9. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J., Heijneker H. L. Size and location of the transforming region in human adenovirus type 5 DNA. Nature. 1974 Oct 25;251(5477):687–691. doi: 10.1038/251687a0. [DOI] [PubMed] [Google Scholar]
- Harlow E., Franza B. R., Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochemsen A. G., Bos J. L., van der Eb A. J. The first exon of region E1a genes of adenoviruses 5 and 12 encodes a separate functional protein domain. EMBO J. 1984 Dec 1;3(12):2923–2927. doi: 10.1002/j.1460-2075.1984.tb02233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochemsen H., Daniëls G. S., Hertoghs J. J., Schrier P. I., van den Elsen P. J., van der EB A. J. Identification of adenovirus-type 12 gene products involved in transformation and oncogenesis. Virology. 1982 Oct 15;122(1):15–28. doi: 10.1016/0042-6822(82)90373-7. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lee F., Mulligan R., Berg P., Ringold G. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature. 1981 Nov 19;294(5838):228–232. doi: 10.1038/294228a0. [DOI] [PubMed] [Google Scholar]
- Mak S., Mak I., Smiley J. R., Graham F. L. Tumorigenicity and viral gene expression in rat cells transformed by Ad 12 virions or by the EcoRI c fragment of Ad 12 DNA. Virology. 1979 Oct 30;98(2):456–460. doi: 10.1016/0042-6822(79)90568-3. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Ruben M., Bacchetti S., Graham F. L. Integration and expression of viral DNA in cells transformed by host range mutants of adenovirus type 5. J Virol. 1982 Feb;41(2):674–685. doi: 10.1128/jvi.41.2.674-685.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Schrier P. I., Van Den Elsen P. J., Hertoghs J. J., Van Der Eb A. J. Characterization of tumor antigens in cells transformed by fragments of adenovirus type 5 DNA. Virology. 1979 Dec;99(2):372–385. doi: 10.1016/0042-6822(79)90016-3. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Senear A. W., Lewis J. B. Morphological transformation of established rodent cell lines by high-level expression of the adenovirus type 2 E1a gene. Mol Cell Biol. 1986 Apr;6(4):1253–1260. doi: 10.1128/mcb.6.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Elsen P., Houweling A., Van der Eb A. Expression of region E1b of human adenoviruses in the absence of region E1a is not sufficient for complete transformation. Virology. 1983 Jul 30;128(2):377–390. doi: 10.1016/0042-6822(83)90264-7. [DOI] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a proteins repress transcription from the SV40 early promoter. Cell. 1985 Mar;40(3):705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]
- Wilson M. C., Fraser N. W., Darnell J. E., Jr Mapping of RNA initiation sites by high doses of uv irradiation: evidence for three independent promoters within the left 11% of the Ad-2 genome. Virology. 1979 Apr 15;94(1):175–184. doi: 10.1016/0042-6822(79)90447-1. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elsen P., Klein B., Dekker B., van Ormondt H., van der Eb A. Analysis of virus-specific mRNAs present in cells transformed with restriction fragments of adenovirus type 5 DNA. J Gen Virol. 1983 May;64(Pt 5):1079–1090. doi: 10.1099/0022-1317-64-5-1079. [DOI] [PubMed] [Google Scholar]
- van den Elsen P., de Pater S., Houweling A., van der Veer J., van der Eb A. The relationship between region E1a and E1b of human adenoviruses in cell transformation. Gene. 1982 May;18(2):175–185. doi: 10.1016/0378-1119(82)90115-9. [DOI] [PubMed] [Google Scholar]
- van der Eb A. J., Graham F. L. Assay of transforming activity of tumor virus DNA. Methods Enzymol. 1980;65(1):826–839. doi: 10.1016/s0076-6879(80)65077-0. [DOI] [PubMed] [Google Scholar]