Abstract
Fascin is an actin-bundling protein that is found in membrane ruffles, microspikes, and stress fibers. The expression of fascin is greatly increased in many transformed cells, as well as in specialized normal cells including neuronal cells and antigen-presenting dendritic cells. A morphological characteristic common to these cells expressing high levels of fascin is the development of many membrane protrusions in which fascin is predominantly present. To examine whether fascin contributes to the alterations in microfilament organization at the cell periphery, we have expressed fascin in LLC-PK1 epithelial cells to levels as high as those found in transformed cells and in specialized normal cells. Expression of fascin results in large changes in morphology, the actin cytoskeleton, and cell motility: fascin-transfected cells form an increased number of longer and thicker microvilli on apical surfaces, extend lamellipodia-like structures at basolateral surfaces, and show disorganization of cell–cell contacts. Cell migration activity is increased by 8–17 times when assayed by modified Boyden chamber. Microinjection of a fascin protein into LLC-PK1 cells causes similar morphological alterations including the induction of lamellipodia at basolateral surfaces and formation of an increased number of microvilli on apical surfaces. Furthermore, microinjection of fascin into REF-52 cells, normal fibroblasts, induces the formation of many lamellipodia at all regions of cell periphery. These results together suggest that fascin is directly responsible for membrane protrusions through reorganization of the microfilament cytoskeleton at the cell periphery.
INTRODUCTION
Fascins represent a family of actin-bundling proteins including sea urchin fascin, HeLa 55-kDa actin-bundling protein, and the Drosophila singed protein (Matsudaira, 1994; Otto, 1994; Edwards and Bryan, 1995). Fascin is evolutionarily conserved, although yeast does not seem to have a fascin homolog. Molecular cloning of sea urchin fascin by J. Bryan and co-workers has shown that fascin has 35% identity at the amino acid level to the product of the Drosophila singed gene (Bryan et al., 1993). Subsequent cloning of human, mouse, and Xenopus fascins (Duh et al., 1994; Holthuis et al., 1994; Mosialos et al., 1994; Edward et al., 1995) has revealed that the amino acid sequences of these fascin proteins are very similar to each other but share no apparent homology with nonfascin actin-bundling proteins (including villin and fimbrin), indicating that the fascins represent a distinct family of actin-binding proteins.
All of the fascin proteins cause aggregation of F-actin into bundles in vitro, the property of which is reflected to the localization of fascin in a variety of cells. Sea urchin fascin is involved in the formation of filopodia by coelomocytes, the phagocytotic defense cells of echinoderms (Otto et al., 1979) and may act in the formation of microvillar cores at fertilization (Otto et al., 1980). We have purified fascin (55-kDa actin-bundling protein) from HeLa cells (Yamashiro-Matsumura and Matsumura, 1985), and found that this human protein is localized mainly at filopodia and membrane ruffles, as well as in stress fibers (Yamashiro-Matsumura and Matsumura, 1986). Fascin is also found in microspikes during spreading of a variety of cells including glioma cells (Lin et al., 1996) and myoblasts (Adams, 1995, 1997). These observations suggest the involvement of fascin in the formation of actin bundles present in filopodia, microspikes, membrane ruffles, and stress fibers.
It is worthy of note that the expression of fascin is highly specific to tissue and cell types. Fascin is abundantly expressed in tissues such as brain and spleen and, at a cellular level, in specific types of cells such as neuronal and glial cells, microcapillary endothelial cells, and antigen-presenting dendritic cells (Duh et al., 1994; Mosialos et al., 1994, 1996; Pinkus et al., 1997). A morphological characteristic common to these specialized normal cells that express high levels of fascin is the development of many membrane protrusions. These observations suggest that fascin plays a role in extending the membranes either for cell motility or for interactions with other types of cells. For example, dendritic cells, which are critical cells for antigen presentation, show numerous, fascin-containing, membrane extensions. Upon maturation, they move to lymph nodes to present the antigen to T-cells. It is interesting to note that coelomocytes of echinoderms containing fascin-positive filopodia are involved in phagocytotic defense, an immune system of echinoderms.
High level expression of fascin is also observed in many transformed cells, including virus-transformed fibroblasts, HeLa (epitheloid carcinoma) cells, and Epstein-Barr virus-infected B lymphocytes (Yamashiro-Matsumura and Matsumura, 1985; Mosialos et al., 1994). Cell transformation is often accompanied by a variety of phenotypical alterations including changes in cell shapes and motility, cell rounding, increased cell motility, loss of anchorage dependency, and the loss of cell–cell contacts. Many of these changes are caused by the alterations in the microfilament cytoskeleton of the cell periphery where fascin is typically found. Indeed, these transformed cells expressing high levels of fascin show an increased activity of membrane extensions, again suggesting the function of fascin in such activities.
By introducing human fascin into LLC-PK1 pig epithelial cells through DNA transfection and protein microinjection, we have examined how fascin contributes to the alterations in membrane protrusions, microfilament organization, and cell motility. LLC-PK1 cells express a low level of fascin, and thus the effects of exogenous expression of fascin were expected to be readily detected. In addition, the molecular weight of pig fascin is slightly higher than that of human fascin, making it easy to distinguish exogenously expressed human fascin from endogenous pig fascin. We have isolated several stable clones expressing human fascin at levels similar to those observed in the transformed cells (such as HeLa cells), as well as in the specialized normal cells. We have found that fascin transfectants develop numerous surface extensions including microspikes and lamellipodia. These alterations appear to cause greatly increased cell motility. Transfectants show a migration activity 8–17 times more than that of parental cells when assayed by modified Boyden chamber. Microinjection of fascin protein also causes membrane extensions, suggesting that fascin is directly responsible for the alterations in the peripheral actin cytoskeleton.
MATERIALS AND METHODS
Cell Culture and Transfection
Cultured cells were: LLC-PK1 cells (a pig epithelial cell line, CRL 1390; American Type Culture Collection, Rockville, MD), REF52 (rat embryo-derived fibroblastic cells), REF-4A (SV-40–transformed REF52 cells), REF-Ad5 (adenovirus-transformed REF52), NRK (normal rat kidney, fibroblastic cells, CRL1570; American Type Culture Collection), Kirsten virus-transformed NRK (CRL1569; American Type Culture Collection), and HeLa cells. LLC-PK1 cells were maintained in M199 medium (Life Technologies, Gaithersburg, MD) containing 3% fetal calf serum in an atmosphere of 5% CO2 and 95% air at 37°C. Other cells were maintained in DMEM containing 10% newborn calf serum.
A SmaI fragment (1.7 kilobases [kb]) of human fascin cDNA (Duh et al., 1994; Mosialos et al., 1994) was cloned into an expression vector, pRc/CMV (Invitrogen, Carlsbad, CA), and the vector was used for transfection of LLC-PK1 cells using a Lipofectin reagent (Life Technologies). Stable clones were isolated after selection with 500 μg/ml of G418 for 2–3 wk.
Immunofluorescence, Western Blotting, and Immunoprecipitation
Antibodies used for immunofluorescence include fascin monoclone 55K-2 (Yamashiro-Matsumura and Matsumura, 1986), β-catenin monoclone (Transduction Laboratories, Lexington, KY) and polyclone (Sigma Chemical, St. Louis, MO), E-cadherin monoclone (Transduction Laboratories), myosin polyclone (provided by Drs. J. Sellers and R. Adelstein, NIH/NHLBI, Bethesda, MD), villin monoclone (Immunotech, Westbrook, ME), vinculin monoclone (Sigma Chemical), and β-tubulin monoclone (Amersham, Arlington Heights, IL). Immunofluorescence involving fascin localization was performed as described previously (Yamashiro-Matsumura and Matsumura, 1986) with cells fixed with methanol. For immunolocalization of myosin and vinculin, or for phalloidin staining, cells were fixed with formaldehyde and permealized with acetone as described previously (Yamashiro-Matsumura and Matsumura, 1986). Phase and immunofluorescence micrographs were taken using Kodak Technical Pan films and T-Max P400 films (Eastman Kodak, Rochester, NY), respectively. In some experiments, images were taken with an AT200 cooled CCD camera (Photometric, Tucson, AZ) and processed with a MicroTome image deconvolution software (VayTek, Fairfield, IA).
Western blotting was performed using an enhanced chemiluminescent system (Amersham as described previously (Yamashiro-Matsumura and Matsumura, 1986). Immunoprecipitation of fascin was performed as described (Yamakita et al., 1996) using an immunoprecipitation buffer described by Tao et al. (1995) with three different polyclonal antibodies (kindly provided by Dr. R. Ishikawa, Gunma University, Gunma, Japan; Dr. P. McCrea, University of Texas MD Anderson Cancer Center, Houston, TX; and Dr. J. C. Adams, University College, London, England). Briefly, cells were lysed in an immunoprecipitation buffer containing 20 mM Tris-HCl, pH 7.6, 150 mM KCl, 0.6 mM CaCl2, 2.0 mM MgCl2, 0.5 mM ATP, and 0.05% Triton X-100, homogenized by several passages through a 25-gauge needle, and centrifuged in an Eppendorf centrifuge for 20 min. A primary antibody and then protein A-Sepharose beads were added to the supernatant. As a control, unrelated polyclonal antibodies, including anti-cdc2, or anti-tropomyosin antibodies were used instead of the anti-fascin antibodies. The antigen/antibody/Protein A complexes were washed extensively with the immunoprecipitation buffer and analyzed by SDS-PAGE followed by Western blotting.
Microinjection
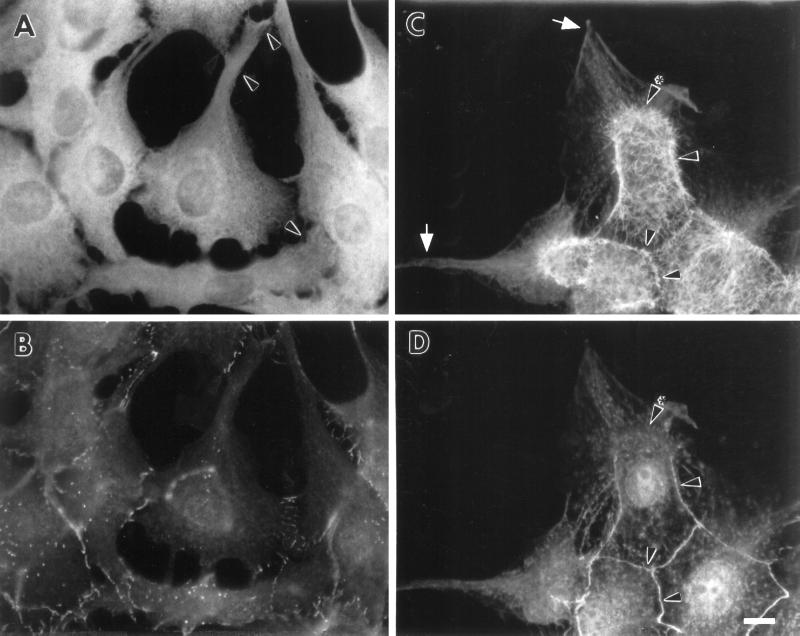
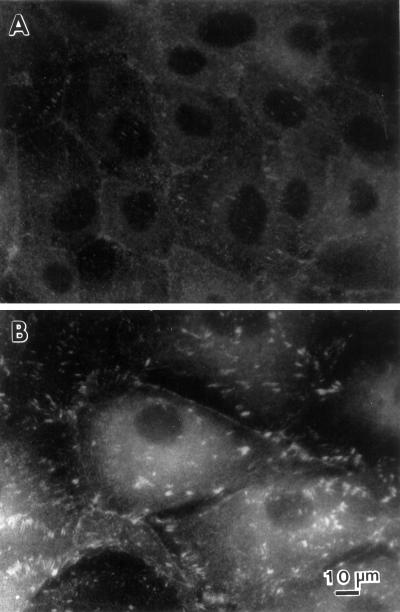
Human fascin protein (5 mg/ml) was purified from HeLa cells by the method described previously (Yamashiro-Matsumura and Matsumura, 1985). Recombinant human fascin was also purified as described by Ono et al. (1997). LLC-PK1 cells were microinjected with purified fascin as described by Yamakita et al. (1990). After 3–16 h incubation, the cells were fixed with formaldehyde and stained with rhodamine phalloidin to examine F-actin structures or fixed with methanol and stained with the monoclonal antibody against human fascin (55k-2) to localize fascin. FITC-dextran was comicroinjected to identify injected cells. In some experiments, microinjected cells were fixed with formaldehyde and double-labeled with phalloidin and the anti-fascin antibody (55k-2). Although fascin staining with formaldehyde-fixed cells was weak and had a nonspecific background, the staining could be used to identify injected cells (Figure 9A).
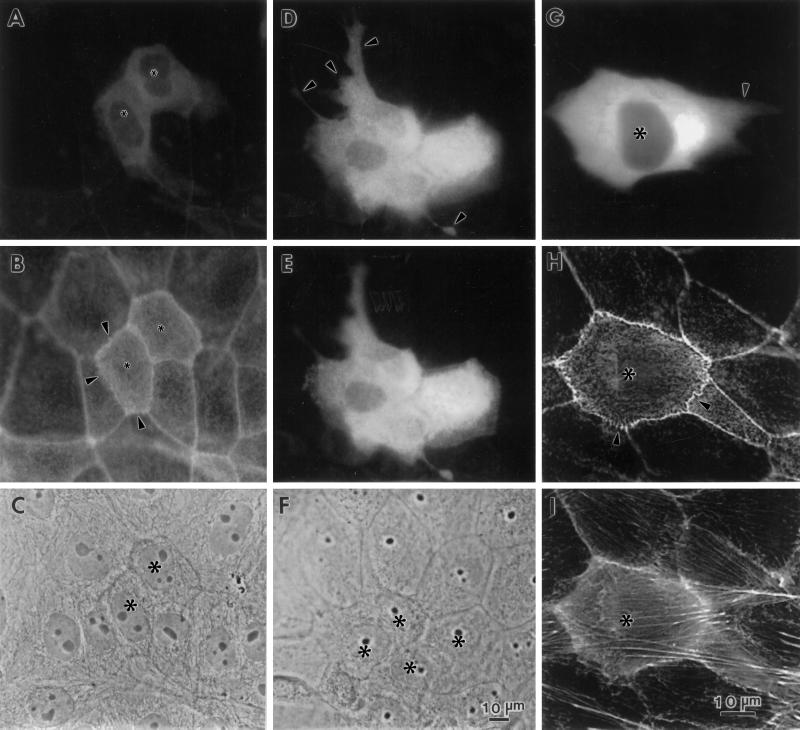
Figure 9.
Microinjection of fascin into LLC-PK1 cells causes morphological alterations similar to those found in DNA transfection. Asterisks indicate microinjected cells. (A–C) Induction of microvilli on apical surfaces. Two LLC-PK1 cells were microinjected with human fascin, fixed with formaldehyde, and stained with rhodamin phalloidin (B) and the 55k-2 fascin antibody (A). (C) Phase contrast. The microinjected cells develop more microvilli on their apical surfaces (B). Note that longer microspikes are found radiating from circumferential bands (arrowheads). Fascin staining in (A) is not of good quality; it is only for the identification of injected cells because the fascin antibody (55k-2) does not work well with formaldehyde-fixed cells and gives a weak staining with nonspecific background. (D–F) Induction of membrane protrusions at basolateral surfaces by fascin microinjection. Four LLC-PK1 cells were microinjected, fixed with methanol, stained with the fascin antibody, and photographed at two different focal planes. (D) Basolateral surface; (E) apical surface; (F) phase contrast. Note that microinjected cells extend basolateral membranes (indicated by arrowheads in D). (G–I) Increase in phalloidin staining by microinjection with fascin. A cell was injected with human fascin together with FITC-dextran, stained with rhodamin phalloidin, and photographed using an AT200-cooled CCD camera (Photometrics). Deconvoluted images of panels H and I were obtained using MicroTome software. (G) FITC-dextran; (H) F-actin organization at a higher focal plane; (I) F-actin at a lower focal plane. F-actin staining is higher with injected cells (asterisk, H and I). Note that circumferential actin bands are partially disorganized (H, indicated by arrowhead) and that microvilli are more developed in the injected cell (H). Bar, 10 μm.
Modified Boyden Chamber Assay
The modified Boyden chamber assay was performed using 24-well Transwell filters (6-mm diameter, 8.0-μm pore size, Costar, Cambridge MA). LLC-PK1 or fascin-transfected cells (0.7 × 104-2 × 104 cells/filter) were plated on the upper sides of Transwell filters in M199 medium supplemented with 3% fetal calf serum but without G418. Cells were incubated at 37°C in a humidified incubator containing 5% CO2 for 18 h, after which filters were fixed with formaldehyde and cells were stained with the Diff-Quik stain kit (Baxter, McGaw Park, IL). After the upper side of each filter had been wiped with a cotton-tipped applicator to remove cells that had not migrated, the filters were viewed under bright field optics. To quantitate migration, stained cells were counted in five fields (under 100× magnification) from each of two filters for each condition. Results were expressed as the mean number of cells counted in each field ± SD.
RESULTS
Fascin Expression Is Increased upon Cell Transformation
Using quantitative Western blotting, we first examined the levels of fascin expression in a variety of normal and transformed cells, including LLC-PK1 (normal epithelial cells), HeLa (transformed epithelial cells), REF-52 (normal fibroblasts) and SV40-transformed REF-52 cells (REF-4A), NRK, and Kirsten virus-transformed NRK cells (CRL1569). Both normal cells of LLC-PK1 and REF52 express a very low level of fascin, about 0.051% of total protein, while NRK shows a slightly higher level (0.076% of total protein) of fascin expression. On the other hand, the transformed cells express 5–12 times more fascin than the level of fascin observed in normal cells: HeLa is the highest, comprising 0.61% of total proteins; CRL 1569 and REF-4A cells show 0.26% and 0.25% of total proteins, respectively. These results, together with the previous result that transformation of B-lymphocytes with Epstein-Barr virus increases the level of fascin mRNA by 200 fold, indicates that cell transformation greatly increases fascin expression.
Morphology of Stable Transfectants Overexpressing Human Fascin
To explore whether a high expression of fascin is involved in the alterations in the microfilament cytoskeleton and cell motility observed with these transformed cells, we increased the expression of fascin in LLC-PK1 cells through DNA transfection and isolated seven stable transfectants. These fascin transfectants are found to express 12 to 17 times as much fascin as the level of parental LLC-PK1 cells (see Western blotting shown in Figure 8), although it should be noted that these increased fascin levels are equivalent to those found in transformed cells such as HeLa cells. We could not isolate stable transfectants with lower levels of fascin expression: although some clones expressed lower levels of fascin immediately after transfection, the fascin expression of such clones increased to high levels during the early stages of selection and cloning.
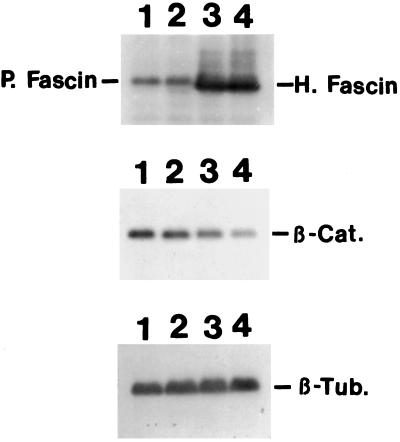
Figure 8.
Decrease in the β-catenin expression in fascin-transfected cells. Total cell lysates of parental LLC-PK1 (lane 1), mock-transfected LLC-PK1 (lane 2), and two clones (lane 3, clone 1; lane 4, clone 2) of fascin transfectants were immunoblotted with antibodies against fascin, β-catenin, or β-tubulin. Fascin antibody (55K-2) detected both endogenous pig fascin, as well as exogenous human fascin as indicated. Clones 1 and 2 express human fascin 12 times and 17 times more than the level of endogenous fascin. In contrast, β-catenin expression of clones 1 and 2 is decreased to 33% and 20%, respectively. Mock-transfected cells show no decrease in β-catenin expression. β-tubulin level was used as an internal control. H, Human fascin; P, pig fascin; β-Cat., β-catenin; β-Tub, β-tubulin.
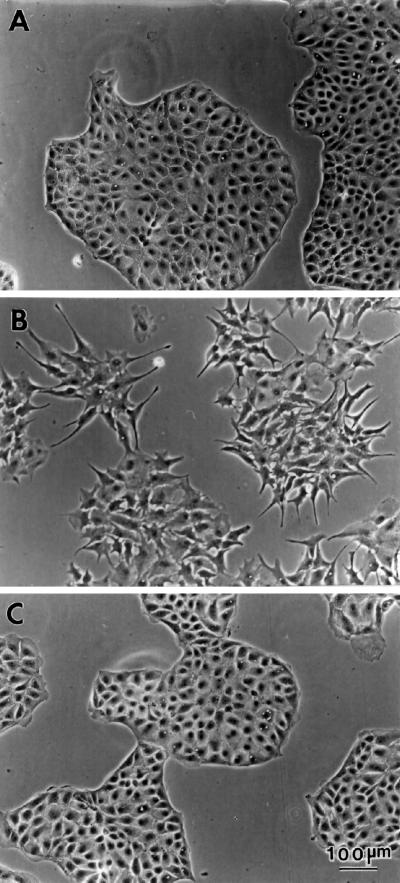
Phase contrast microscopy at low magnification (Figure 1) has revealed several prominent morphological alterations of the fascin transfectants. First, fascin transfectants develop many membrane protrusions. As Figure 1B shows, transfected cells show many elongated membrane projections, resulting in a morphology similar to a fibroblast. In contrast, parental cells (A) show a typical epithelial sheet with a relatively smooth edge and few projections. The development of membrane extensions appears to cause the flatter morphology of transfected cells: transfected cells occupy roughly twice as much area as parental cells (measured by the ImagePro image analysis software). The additional prominent change observed with fascin transfection is the disorganization of cell–cell contacts. As a result, transfectants do not form a dome whereas parental cells form a dome after confluency. It should be noted that all seven transfectants isolated so far show similar morphology, suggesting that fascin expression is involved in the morphological alterations described above. Mock transfection (C), on the other hand, has no effect on the morphological characteristics of the parental cell line.
Figure 1.
Phase contrast images of LLC-PK1, fascin-transfected, and mock-transfected cells. (A) Parental LLC-PK1 pig epithelial cells; (B) fascin-transfected cells; (C) mock-transfected cells. Note that transfected cells show many membrane extensions and that cell–cell contacts are disorganized. Bar, 100 μm.
Alterations in the Microfilament Cytoskeleton
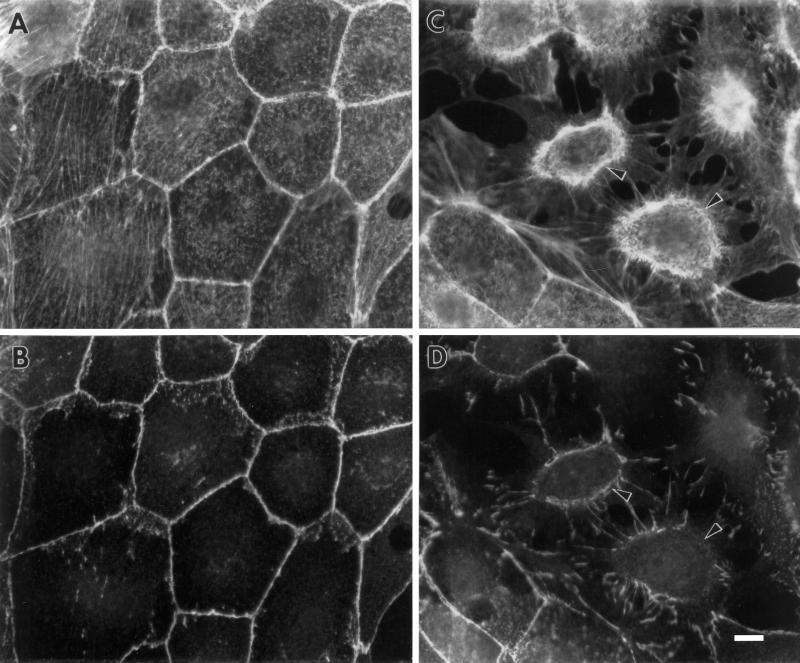
Fascin transfection greatly alters the organization of the actin cytoskeleton while affecting microtubules and intermediate filaments minimally (our unpublished observations). We have first examined the organization of F-actin by phalloidin staining (Figure 2) and found three major alterations. First, fascin transfectants develop membrane protrusions at lower cell surfaces (arrowhead in Figure 2B, fluorescent image taken at a lower focal plane). These membrane extensions are likely to be formed by the extension of basolateral surfaces (see below and MICROINJECTION OF FASCIN), which seems to explain the flatter morphology of fascin-transfected cells. On the other hand, parental cells (Figure 2A) show an epithelial cell sheet with relatively smooth periphery.
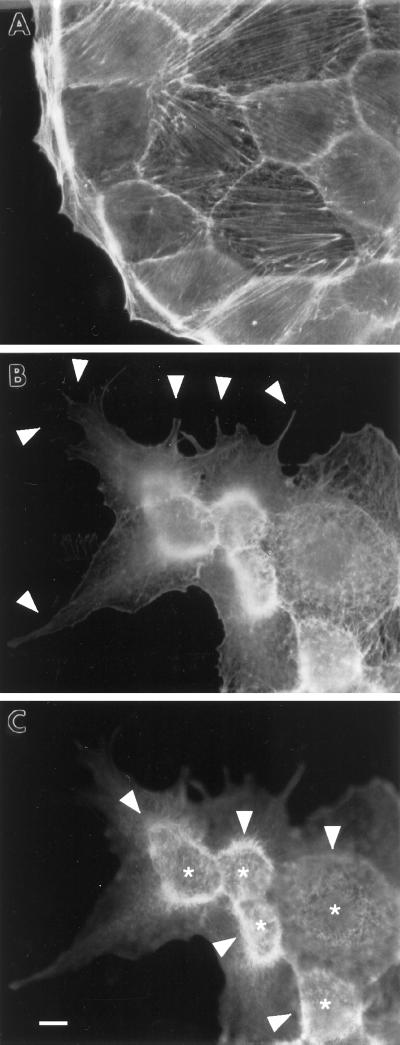
Figure 2.
Formation of membrane extensions and microspikes by fascin transfection. Parental LLC-PK1 and fascin-transfected cells were stained with FITC-phalloidin to examine F-actin organization at the periphery. (A) Image of parental LLC-PK1 cells; (B) image of fascin-transfected cells taken at a lower focal plane; (C) image of fascin-transfected cells taken at a higher focal plane. Note that fascin transfection induces the membrane extension at lower surfaces (arrowhead in B), as well as the formation of long and thick microspikes on upper surfaces (asterisk in C). Circumferential actin bands of parental LLC-PK1 cells appear to transform into ring-like structure (arrowhead in C) from which microfilament bundles are radiating. Bar, 10 μm.
A second morphological alteration caused by fascin transfection is the formation of long, thick microspikes on the upper surfaces of transfectants (Figure 2C, image taken at a higher focal plane). Instead of circumferential bands of parental cells, fascin transfectants show ring-like structures (arrowhead in Figure 2C) on the upper surfaces, from which many long actin bundles are radiating. In addition, numerous microspikes are formed in an area (asterisk) surrounded by the “ring.” These microspikes are much longer and thicker than microvilli present on apical surfaces of parental cells. Because β-catenin antibody stains the “ring” (see Figure 3 below), the surface surrounded by the “ring” is likely to correspond to a previously apical surface. These microspikes are stained with the villin antibody (our unpublished observations), indicating that fascin expression greatly extends the length of microvilli on the apical surfaces of the original cells.
Figure 3.
Disorganization of cell–cell contacts shown by fascin transfectants. Cells were double-labeled with fluorescent phalloidin and anti-β-catenin antibody. (A and B) LLC-PK1 cells; (C and D) fascin-transfected cells. (A and C) F-actin localization by phalloidin staining; (B and D) β-catenin localization. Note that cell–cell contacts are disorganized in fascin transfectants. The “rings” of transfectants (arrowhead in C and D) are stained with both the β-catenin antibody and phalloidin, confirming that they are derived from circumferential actin bands as a result of disorganization of cell–cell contacts of parental LLC-PK1 cells. Bar, 10 μm.
Third, the circumferential bands are mostly disorganized in fascin-transfected cells, resulting in the loss of cell–cell contacts. As Figure 3 shows, parent cells show typical polygonal bands in cell–cell contacts that are stained with both fluorescent phalloidin and anti-β-catenin antibody (A and B). In fascin-transfected cells (C and D), such circumferential actin bands appear to be transformed into the “ring” (arrowheads in Figure 3, C and D) because they are also stained by both phalloidin and the β-catenin antibody. This observation confirms that microspikes induced by fascin transfection are formed on apical surfaces, while the induction of membrane protrusions occurs at basolateral surfaces.
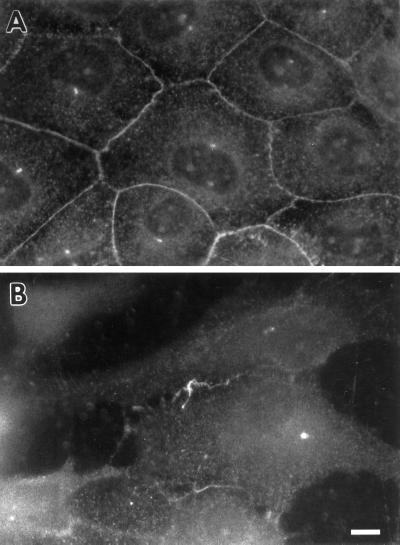
The disorganization of cell–cell contacts in fascin transfectants is also clearly seen by staining with an antibody against E-cadherin, another component of cell–cell contacts. As Figure 4 shows, E-cadherin shows disrupted localization similar to that of β-catenin although E-cadherin staining is more diffuse throughout the cytoplasm than β-catenin staining.
Figure 4.
Localization of E-cadherin in LLC-PK1 and fascin transfectants. (A) LLC-PK1 cells; (B) fascin-transfected cells. The organization of E-cadherin is also disrupted. Bar, 10 μm.
Organization of Fascin in Parental and Fascin-transfected Cells
To explore how fascin is involved in the above alterations in the actin cytoskeleton, we examined the localization of fascin in both parental and fascin-transfected cells. For double labeling we chose β-catenin and fascin for the following two reasons: First, fascin has been reported to bind to β-catenin (Tao et al., 1995); Second, the antibody to fascin requires methanol fixation for immunolocalization, and thus we cannot colocalize F-actin or myosin, the localization of which requires formaldehyde fixation.
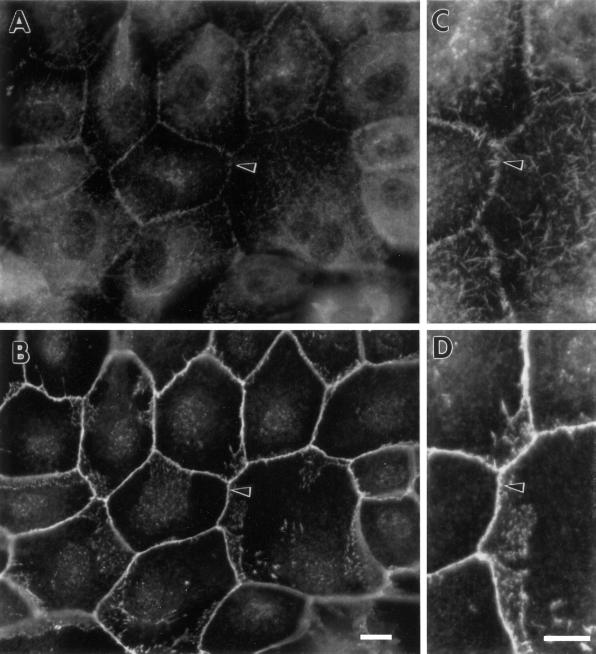
Figure 5 demonstrates the localization of fascin in a normal situation, i.e., in LLC-PK1 cells. Although fascin staining is very weak, we can localize fascin in cell–cell contacts, as well as in microvilli on apical surfaces (A). Fascin has been reported to colocalize with β-catenin in cell–cell contacts of A431 cells (Tao et al., 1995). The localization of fascin in cell–cell contacts of LLC-PK1 cells, however, differs significantly from that of β-catenin. While β-catenin antibody continuously stains adhesion belts, fascin staining appears discontinuous. Close examination (see high magnification photographs of panels C and D) has revealed that fascin antibody stains short spikes radiating upward from adhesion belts but not the adhesion belts (see arrowhead in panels C and D).
Figure 5.
Organization of fascin and β-catenin in parental LLC-PK1 cells. (A and C) Fascin localization; (B and D) β-catenin localization. (C) and (D) are high magnification images corresponding to (A) and (B), respectively. Note that fascin is localized in short microspikes radiating from the adhesion belts while β-catenin is continuously localized on the adhesion belts (arrowhead). Bar, 10 μm.
In fascin transfectants, cytoplasmic staining of fascin is greatly increased, although the staining is particulate rather than entirely diffuse (Figure 6A). In addition, fascin appears to be localized in membrane extensions (arrowheads in A). To reduce the background, fascin-transfected cells were first extracted with a low concentration (0.03%) of Triton X-100 and then double stained with anti-fascin and anti-β-catenin antibodies. As Figure 6C shows, fascin exhibits two types of localization. First, it is found in long, thick microfilament bundles radiating from the “ring” (indicated by arrowhead), as well as in numerous microspikes present on apical surfaces surrounded by β-catenin. Second, fascin is present in membrane protrusions (arrow in C). These observations strongly suggest that fascin overexpression facilitates the formation of fascin-containing actin bundles in LLC-PK1 cells together with membrane extensions. It should be noted that β-catenin staining is less prominent where fascin-containing microfilament bundles are more developed (see arrowhead with asterisk in Figure 6, C and D).
Figure 6.
Localization of fascin and β-catenin in fascin-transfected cells. Intact (A and B) or Triton-permealized (C and D) fascin transfectants were double labeled with antifascin and anti-β-catenin antibodies. (A and C) fascin; (B and D) β-catenin. In intact cells (A and B), fascin staining is not entirely diffuse but appears particulate (A). In addition, fascin shows the localization in membrane extensions (arrowhead in A). Triton permealization makes fascin’s localization in microvilli and membrane protrusions very clear (C). Fascin localizes in long and thick microvilli (arrowhead in C) radiating from “rings,” as well as in membrane extensions (arrow in C). Note that less β-catenin is found where more fascin microfilament bundles are developed (arrowhead with asterisk in C and D). Bar, 10 μm.
Localization of Vinculin
Fascin transfection induces the extension of basolateral membranes. It is possible that the extension may cause alterations in focal contacts. We have thus examined the localization of vinculin. As Figure 7 shows, more and larger focal contacts are found in fascin-transfected cells (B), in comparison to those found in parental cells (A). These results indicate that the basolateral membrane expansion accompanies the development of focal contacts, the organization of which is reminiscent of that seen in fibroblasts.
Figure 7.
Vinculin staining of LLC-PK1 and fascin-transfected cells. (A) LLC-PK1; (B) fascin-transfectants. More and larger focal contacts are seen in transfected cells. Bar, 10 μm
Increased Cell Migration of Fascin-transfected Cells
The induction of lamellipodia and microspikes by fascin transfection suggests that fascin transfectants may gain increased cell motility. We have thus examined the cell migration activity of wild and fascin transfectants using a modified Boyden chamber. Varying numbers of fascin-transfected cells were plated on upper chambers, and cells that had migrated through 8-μm holes were counted after 18 h incubation. As a control, the same numbers of parental LLC-PK1 were plated and counted in the same way. As Table 1 shows, the number of migrated cells was 8–17 times greater with fascin-transfected cells than that with wild LLC-PK1 cells. This finding indicates that cell motility is greatly enhanced by the expression of fascin.
Table 1.
Cell migration activity of parental and fascin-transfected cells
| Number of cells added in upper chamber | LLC-PK1 cellsa | Fascin transfectantsa |
|---|---|---|
| 2.0 × 104 | 10 ± 1.6 | 82 ± 8 |
| 1.4 × 104 | 7 ± 3 | 121 ± 58 |
| 0.7 × 104 | 1.6 ± 0.7 | 16 ± 9 |
Number of migrated cells measured by modified Boyden chamber method.
Decrease in the Expression of β-Catenin in Fascin-transfected Cells
One of the prominent phenotypic alterations of fascin transfection is the disorganization of cell–cell contacts. We have thus examined whether the level of expression of β-catenin is altered. We used parental cells and two transfectants (clone 1 and 2) expressing different levels of human fascin (Figure 8). Because tubulin organization and its staining intensity seem unchanged upon fascin transfection, the reactivity of the tubulin antibody was used as an internal standard for the normalization between parental and fascin-transfected cells. Quantitative Western blotting has revealed that clone 1 and 2 express fascin 12 and 17 times, respectively, more than the level of endogenous fascin in parental LLC-PK1 cells. It should be noted that these increased levels of fascin expression correspond to 0.61 and 0.77% of total protein, respectively, which are the same levels found in transformed cells such as HeLa cells. The expression levels of β-catenin are decreased to one-third and one-fifth of the level of parental cells in clone 1 and 2, respectively. We have also examined the level of E-cadherin in clone 2. Despite the disorganization of E-cadherin organization in transfected cells, the expression level of E-cadherin is decreased by only 20% (our unpublished results).
Fascin has been reported to bind to β-catenin in HeLa cells and brain (Tao et al., 1995). We thus examined whether fascin is associated with β-catenin in LLC-PK1 cells or fascin-transfected cells. Fascin was immunoprecipitated using three different polyclonal antibodies (kindly provided by Dr. R. Ishikawa, Dr. P. McCrea, and Dr. J.C. Adams), and the immunoprecipitates were immunoblotted with the β-catenin antibody. As controls, unrelated polyclonal antibodies such as anti-tropomyosin or anti-cdc2 antibody were used at the same time. We have found that the level of β-catenin in the fascin immunoprecipitates is very low and indistinguishable from those present in the control immunoprecipitates using anti-cdc2 or anti-tropomyosin antibodies (our unpublished results). This result suggests that the binding between fascin and β-catenin may not be significant in these cell lines, LLC-PK1 cells, and fascin transfectants. The lack of association appears to reflect the difference in the immunofluorescent localization between fascin and β-catenin (Figure 5).
Microinjection of Fascin
To explore whether fascin directly causes the morphological alterations described above, we microinjected a fascin protein into parental cells, LLC-PK1. Microinjected cells were stained with either phalloidin (Figure 9, B, H, and I) or with the fascin monoclonal antibody (A, D, and E).
We have observed three major effects as early as 3 h after microinjection of fascin protein. First, thicker and longer microfilament bundles are formed on apical surfaces. Figure 9, A and B, represents two fascin-injected cells (asterisks) that were stained with fascin antibody and rhodamin phalloidin, respectively. Figure 9A identifies the two cells injected with fascin. The phalloidin staining has revealed that the fascin-injected cells develop more microvilli on their apical surfaces than surrounding uninjected cells. Similar induction of more microvilli on the apical surface is also observed in a fascin-injected cell shown in Figure 9H (asterisk).
Second, fascin microinjection results in the protrusion of the basolateral membranes. Figure 9, D and E, shows micrographs of four injected cells taken at low and high focal planes, respectively. The micrograph taken at a lower focal plane (D) indicates that injected cells extend the basolateral membranes, resulting in the formation of lamellipodia- or filopodia-like structures (arrowhead). Similar extension of membrane is also observed with a fascin-injected cell shown in Figure 9G (arrowhead). A third alteration observed with fascin microinjection is that phalloidin staining becomes higher in fascin-injected cells. Panels H and I are deconvoluted images taken at the apical and basolateral focal planes. In both images, fascin-microinjected cells show higher staining with fluorescent phalloidin. Because they are deconvoluted images, the higher staining is not due to a difference in the thickness of fascin-injected cells, but rather suggests that the F-actin content is increased by fascin.
The first two morphological effects caused by protein microinjection, i.e., the formation of microvilli on apical surfaces and the extension of basolateral membranes, are quite similar to two of the three morphological alterations seen with fascin DNA transfection (see Figures 2 and 3). This suggests that fascin has a direct role in inducing these two types of structural changes upon transfection. It should be noted that lamellipodia-like protrusions occur only at basolateral surfaces but not on apical surfaces. These differential responses to fascin microinjection reflect the distinct natures of apical and basolateral surfaces of polarized epithelial cells.
Fascin transfectants exhibit an additional alteration, the disorganization of cell–cell contacts. The cell–cell contacts of fascin-microinjected cells, however, appear mostly intact although, in a few cases, the actin organization at circumferential belts is subtly but significantly disturbed (see arrowheads in Figure 9H). In addition, microspikes are more developed, radiating from the adhesion belts (arrowheads in B), which could be an early stage of the disorganization of cell–cell contacts seen in DNA transfection. These observations suggest that the disorganization of cell–cell contacts may require the prolonged presence of fascin.
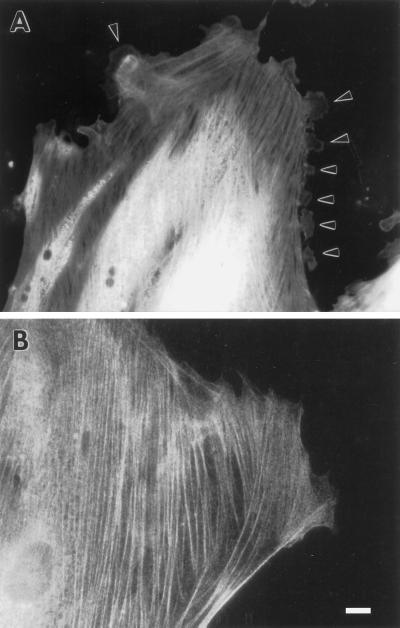
To examine whether fascin causes similar morphological alterations in the other type of cultured cell, i.e., fibroblasts, we microinjected fascin protein into REF52 cells. We chose REF52 cells because they contain a very low level of fascin and should thus be relatively sensitive to such manipulation. As Figure 10 shows, microinjection of fascin induces the formation of a variety of membrane protrusions at the regions of the cell periphery (A, indicated by arrowhead), including lamellipodia, filopodia, and in a few cases, long, branched microvilli. As a control, we microinjected caldesmon (B) and observed no alterations in the morphology at the periphery. These observations suggest that extension of the membrane is fascin’s primary function in both epithelial and fibroblastic cells.
Figure 10.
Induction of membrane protrusions by microinjection of fascin into REF-52 cells. (A) Recombinant human fascin was microinjected into REF-52 fibroblastic cells and stained with fascin antibody. (B) As a control, caldesmon was injected and stained with caldesmon antibody. Note that fascin microinjection causes numerous protrusions along all sides of the cell periphery of REF-52 cells (arrowhead in A). Bar, 10 μm.
DISCUSSION
Fascin shows greatly increased expression both in specialized normal cells and in many transformed cells. The forced expression of fascin to similar high levels in LLC-PK1 epithelia results in large alterations in morphology, as well as cell motility: fascin transfectants form longer and thicker microspikes on apical surfaces, develop lamellipodia-like structures on basolateral surfaces, show the disorganization of cell–cell contacts, and exhibit greatly increased cell motility. Microinjection of fascin in LLC-PK1 cells causes similar morphological alterations (Figure 9): lamellipodia are formed at basolateral surfaces, and long and thick microvilli are developed on apical surfaces, although massive disorganization of cell–cell contacts is not readily observed in protein-injected cells. These effects of fascin injection are not restricted to epithelial cells: normal fibroblasts, REF-52 cells, induce many lamellipodia at the cell periphery (Figure 10). These results, together, suggest that fascin, when expressed at high levels, plays a primary role in the development of membrane protrusions.
This notion of fascin’s primary role is supported by the characteristic properties shown by specialized cells expressing a high level of fascin, including neuronal and glial cells, microcapillary endothelial cells, and antigen-presenting dendritic cells (Duh et al., 1994; Mosialos et al., 1994, 1996; Pinkus et al., 1997). All of these cells exhibit membrane protrusions and are highly motile just like fascin-transfected cells. It is worthy of note that specific migratory cells of developing egg chambers of Drosophila express a high level of singed protein, a Drosophila homolog of fascin (Cant et al., 1994). The involvement of fascin in cell motility and membrane protrusions is further supported by the recent report of Adams (1997) that cell motility of C2C12 myoblasts is correlated with the formation of fascin-containing microspikes on thrombospondin-1 matrix.
How does fascin promote the formation of membrane protrusions such as microspikes and lamellipodia, the formation of which involves the alterations in the peripheral actin cytoskeleton? Actin binding and bundling activities of fascin are undoubtedly involved in these changes. An actin bundling activity of fascin easily explains the development of microvilli on the apical surfaces of LLC-PK1 transfectants (see Figures 2, 3, and 9), which is consistent with the analysis of Drosophila singed mutations, where deletion of the singed protein (Drosophila fascin) results in a short, curved bristle shaft with a decreased number of microfilament bundles (Poodry, 1980).
The formation of membrane protrusions like lamellipodia should require actin polymerization followed by the stabilization of actin filaments in the area of membrane extensions (Condeelis, 1992, 1993; Welch et al., 1997). Fascin may affect both processes. First, fascin could stabilize membrane protrusions by acting as an actin cross-linking protein. Indeed, human fascin forms both actin bundles and actin gel in vitro depending on the molar ratio of fascin to actin (Yamashiro-Matsumura and Matsumura, 1985). At a low ratio such as 1:18, fascin forms actin gel while at a higher ratio, fascin makes actin bundles. Second, fascin appears to alter the equilibrium of actin polymerization as suggested by the increased phalloidin staining of cells microinjected with fascin (see Figure 9). Preliminary study, however, showed that fascin did not change the rate of actin polymerization or the critical concentration in vitro (our unpublished results). Perhaps, the cross-linking or bundling by fascin may inhibit actin depolymerization, just like Dictyostelium 30-kDa actin-bundling protein (Zigmond et al., 1992). Alternatively, cross-linking may reduce the mobility of actin filaments, thereby inhibiting annealing of short filaments when uncapped. This would prevent the loss of filament ends, thereby facilitating polymerization. α-Actinin has been reported to show such activity in vitro (Colombo et al., 1993). In any case, fascin’s effect on actin polymerization should be dynamic because fascin transfectants show increased cell migration through 8-μm holes. We are in the process of examining how fascin affects depolymerization or annealing.
The level of β-catenin is greatly decreased in fascin-transfected cells. While the exact reason for the down-regulation of β-catenin is not clear, fascin may be responsible. The disorganization of cell–cell contacts could release β-catenin from the cytoskeleton to the cytoplasm, and cytoplasmic β-catenin would be more unstable than cytoskeletal β-catenin. For example, the degradation of β-catenin occurs when adenomatous polyposis coli binds to β-catenin that is not associated with cell–cell contacts (Munemitsu et al., 1995; Gumbiner, 1997; Morin et al., 1997). Alternatively, the association between β-catenin and fascin (Tao et al., 1995) might cause degradation of β-catenin as did the association of adenomatous polyposis coli with β-catenin. However, we could not detect β-catenin in fascin immunoprecipitates in LLC-PK1 cells or in fascin transfectants. It is possible that the association may not be stable in our cells. The down-regulation of β-catanin may also be caused by the changes in cell morphology shown by fascin transfectants. In particular, the development of more and larger focal adhesions could change the signal transduction pathways, leading to the alterations in gene expression including β-catenin.
The effects of fascin overexpression are very different from those shown by the overexpression of other actin cross-linking or bundling proteins such as α-actinin or villin. For example, overexpression of α-actinin in fibroblasts has been reported to decrease cell motility (Gluck and Ben-Ze’ev, 1994). While villin overexpression was reported to induce microvilli on the surfaces of transfected fibroblasts, there seems to be no effect on the formation of lamellipodia (Friederich et al., 1989). These observations indicate that fascin plays a unique role in the organization of the actin cytoskeleton and cell motility and apparently explain why the expression of fascin is highly specific to certain tissues and cells.
The high expression of fascin in transformed cells suggests that fascin may play an important role in tumor pathophysiology. This speculation seems to be accurate. In collaboration with us, Dr. Gown has examined the expression of fascin in about 100 breast cancer cases and found that fascin expression is restricted to the high-grade tumors, which are more proliferative and metastatic (Sun et al., 1997). Thus, fascin is likely to be involved in the metastasis of breast cancer, and fascin could be an important marker for breast cancer pathology.
ACKNOWLEDGMENTS
We thank Drs. G. Plopper and V. Quaranta (The Scripps Research Institute, La Jolla, CA) for suggesting the cell migration assay. We also thank Drs P. McCrea (University of Texas MD Anderson Cancer Center, Houston, TX), R. Ishikawa (Gunma University School of Medicine, Gunma, Japan), and J.C. Adams (University College, London, England) for kindly providing the polyclonal antibodies against fascin; Drs. R. Adelstein and J. Sellers (NIH/NHLBI, Bethesda, MD) for antimyosin antibody; and Dr. F. Deis for his careful reading of this manuscript. This work is supported by grant R37 CA-42742 from NIH.
REFERENCES
- Adams JC. Formation of stable microspikes containing actin and the 55 kDa actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1: implications for the anti-adhesive activities of thrombospondin-1. J Cell Sci. 1995;108:1977–1990. doi: 10.1242/jcs.108.5.1977. [DOI] [PubMed] [Google Scholar]
- Adams JC. Characterization of cell-matrix adhesion requirements for the formation of fascin microspikes. Mol Biol Cell. 1997;8:2345–2363. doi: 10.1091/mbc.8.11.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J, Edwards R, Matsudaira P, Otto J, Wulfkuhle J. Fascin, an echinoid actin-bundling protein, is a homolog of the Drosophila singed gene product. Proc Natl Acad Sci USA. 1993;90:9115–9119. doi: 10.1073/pnas.90.19.9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant K, Knowles BA, Mooseker MS, Cooley L. Drosophila singed, a fascin homolog, is required for actin bundle formation during oogenesis and bristle extension. J Cell Biol. 1994;125:369–380. doi: 10.1083/jcb.125.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo R, DalleDonne I, Milzani A. Alpha-actinin increases actin filament end concentration by inhibiting annealing. J Mol Biol. 1993;230:1151–1158. doi: 10.1006/jmbi.1993.1232. [DOI] [PubMed] [Google Scholar]
- Condeelis J. Are all pseudopods created equal? Cell Motil Cytoskeleton. 1992;22:1–6. doi: 10.1002/cm.970220102. [DOI] [PubMed] [Google Scholar]
- Condeelis J. Life at the leading edge: the formation of cell protrusions. Annu Rev Cell Biol. 1993;9:411–444. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- Duh F-M, et al. cDNA cloning and expression of the human homolog of the sea urchin fascin and Drosophila singed genes which encodes an actin-bundling protein. DNA Cell Biol. 1994;13:821–827. doi: 10.1089/dna.1994.13.821. [DOI] [PubMed] [Google Scholar]
- Edward RA, Herrera-Sosa H, Otto J, Bryan J. Cloning and expression of a murine fascin homolog from mouse brain. J Biol Chem. 1995;270:10764–10770. doi: 10.1074/jbc.270.18.10764. [DOI] [PubMed] [Google Scholar]
- Edwards RA, Bryan J. Fascins, a family of actin bundling proteins. Cell Motil Cytoskeleton. 1995;32:1–9. doi: 10.1002/cm.970320102. [DOI] [PubMed] [Google Scholar]
- Friederich E, Huet C, Arpin M, Louvard D. Villin induces microvilli growth and actin redistribution in transfected fibroblasts. Cell. 1989;59:461–475. doi: 10.1016/0092-8674(89)90030-5. [DOI] [PubMed] [Google Scholar]
- Gluck U, Ben-Ze’ev A. Modulation of alpha-actinin levels affects cell motility and confers tumorigenicity on 3T3 cells. J Cell Sci. 1994;107:1773–1782. doi: 10.1242/jcs.107.7.1773. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. A balance between beta-catenin and APC. Curr Biol. 1997;7:R443–446. doi: 10.1016/s0960-9822(06)00214-4. [DOI] [PubMed] [Google Scholar]
- Holthuis JC, Schoonderwoert VT, Martens GJ. A vertebrate homolog of the actin-bundling protein fascin. Biochim Biophys Acta. 1994;1219:184–188. doi: 10.1016/0167-4781(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Lin XH, Grako KA, Burg MA, Stallcup WB. NG2 proteoglycan and the actin-binding protein fascin define separate populations of actin-containing filopodia and lamellipodia during cell spreading and migration. Mol Biol Cell. 1996;7:1977–1993. doi: 10.1091/mbc.7.12.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Actin crosslinking proteins at the leading edge. Semin Cell Biol. 1994;5:165–174. doi: 10.1006/scel.1994.1021. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC [see comments] Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Mosialos G, Birkenbach M, Ayehunie S, Matsumura F, Pinkus GS, Kieff E, Langhoff E. Circulating human dendritic cells differentially express high levels of a 55-kd actin-bundling protein. Am J Pathol. 1996;148:593–600. [PMC free article] [PubMed] [Google Scholar]
- Mosialos G, Yamashiro S, Baughman RW, Matsudaira P, Vara L, Matsumura F, Kieff E, Birkenbach M. Epstein-Barr virus infection induces expression in B lymphocytes of a novel gene encoding an evolutionarily conserved 55-kilodalton actin-bundling protein. J Virol. 1994;68:7320–7328. doi: 10.1128/jvi.68.11.7320-7328.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Yamakita Y, Yamashiro S, Matsudaira PT, Gnarra JR, Obinata T, Matsumura F. Identification of an actin binding region and a protein kinase C phosphorylation site on human fascin. J Biol Chem. 1997;272:2527–2533. doi: 10.1074/jbc.272.4.2527. [DOI] [PubMed] [Google Scholar]
- Otto JJ. Actin-bundling proteins. Curr Opin Cell Biol. 1994;6:105–109. doi: 10.1016/0955-0674(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Otto JJ, Kane RE, Bryan J. Formation of filopodia in coelomocytes: localization of fascin, a 58,000 dalton actin cross-linking protein. Cell. 1979;17:285–293. doi: 10.1016/0092-8674(79)90154-5. [DOI] [PubMed] [Google Scholar]
- Otto JJ, Kane RE, Bryan J. Redistribution of actin and fascin in sea urchin eggs after fertilization. Cell Motil. 1980;1:31–40. doi: 10.1002/cm.970010104. [DOI] [PubMed] [Google Scholar]
- Pinkus GS, Pinkus JL, Langhoff E, Matsumura F, Yamashiro S, Mosialos G, Said JW. Fascin, a sensitive new marker for Reed-Sternberg cells of Hodgkin’s Disease. Evidence for a dendritic or B cell derivation? Am J Pathol. 1997;150:543–562. [PMC free article] [PubMed] [Google Scholar]
- Poodry CA. Epidermis: Morphology and Development. Vol. 2. M. Ashburner and T.R.F. Wright, New York: Academic Press; 1980. [Google Scholar]
- Sun S, Amenta P, Yamashiro S, Yamakita Y, Matsumura F, Gown AM. Expression of fascin in breast cancer correlates with tumor grade, increased cell proliferation and p53 tumor suppressor gene overexpression. Lab Invest. 1997;76:26A. (Abstract). [Google Scholar]
- Tao, Y.-S., Edwards, R., Bryan, J., and McCrea, P.D. (1995). The actin-bundling protein fascin associates with beta-catenin. Mol. Biol. Cell 6(suppl), 300a (Abstract). [DOI] [PMC free article] [PubMed]
- Welch MD, Mallavarapu A, Rosenblatt J, Mitchison TJ. Actin dynamics in vivo. Curr Opin Cell Biol. 1997;9:54–61. doi: 10.1016/s0955-0674(97)80152-4. [DOI] [PubMed] [Google Scholar]
- Yamakita Y, Ono S, Matsumura F, Yamashiro S. Phosphorylation of human fascin inhibits its actin-binding and -bundling activities. J Biol Chem. 1996;271:12632–12638. doi: 10.1074/jbc.271.21.12632. [DOI] [PubMed] [Google Scholar]
- Yamakita Y, Yamashiro S, Matsumura F. Microinjection of nonmuscle and smooth muscle caldesmon into fibroblasts and muscle cells. J Cell Biol. 1990;111:2487–2498. doi: 10.1083/jcb.111.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro-Matsumura S, Matsumura F. Purification and characterization of an F-actin-bundling 55-kilodalton protein from HeLa cells. J Biol Chem. 1985;260:5087–5097. [PubMed] [Google Scholar]
- Yamashiro-Matsumura S, Matsumura F. Intracellular localization of the 55-kD actin-bundling protein in cultured cells: spatial relationships with actin, alpha-actinin, tropomyosin, and fimbrin. J Cell Biol. 1986;103:631–640. doi: 10.1083/jcb.103.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH, Furukawa R, Fechheimer M. Inhibition of actin filament depolymerization by the Dictyostelium 30,000-D actin-bundling protein. J Cell Biol. 1992;119:559–567. doi: 10.1083/jcb.119.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]