Summary
Our understanding of the origins and the biological functions of different peripheral B cell subsets continues to evolve. Some understanding has been obtained regarding the synergy between BCR derived signals and other receptors and signaling pathways that drive the development of follicular, marginal zone and B-1 B cells, but this remains a complex and poorly understood issue. More recent information regarding the origins of B-1 and B-2 B cells, the ability of follicular B cells to mature both in the bone marrow and the spleen, the existence of a definable precursor for MZ B cells, and the ability of follicular B cells to occupy two distinct niches are all highlighted in this review.
Introduction
Naïve B cells are generally divided into three subsets, B-1 B cells, follicular B cells, and marginal zone (MZ) B cells. B-1 B cells are typically subdivided into B-1a and B-1b B cells. The origins and development of these unique subsets will be considered below following a discussion of recent developments regarding the functions of these cell types. Individual subsets have been best defined in rodents, and unique sub-set specific functions can therefore most clearly be assigned in mice. Cells of different subsets vary in terms of their location, their ability to migrate, and in the likelihood that they will be activated in a T-dependent or a T-independent fashion.
Beyond the widely recognized role of peripheral B cells in mediating humoral immune responses, B cells can also secrete cytokines and have the potential to present antigen to naïve T cells. Functions of B cells that are less well understood include the potential role of some B cell populations as regulatory cells inhibiting tissue specific inflammation, the putative role of activated cytokine producing B cells in the activation of T cells that drive inflammation, and the likely role of B cells in the induction of tertiary lymphoid organs at sites of disease related inflammation.
Follicular B cells occupy two functional niches
Naïve, mature follicular B cells occupy two niches during their recirculatory wanderings. Once they mature and attain the ability to recirculate in the spleen (or in the bone marrow itself), they migrate repeatedly through the blood and the lymph to B cell areas of lymph nodes, Peyer’s patches, and the spleen. Naïve follicular B cells residing in the well-established “follicular niche” may present T-dependent antigens to activated T cells. The follicular niche therefore represents the major site at which recirculating B cells mediate T-dependent immune responses to protein antigens.
During a T-dependent B cell response “danger” is interpreted in part by dendritic cells. The contribution of TLR ligands on B cells themselves to T-dependent B cell activation has been a source of controversy (1, 2), but it is likely that follicular B cells activated in the follicular niche by T-dependent antigens of microbial origin receive synergistic signals delivered via the BCR, CD40, and TLRs. Differentiation of activated B cells into antibody secreting cells may be facilitated by TLR activation on follicular B cells, but only in the context of prior BCR and CD40 activation. Follicular B cells in mice express the same constellation of Toll like receptors that MZ B cells and B-1 B cells do. They can be induced to proliferate following exposure to TLR ligands but, unlike MZ B cells and B-1 B cells, follicular B cells lack the intrinsic ability to differentiate into antibody secreting cells if stimulated only by TLR ligands (3). Early in a T cell - dependent response, activated follicular B cells can also differentiate into short-lived plasma cells that do not migrate to distant sites. In contrast, activated germinal center B cells may, as they differentiate into plasmablasts, obtain the ability to migrate to the bone marrow where they may be sustained as long-lived plasma cells by the interaction of APRIL and/or BAFF produced by stromal cells with BCMA, a receptor of the BAFF-R family, on plasma cells (4).
Apart from homing to follicles in conventional secondary lymphoid organs, follicular B cells also home to the bone marrow where they form discrete collections around vascular sinusoids (5, 6). This “perisinusoidal niche” is made up of the same re-circulating B cells that at different temporal periods in their wanderings reside in follicles. While a requirement for BAFF has been established in the context of survival in the follicular niche, it is unclear whether BAFF nurtures B cells in the perisinusoidal niche. Perisinusoidal B cells can be activated by blood borne microbes in a T cell - independent manner, and differentiate into IgM-secreting AFCs, but they are unable to induce AID, possibly because they are not architecturally configured to readily interact with helper T cells which are relatively scarce in this compartment. So far all the available evidence for the activation of bone marrow perisinusoidal B cells has been obtained from studies in which live bacteria were injected intravenously. It is assumed that some additional signals are provided by the injected microbe, but the nature of the presumed “second signals” for T-independent B cell activation in the bone marrow remain to be established.
Functional roles of Marginal zone B cells
MZ B cells are considered to be innate-like cells that can be induced to differentiate into short-lived plasma cells in the absence of BCR ligation. MZ B cells can also mediate the transport of antigen in immune complexes into splenic follicles, may be involved in T-dependent B cell responses, and may participate in immune responses to lipid antigens. In rodents, MZ B cells reside primarily in the vicinity of the marginal sinus of the spleen where they are positioned by the activity of S1P1 and S1P3, receptors for sphingosine1-phosphate (7–9). Although these cells were long viewed as being sessile, it has been demonstrated that they can transport antigen in immune complexes from the vicinity of the marginal sinus to follicular B cells in the splenic follicle (9, 10), and it is presumed that they can shuttle between these two locations. The high levels of CD21 expressed on MZ B cells presumably evolved to facilitate immune complex capture. In rodents, MZ B cells primarily appear to mediate T-independent responses to antigens in blood-born pathogens. During these pathogen driven responses, LPS and possibly other bacterial products may decrease integrin adhesiveness and drive the migration of activated B cells out of the MZ into the red pulp of the spleen where they may differentiate into short-lived plasma cells (11). In human lymph nodes B cells in an outer extra-follicular rim have also long been called MZ B cells. These human cells with an IgM+ memory B cell phenotype could potentially also play a role in antigen capture and transport into lymph node B cell follicles, in a manner analogous to that postulated for mouse splenic MZ B cells.
In addition to their important role in T-independent responses, MZ B cells may also participate in T-dependent immune responses to protein antigens, as well as in responses to lipid antigens. Although MZ B cells can potentially contribute to T-dependent responses to protein antigens by helping to deliver these antigens to follicular B cells, they may also be directly activated by T dependent antigens and receive T cell help. By virtue of their higher-level expression of MHC class II, B7-1 and B7-2, MZ B cells are better equipped to present antigen to and activate T cells than follicular B cells (12, 13). The potential for MZ B cells to mediate T-dependent responses has been described in cell transfer studies, but direct in vivo evidence for the participation of MZ B cells in T-dependent activation and germinal center formation is difficult to come by. Since recirculating transitional and follicular B cells serve as precursors of MZ B cells, it is difficult to distinguish between the T-dependent activation of precursors that reside at least transiently in follicles and the T-dependent activation of MZ B cells in the MZ itself.
MZ B cells also express high levels of CD1d, which could potentially be involved in the presentation of lipid antigens to NKT cells. NKT cells may activate MZ B cells in the spleen via CD40L – CD40 interactions in order to induce rapid class switched and potentially somatically mutated antibody responses (14).
Immune functions mediated by B-1 B cells
Most of our knowledge about the function of B-1 B cells is based on studies in rodents. A B-1 B cell population has not been clearly defined in humans, and indeed attempts to identify a B-1 compartment in a number of mammals have not met with success (15). In mice, although B-1a and B-1b B cells originate from different progenitors, they both seed the peritoneal and pleural cavities. The pleuro-peritoneal milieu appears to influence the functional characteristics of both B-1a and B-1b B cells as well as of the relatively small proportion of B-2 cells that reside in these sites (16, 17). B-1 cells can contribute to the generation of IgM responses to T-independent antigens such as phosphorylcholine, an antigen on many pathogenic bacteria. B-1 B cells can home from the peritoneum to mesenteric lymph nodes and to the intestinal lamina propria. The exact route and mechanism of migration is still a subject of debate. B-1 cells may leave the peritoneal cavity via the omentum, seed the parathymic lymph nodes and reach the blood stream via lymphatics and the thoracic duct (18). An alternative route from the peritoneum may involve egress via the omentum and direct trafficking to the intestine. TLR signaling can induce a downmodulation of integrin adhesiveness and of CD9 expression, thus facilitating egress of B-1 cells from the peritoneum. Activation and differentiation of B-1 cells can also be induced by cytokines such as IL-5 and IL-10. CXCL 13 produced by omental cells may contribute to egress of B-1 cells (in addition to also facilitating the entry of these cells into the peritoneum). The role of S1P1 on peritoneal B cells in mediating egress from the peritoneal cavity is presently unclear (19, 20). It has also been suggested that B-1 cells may need to traffic through the spleen for self-renewal to occur, but the requirement for the spleen for B-1 B cell self-renewal remains controversial (21, 22). At mucosal sites B-1 B cells can contribute to the generation of T-independent IgA responses.
Commensal bacteria presented by dendritic cells in the intestinal lamina propria may contribute to the activation of B-1 B cells. LPS and other TLR ligands can induce both the proliferation of B-1 B cells as well as their differentiation into IgM secreting short-lived plasma cells. Antigen specific B-1 B cells may be induced to switch in a T-cell independent manner into IgA secreting cells (23).
A potential role for γδ T cells in B-1 cell activation has been suggested, but there is no evidence that B-1 cells are ever activated in a conventional αβ T cell-dependent manner. While B-1a B cells contribute to innate-like immune responses B-1b B cells contribute to adaptive immunity (24, 25). They may represent a specialized type of IgM memory cell that originates from B-2 cells in a T-independent manner (24). These cells have been best characterized in a murine relapsing fever model involving chronic infection with Borrelia hermsii. Exactly where the B-2 cells that differentiate into B-1b B cells are activated in a T-independent manner is unclear but activation could potentially occur in the blood itself or in the bone marrow. It is unclear as to what products of the Borrelia spirochetes contribute to the acquisition of the ability of these cells to migrate to the peritoneal cavity and develop into B-1b B cells. These B-1b B cells can respond to blood-borne Borrelia challenge by producing protective antigen-specific IgM (24).
Development and selection
Cellular pathways for B cell maturation in the periphery
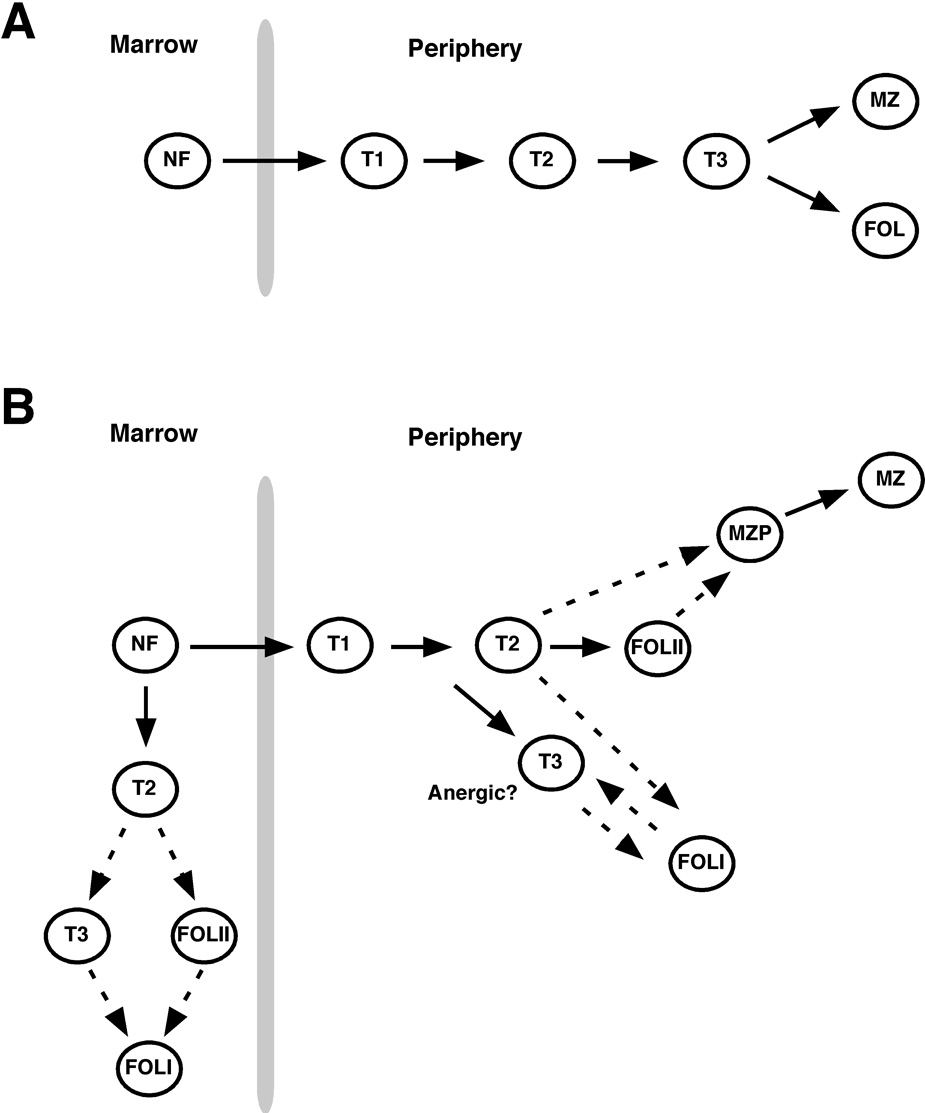
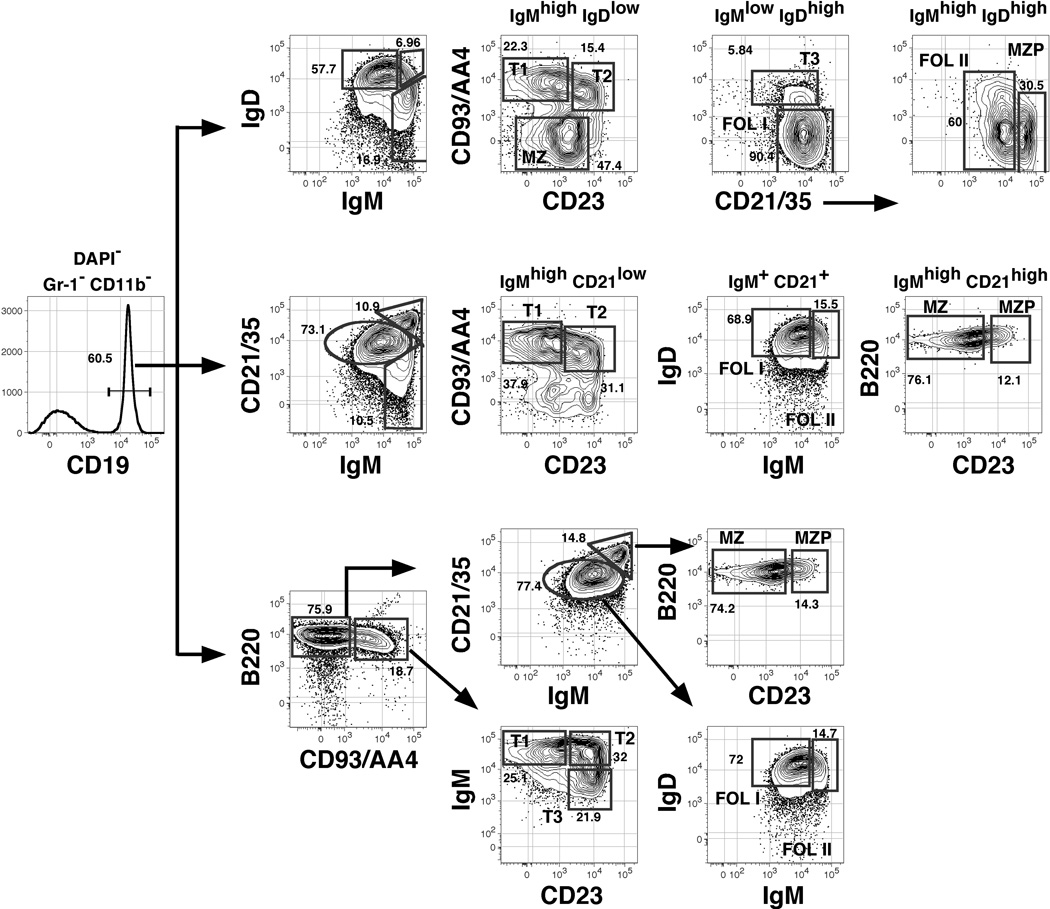
Newly formed B cells are generated in the bone marrow and either mature further at this site or in the periphery (Figure 1). Functionally immature B cells are defined by their short half-lives, their dominance during early phases of reconstitution of the peripheral B cell pool, and their propensity to undergo apoptosis rather than proliferate following BCR engagement (26, 27). Carsetti and colleagues first proposed that immature or “transitional” B cells in the adult spleen may be subdivided into two distinct subsets (T1 and T2) (28). The “T2” population as originally described contains considerable numbers of MZ B cell precursors (MZPs) (29), but studies showing that transitional B cells in the adult spleen can be divided into at least three subsets confirmed that peripheral B cell maturation may occur in a step-wise process (26). Unlike mature B cells, cells within all three transitional subsets express the B-lineage precursor marker CD93/AA4. Cells within each population also express low to intermediate levels of CD21 (and thus are distinct from MZPs), and are resolved by differential surface expression of IgM and CD23 into the IgMhigh CD23− (T1), IgMhigh CD23+ (T2) and IgMlow CD23+ (T3) subsets (26). Because alternative markers are sometimes employed to identify maturing B cells in the bone marrow and the periphery, we have summarized the surface phenotype of each subset in Table 1. Moreover, since multiple flow cytometric gating strategies have emerged to identify these and additional splenic B cell subsets (26, 30, 31), we devised an approach to resolve each population with a single test tube and via three different gating strategies. These data incorporate the general approaches employed by the Kearney, Allman, and Pillai laboratories, and are illustrated in Figure 2.
Figure 1. Peripheral B cell maturation from the bone marrow.
Past (A) and current (B) models for developmental stages in peripheral B cell maturation form the marrow are shown. Question marks indicate predicted precursor-successor relationships that have yet to be adequately tested. Cell surface markers employed to distinguish each population are summarized in Table 1.
Table 1.
Cell surface phenotypes for identification of BONE MARROW and splenic Blineage subpopulations
| Location | B cell subset | Phenotype* |
|---|---|---|
| Bone marrow | Newly formed, immature | AA4+ CD23− sIgMhigh sIgD−/low HSAhigh CD62L− CD21/35−/low |
| T2-like | AA4+ CD23+ sIgMhigh sIgDhigh HSAhigh CD62L+ CD21/35low | |
| Mature | CD23+ AA4− sIgMlow sIgDhigh HSAlow CD62L+ CD21/35low | |
| Spleen | Transitional T1 | CD23− AA4+ sIgMhigh sIgD−/low HSAhigh CD62L− CD21/35−/low |
| Transitional T2 | CD23+ AA4+ sIgMhigh sIgDhigh HSAhigh CD62L+ CD21/35low | |
| Transitional T3 | CD23+ AA4+ sIgMlow sIgDhigh HSAhigh CD62L+ CD21/35low | |
| Follicular type I | CD23+ AA4− sIgMlow sIgDhigh HSAlow CD62L+ CD21/35int. | |
| Follicular type II | CD23+ AA4−/low sIgMhigh sIgDhigh HSAlow CD62L+ CD21/35int. | |
| MZP | CD23+ AA4−/low sIgMhigh CD1d+ sIgDhigh HSA+ CD21/35high | |
| MZ | CD23− AA4− sIgMhigh CD1d+ sIgDlow HSA+ CD21/35high |
Note; all cells in question are CD19+ B220+ sIgM+.
Figure 2. Resolution of developing and mature subpopulations of splenic B cells.
Splenocytes from a single 8-week old female C57BL/6 mouse were stained in a single tube with antibodies to the indicated cell surface molecules before analysis of 200,000 events on an LSRII flow cytometer. All non-viable cells were eliminated from this analysis by gating on cells that failed to stain with the UV-excited DNA dye DAPI, and myeloid-lineage cells were eliminated by gating on CD11b− Gr-1− cells (gates not shown). CD19+ cells were identified as shown (left-most plot) and three gating strategies were employed to identify the indicated subsets. Gating strategies are from Cariappa et al. (top) (30), Martin and Kearney, and Srivastava et al., (middle) (29, 31), or Allman et al., (bottom) (26).
Cellular pathways for B cell maturation in the bone marrow
Previous models of sIgM+ peripheral B cell maturation predicted a linear developmental sequence that culminated in a binary cell fate decision in which late transitional B cells yield either follicular or MZ B cells (Fig. 1A). However, we suggest that mature B cells derive from one of at least two cellular pathways, one in which T2-like cells arise and further mature in the bone marrow, and a second analogous pathway in the periphery. Maturation via either route is characterized by the acquisition of responsiveness to multivalent antigen, up-regulation of surface markers associated with B cell maturation including IgD and CD23, dependency on the BLyS/BAFF receptor BR-3, and tonic as well as self-antigen dependent BCR signaling. The main evidence for this model stems from data showing that newly formed B cells in the bone marrow, defined in part by their relatively rapid turnover rate, are functionally heterogeneous with respect to multiple criteria typically employed to discern immature and mature B cells. For instance, whereas the least mature surface IgMhigh B cells in the bone marrow and the adult spleen are CD23− IgDlow, non-responsive to the anti-apoptotic cytokine BLyS/BAFF, and fail to respond effectively to multivalent antigens, a subpopulation of recently formed bone marrow B cells, similar to splenic T2 cells, are CD23+ IgDhigh and responsive to BLyS and multivalent antigens (6, 32).
Assuming that BCR signaling plays an essential role in the earliest phases of B cell maturation, the identification of T2-like bone marrow B cells suggests that B cell maturation may occur at different rates for individual clones, with certain BCRs driving maturation before emigration from the marrow. Further, because the majority of B cells mature in the periphery, most BCRs may not elicit or produce the appropriate maturation signals until after cells exit the marrow. Alternatively, early maturation may be a stochastic process unrelated to BCR specificity. This issue may only be resolved with a comprehensive characterization of the expressed BCR repertoire within each transitional B cell subset in the bone marrow and the spleen. Regardless, at present the weight of evidence indicates that mature B cells may derive from T2-like cells in the bone marrow or the periphery.
The tolerance issue: deletion, editing, and anergy among peripheral B cells
In adult mice a large fraction of recently generated bone marrow B cells fails to enter one of the long-lived B cell compartments. Whether this reflects negative selection of self-reactive clones versus failed positive selection remains an intriguing and critical question. In addition, the fate of self-reactive cells may be influenced by local levels of BAFF, as heightened BAFF levels promotes the survival and differentiation of self-reactive anergic B cells (33, 34). This notion is more thoroughly explored in the accompanying review by Stadanlick and Cancro (this issue).
Wardemann and Nussensweig and colleagues characterized the V-gene usage and specificity of more than 200 clones derived from immature and mature B cells from human bone marrow and peripheral blood (35). These heroic experiments illustrate that in healthy people 55 to 75% of immature B cells are self-reactive. Therefore, although untested in mice, the data suggest the possibility that cellular attrition within immature B cell pools may reflect the clonal deletion of self-reactive clones. Considerable evidence, discussed elsewhere in this issue, suggests that receptor editing is a major mechanism of central tolerance during B cell development. It therefore remains unclear as to what proportion of developing self reactive cells are subjected to clonal deletion versus receptor editing.
A third mechanism of tolerance described during B cell development is anergy. Because anergic B cells generated in Ig transgenic mice fail to thrive when forced to develop alongside non-anergic B cells (36), the extent to which anergy contributes to the self non-self discrimination problem remains open to debate. Interestingly, recent work suggests that the splenic T3 population is enriched for anergic (and self-reactive) clones (37) (see Fig. 1B). This idea is consistent with the notion that ligation of the BCR by self-antigen on immature sIgMhigh B cells within the T1 or T2 subset would likely down-modulate surface IgM levels, resulting in sIgMlow cells that otherwise fit the description of late transitional B cells. However, because cells within the T3 population exhibit a rather rapid 50% renewal rate of 4 days, we suggest that on average such cells fail to survive beyond this time frame (or differentiate into more mature cells that are sIgDhighsIgMlow), leaving open the question as to whether this population is a developmental intermediate, or a collection of anergic cells.
The positive selection issue: The follicular versus MZ B cell decision
Continuous BCR signaling is required for the survival of peripheral B cells including all follicular and MZ B cells (38). While this might indicate that the outcome of low-level or tonic BCR signaling is equal for follicular and MZ B cells, other experiments suggest that BCR specificity, and therefore BCR signaling, plays a key role in the follicular versus MZ cell fate decision. Both follicular and MZ B cells express a diverse array of V-gene segments. However, experiments with IgH transgenic mice showed that immature B cells specific for phosphorylcholine selectively yield MZ B cells (31). Likewise, B cells specific for Thy-1 are selected into the B-1 compartment in normal mice and to the MZ B cell pool in T cell deficient mice (39). Because phosphorylcholine is a constituent of the cell wall of encapsulated bacteria, which comprise a significant fraction of the normal gut flora, and Thy-1 is clearly a self-antigen, these experiments suggest that weakly self-reactive immature B cells may avoid negative selection and preferentially colonize the MZ. This notion is further supported by Weigert and colleagues who employed transgenic IgH plus Ig. “knock-in” mice in which the majority of B cells are DNA-reactive. In this system, the “56R” IgH transgene encodes DNA reactivity when paired with a number of light chains including λ1. Thus, editing events in immature B cells bearing the IgH and Igk transgenes often result in λ1 expression, and these cells can retain measurable DNA reactivity and selectively colonize the MZ (40). Interestingly, in this system many λ1+ MZ B cells co-express kappa light chains. This phenomenon, termed light chain isotype inclusion, indicates that unique selection pressures promote the maintenance of the MZ B cell pool. That distinct mechanisms govern the maintenance of follicular and MZ B cells is supported by data from Hao and Rajewsky showing that MZ B cells are selectively maintained following deletion of the RAG2 gene in adult mice (41).
The notion that the BCR regulates the follicular versus MZ B cell fate decision is further supported by genetic experiments involving the deletion or inactivation of genes encoding key components of the BCR signal transduction network. The resulting mutants can be categorized based on the degree with which they affect BCR-mediated signaling. Complete abrogation of BCR signaling leads to the loss of all splenic B cells (38). In contrast partial inactivation of BCR signaling, perhaps by crippling only one of multiple second messenger pathways typically activated by the BCR, can lead to selective loss of B-1 cells, and often lead to a decline in overall peripheral B cell survival (31, 42, 43). Other mutations that result in diminished BCR signaling can affect B-1 and follicular B cell development (primarily affecting sIgDhighsIgMlow FOL I B cells but not sIgDhighsIgMhigh FOL II B cells; 30) without affecting MZ B cell differentiation (44). Thus, whereas diminished BCR signaling can negate follicular and B-1 B cell development in some systems, in others diminished signaling does not, and if anything preferentially promotes the MZ B cell fate. One possible explanation for this complexity is that mutations in some signaling molecules that are believed to be downstream of the BCR may have more pronounced effects on signaling events that emanate from receptors other than the BCR that also influence peripheral B cell fate. Unraveling this puzzle will undoubtedly require a better understanding of the signaling pathways mediated by the BCR in the context of positive selection.
Notch signaling in MZ B cell development
Aside from the BCR, one key cell surface receptor required for MZ B cell development is Notch2. In mice and people the Notch family consists of four receptors (Notch1–4) and five cell surface ligands (see (45) for review). Engagement of Notch receptors by ligand induces two proteolytic cleavage events within Notch proteins; ADAM-protease mediated cleavage of the ectodomain followed by gamma secretase mediated release of the Notch intracellular domain (ICN). ICN then translocates to the nucleus where it forms a transcriptional activation complex with the transcription factor CSL (also termed RBP-Jκ) and the co-activator MAML1. Analyses of knockout mice provide clear evidence that Notch2 signaling via engagement by the Notch2 ligand Delta like-1 (DL-1) is critical for MZ B cell development, as MZ B cells are not detected in Notch2−/− and DL-1−/− mice (46, 47). It appears that Notch2-DL1 interactions are a key and perhaps limiting step in the formation of a unique MZ B cell niche. Thus, given that the MZ compartment may be enriched for self-reactive clones, understanding the role of Notch signaling in MZ B cell development may prove useful in combating antibody-mediated autoimmune syndromes.
Multiple questions remain concerning the mechanism whereby Notch signaling promotes the MZ B cell fate. First, the cell types expressing cell surface DL1 that induce Notch activation on developing B cells remain to be defined, although it is likely that these cells are found in the spleen and that equivalent cells do not exist in the bone marrow or in other lymphoid organs. Indeed, although peripheral B cells may express DL1, DL1-mediated MZ B cell differentiation appears to be mediated by a rare and poorly defined hematopoietic stem cell-derived stromal cell (47). When and where maturing B cells are exposed to these DL1+ cells, and whether such interactions invariably drive MZ B cell differentiation, remains unclear. Nonetheless, exposure of immature B cells to these potentially rare cells may constitute a key and rate limiting step responsible for regulating and maintaining the size of the MZ B cell pool.
A second question concerns the genetic targets of Notch2 signaling underpinning MZ B cell differentiation and whether Notch activity modifies other signaling pathways that govern the MZ fate. Genetic experiments show that Notch2 activity somehow intersects with the NF-κB pathway, as deletion of a single allele for Notch2 and the NF-κB component p50 results in complete loss of MZ B cells without affecting numbers of follicular B cells (48). Constitutive activation of the canonical NF-κB pathway can overcome the need for BAFF-BAFF-R signaling for follicular B cell survival and MZ B cell generation (49). It appears likely therefore that BAFF-R activation (inducing active NF-κB1 containing heterodimers) may synergize with Notch-2 to mediate the development of MZ B cells. Some synergism between BCR signaling and Notch2 might also contribute to MZ B cell development, since co-stimulation of the BCR and Notch2 on mature B cells results in synergistic B cell activation (50). Since BCR signaling is highly dependent on the NF-κB pathway, and Notch signaling sustains NF-κB activity in T cells (51), it is tempting to consider whether BCR, BAFF-R, and Notch signaling collaborate to promote B cell activation and MZ B cell development by together amplifying and/or sustaining NF-κB activation. This model appears consistent with the lack of MZ B cells in Notch2−/−, p50−/−, and Notch2+/− p50+/− mice, and the observed synergy between Notch2 and the BCR in standard B cell activation assays.
B-1 B cells
Although the development of every B cell requires successive rearrangement and expression of functional Ig heavy and light chain genes, there are key differences in the expressed antibody repertoire in fetal and adult life (see (52) for review). Most notably, whereas usage of VH gene segments in adult splenic B cells is stochastic, fetal-derived B cells preferentially utilize DH-proximal VH gene segments. Moreover, whereas terminal deoxynucleotidyl transferase (TdT) mediates N-addition in adult B cell precursors, fetal-derived B and T cells lack N-additions and B-lineage precursors in the fetal liver do not express TdT. These findings strongly suggest that the fetal-derived antibody repertoire is less complex compared to the V-gene repertoire of the adult B cell pool.
Whether CD5+ B-1 B cells derive largely from fetal progenitors has been hotly debated for years (52). Despite progenitor transfer experiments suggesting that B1 B cells derive from mostly from fetal precursors, because CD5 expression can be induced on adult splenic B cells following BCR crosslinking, and the vast majority of CD5+ peritoneal cavity B cells in 6–10 month-old mice contain N-additions, others have suggested that relatively strong positive selection signals drive adult transitional B cells into the B-1 compartment. Both viewpoints require reevaluation due to Dorshkind and colleagues who characterized a novel B-1-lineage dedicated precursor. Significantly, although B-1 dedicated precursors were highly enriched among fetal precursors, these cells were also detected in adult bone marrow (53). These findings raise several fundamental questions concerning B-1 B cell origins. Do all B-1 B cells derive from this unique progenitor pool? Do B-lineage precursors derived from this pool acquire cell surface markers expressed by canonical B-lineage progenitors? Do B-1 dedicated precursors in the adult express TdT? Experiments addressing these questions should allow a better understanding of the origins of B-1 B cells and how their unique developmental properties affect their function.
Conclusions
From these and many additional studies it has become apparent that all B cells are not created equal. Instead, each mature B cells falls into one of several functionally distinct subpopulations. Thus, although all B cells ultimately serve to yield antibody-secreting cells, many quantitative and qualitative features of the antibody response can vary greatly for cells within each compartment. As we have discussed, these unique functional attributes likely reflect the combined influence of multiple factors including disparate anatomic location, homing and recirculation properties, developmental mechanisms, and activation requirements. Understanding how these disparate factors are integrated to ensure protection against diverse pathogens will continue to be an important and challenging area of investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 2.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genestier L, Taillardet M, Mondiere P, Gheit H, Bella C, Defrance T. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007;178:7779–7786. doi: 10.4049/jimmunol.178.12.7779. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cariappa A, Mazo IB, Chase C, Shi HN, Liu H, Li Q, Rose H, Leung H, Cherayil BJ, Russell P, et al. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity. 2005;23:397–407. doi: 10.1016/j.immuni.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Cariappa A, Chase C, Liu H, Russell P, Pillai S. Naive recirculating B cells mature simultaneously in the spleen and bone marrow. Blood. 2007;109:2339–2345. doi: 10.1182/blood-2006-05-021089. [DOI] [PubMed] [Google Scholar]
- 7.Cinamon G, Matloubian M, Lesneski MJ, Xu Y, Low C, Lu T, Proia RL, Cyster JG. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5:713–720. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 8.Vora KA, Nichols E, Porter G, Cui Y, Keohane CA, Hajdu R, Hale J, Neway W, Zaller D, Mandala S. Sphingosine 1-phosphate receptor agonist FTY720-phosphate causes marginal zone B cell displacement. J Leukoc Biol. 2005;78:471–480. doi: 10.1189/jlb.0904487. [DOI] [PubMed] [Google Scholar]
- 9.** Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. This study reveals the mechanisms involved in MZ B cell mediated transport of immune complexes from the marginal zone into splenic follicles
- 10.Ferguson AR, Youd ME, Corley RB. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int Immunol. 2004;16:1411–1422. doi: 10.1093/intimm/dxh142. [DOI] [PubMed] [Google Scholar]
- 11.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 12.Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J Immunol. 2004;172:803–811. doi: 10.4049/jimmunol.172.2.803. [DOI] [PubMed] [Google Scholar]
- 13.Song H, Cerny J. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J Exp Med. 2003;198:1923–1935. doi: 10.1084/jem.20031498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leadbetter EA, Brigl M, Ilaironov P, Cohen N, Luteran MC, Pillai S, Besra GS, Brenner MB. NK T cells provide lipid antigen-specific cognate help for B cells. Proc. Natl. Acad. Sci USA. doi: 10.1073/pnas.0801375105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiriot A, Drapier AM, Vieira P, Fitting C, Cavaillon JM, Cazenave PA, Rueff-Juy D. The Bw cells, a novel B cell population conserved in the whole genus Mus. J Immunol. 2007;179:6568–6578. doi: 10.4049/jimmunol.179.10.6568. [DOI] [PubMed] [Google Scholar]
- 16.Hastings WD, Tumang JR, Behrens TW, Rothstein TL. Peritoneal B-2 cells comprise a distinct B-2 cell population with B-1b-like characteristics. Eur J Immunol. 2006;36:1114–1123. doi: 10.1002/eji.200535142. [DOI] [PubMed] [Google Scholar]
- 17.Berberich S, Forster R, Pabst O. The peritoneal micromilieu commits B cells to home to body cavities and the small intestine. Blood. 2007;109:4627–4634. doi: 10.1182/blood-2006-12-064345. [DOI] [PubMed] [Google Scholar]
- 18.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 19.Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, Fagarasan S. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunisawa J, Kurashima Y, Gohda M, Higuchi M, Ishikawa I, Miura F, Ogahara I, Kiyono H. Sphingosine 1-phosphate regulates peritoneal B-cell trafficking for subsequent intestinal IgA production. Blood. 2007;109:3749–3756. doi: 10.1182/blood-2006-08-041582. [DOI] [PubMed] [Google Scholar]
- 21.Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195:771–780. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kretschmer K, Stopkowicz J, Scheffer S, Greten TF, Weiss S. Maintenance of peritoneal B-1a lymphocytes in the absence of the spleen. J Immunol. 2004;173:197–204. doi: 10.4049/jimmunol.173.1.197. [DOI] [PubMed] [Google Scholar]
- 23.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 24.* Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. This study established that B-1b B cells constitute a T-independent memory IgM population derived from B2 precursors
- 25.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 27.Norvell A, Mandik L, Monroe JG. Engagement of the antigen-receptor on immature murine B lymphocytes results in death by apoptosis. J Immunol. 1995;154:4404–4413. [PubMed] [Google Scholar]
- 28.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava B, Quinn WJ, 3rd, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J Exp Med. 2005;202:1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cariappa A, Boboila C, Moran ST, Liu H, Shi HN, Pillai S. The recirculating B cell pool contains two functionally distinct, long-lived, posttransitional, follicular B cell populations. J Immunol. 2007;179:2270–2281. doi: 10.4049/jimmunol.179.4.2270. [DOI] [PubMed] [Google Scholar]
- 31.Martin F, Kearney JF. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 2000;12:39–49. doi: 10.1016/s1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
- 32.Lindsley RC, Thomas M, Srivastava B, Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109:2521–2528. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- 33.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 34.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 36.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 37.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, Cambier JC. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Wen L, Brill-Dashoff J, Shinton SA, Asano M, Hardy RR, Hayakawa K. Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 2005;23:297–308. doi: 10.1016/j.immuni.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Li H, Weigert M. Autoreactive B cells in the marginal zone that express dual receptors. J Exp Med. 2002;195:181–188. doi: 10.1084/jem.20011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hao Z, Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J Exp Med. 2001;194:1151–1164. doi: 10.1084/jem.194.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otero DC, Anzelon AN, Rickert RC. CD19 function in early and late B cell development: I. Maintenance of follicular and marginal zone B cells requires CD19-dependent survival signals. J Immunol. 2003;170:73–83. doi: 10.4049/jimmunol.170.1.73. [DOI] [PubMed] [Google Scholar]
- 43.Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, Humphries LA, Rawlings D, Reynolds H, Vigorito E, et al. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002;196:753–763. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K, Pillai S. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14:603–615. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 45.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 46.Saito T, Chiba S, Ichikawa M, Kunisato A, Asai T, Shimizu K, Yamaguchi T, Yamamoto G, Seo S, Kumano K, et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 2003;18:675–685. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 47.Hozumi K, Negishi N, Suzuki D, Abe N, Sotomaru Y, Tamaoki N, Mailhos C, Ish-Horowicz D, Habu S, Owen MJ. Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat Immunol. 2004;5:638–644. doi: 10.1038/ni1075. [DOI] [PubMed] [Google Scholar]
- 48.Moran ST, Cariappa A, Liu H, Muir B, Sgroi D, Boboila C, Pillai S. Synergism between NF-kappa B1/p50 and Notch2 during the development of marginal zone B lymphocytes. J Immunol. 2007;179:195–200. doi: 10.4049/jimmunol.179.1.195. [DOI] [PubMed] [Google Scholar]
- 49.** Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. This study reveals that canonical NF-κB activity downstream of the BAFF receptor contributes to the both follicular B cell maintenance and MZ B cell development
- 50.Thomas M, Calamito M, Srivastava B, Maillard I, Pear WS, Allman D. Notch activity synergizes with B-cell-receptor and CD40 signaling to enhance B-cell activation. Blood. 2007;109:3342–3350. doi: 10.1182/blood-2006-09-046698. [DOI] [PubMed] [Google Scholar]
- 51.Shin HM, Minter LM, Cho OH, Gottipati S, Fauq AH, Golde TE, Sonenshein GE, Osborne BA. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. Embo J. 2006;25:129–138. doi: 10.1038/sj.emboj.7600902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7:213–219. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 53.** Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. This study characterized a novel B-1 B cell precursor in fetal liver and a corresponding cell in adult bone marrow