Abstract
Both amide-to-ester and amide-to-E-olefin backbone amide mutation methods were employed to perturb the same H-bond (formed by the NH of F23 and the CO of R14) in the pin WW domain. Comparison of the thermodynamic folding energies of the ester mutant and the E-olefin mutant, accounting for the transfer free energy differences measured on relevant model compounds, yielded an estimated value of 0.3 kcal/mol for the O-O repulsion term (ΔGO-Orep) in a β-sheet context. The value of ΔGO-Orep enabled us to calculate the intrinsic F23-R14 H-bond free energy to be 1.3 kcal/mol.
There is growing interest in deciphering the contributions of backbone-backbone hydrogen bonding to protein folding energetics.1 Since backbone hydrogen bonds are formed between main chain amides (Fig 1A), they can be perturbed by replacing the amide bond of interest in a protein with an iso-structural moiety which has reduced or lacks H-bonding capacity. Currently, the most convenient approach to perturb backbone H-bonding is to replace amides with esters (Fig 1A).2 An amide-to-ester (A-to-E) mutation eliminates the H-bond donor (N-H) and weakens the H-bond acceptor (C=O). A-to-E mutations are conservative in that the trans conformation of the linkage is maintained, as well as the ϕ, ψ dihedral angle preference of the flanking substructure. One concern with this approach is the possible electrostatic repulsions introduced between the O replacing the NH and carbonyl oxygen of the acceptor amide (Fig 1A; red line). The magnitude of this O-O repulsion is unclear, which complicates the extraction of H-bond energies from A-to-E perturbation thermodynamic data.1d, 2c, d It has been proposed that an amide-to-E-olefin (A-to-O) mutation is the ideal peptide bond perturbation.3 An A-to-O mutation in a protein eliminates one H-bond donor (NH) and one H-bond acceptor (CO) without introducing electrostatic repulsions. However, this strategy has rarely been realized due to the difficulties associated with stereospecific synthesis of alkene-containing isosteres and incorporating them into proteins. Recently, our group has reported a convenient protocol for the preparation of the Phe-Phe E-olefin dipeptide isostere and its incorporation into proteins.4 Herein, we report perturbation of the Phe22-Phe23 amide bond in the Pin WW domain employing both A-to-O and A-to-E mutations (Fig 1B). An energetic comparison of the ester mutant and E-olefin mutant enables us to quantify the repulsive O-O interaction introduced by A-to-E mutations and to establish the H-bond energy.
Figure 1.
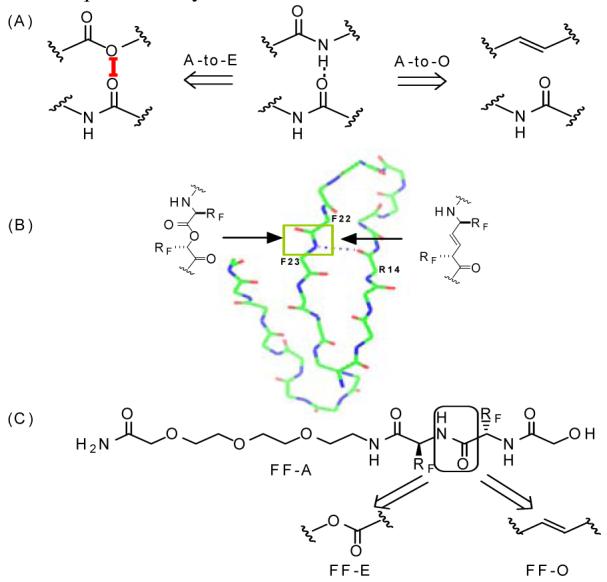
(A) The backbone A-to-E mutation eliminates a H-bond donor and weakens the acceptor; The A-to-O mutation eliminates both the H-bond donor and acceptor. (B) The backbone amide perturbation strategy in the Pin WW domain, wherein a H-bond donor is removed using both A-to-E and A-to-O mutations. (C) Structure of the Phe-Phe dipeptide derivatives designed to evaluate the desolvation energy differences between the amide, ester and E-olefin derivatives. PEG fragments were appended to increase water solubility.
The Pin WW domain, the ligand binding domain of the human Pin 1 protein, is one of the smallest and best-studied β-sheet proteins.2c, 5, 6 It is a 34-residue polypeptide that folds into a twisted 3-stranded anti-parallel β-sheet structure (Fig 1B). Mutational studies show that the Pin WW domain is highly tolerant to side chain mutations at nearly every position.6 Taking advantage of this fact, we carried out this study with the Val22Phe, Tyr23Phe variant of the PIN WW domain, because we had already prepared the Phe-Phe E-olefin dipeptide isostere required to perform the A-to-O mutation. The V22F/Y23F mutant is slightly destabilized compared to WT Pin, with the folding free energy lower by 0.7 kcal/mol. Far-UV circular dichroism spectroscopy, fluorescence spectroscopy, and NMR (vide infra, Fig. 2) data show that the double mutant reversibly folds into the same beta-sheet structure as the wide type WW domain (Table 1, supporting information).
Figure 2.
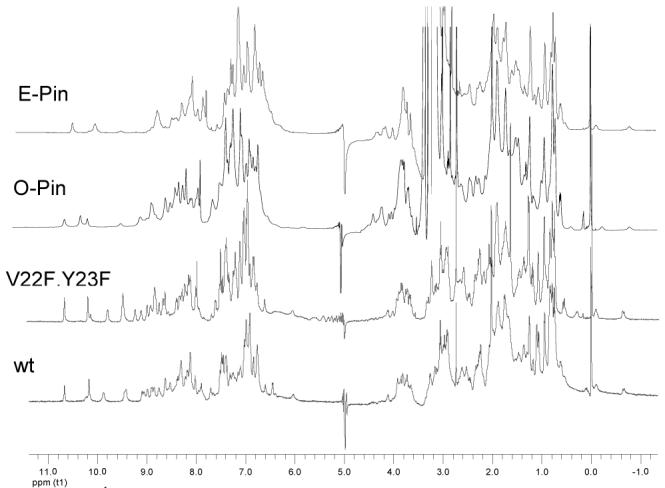
1H NMR spectra (500 MHz, 283K) of the wt Pin WW domain and variants thereof. Spectra of all variants exhibit two characteristic up field shifted native state resonances, assigned above in the text. Protein samples were prepared in 20 mM sodium phosphate buffer, pH 7.2, H2O/D2O 9:1 (v/v). 1.0 M TMAO was added to the buffer of E-pin and O-pin to ensure complete folding.
Table 1.
Thermodynamic parameters of the Pin WW Domain Variants
| Protein | Tma (°C) | m value b (kcal/(mol·M)) | ΔGfb (kcal/mol) | ΔΔGf (kcal/mol) |
|---|---|---|---|---|
| wt Pin | 59.8 | 1.04 | -3.3 ± 0.1 | 0 |
| V22F/Y23F | 45.9 | 0.84 | -2.6 ± 0.1 | 0.7 ± 0.1c |
| O-Pin | 27.6 | 1.51 | -1.8 ± 0.1 | 0.8 ± 0.1d |
| E-Pin | 20.0 | 1.55 | -1.3 ± 0.1 | 1.3 ± 0.1d |
calculated by fitting thermal denaturation curves.
calculated by fitting chaotrope denaturation curves.
For the control protein, ΔΔGf = ΔGf,V22F/Y23F - ΔGf,wt.
For the O-Pin and E-Pin, ΔΔGf = ΔGf,mut - ΔGf,V22F/F23F.
The NH of F23 makes an H-bond to the carbonyl of R14 (Fig 1B), while the CO of F22 is not hydrogen bonded and is exposed to solvent, according to solution and solid state structural data.7 This particular H-bond formed by F23 and R14 was perturbed by applying A-to-E and A-to-O mutations to the amide bond comprising F22 and F23. Both ester and olefin-containing Pin WW domain variants, referred to as E-Pin and O-Pin hereafter, were prepared by manual solid-phase peptide synthesis utilizing the Boc/benzyl strategy. The ester bond in E-Pin was introduced by using the α-hydroxy acid equivalent of phenylalanine-23 as a building block.2c The Phe-Phe E-olefin dipeptide isostere was incorporated into the O-Pin chain in place of F22-F23 according to the reported protocol.4 HF cleavage afforded the crude peptides, which were purified by RP-HPLC and characterized by MALDI-TOF mass spectrometry.
The backbone mutants were subjected to spectroscopic analysis to ensure that they adopt a normally folded structure. Both E-Pin and O-Pin exhibit a far-UV CD maximum at 227 nm (Fig S1) and a fluorescence emission maximum at 342 nm upon excitation at 295 nm (Fig S2), characteristics of the 3-stranded β-sheet structure of the wt and the V22F/Y23F WW domains. Most importantly, the 1H NMR spectra of the E-Pin and O-Pin display dispersed resonances in both amide and aliphatic regions, similar to those of wt and V22F/Y23F Pin WW domains (Fig 2). The spectra of the mutants also exhibit two small up-field resonances (-0.1, -0.6 ppm) originating from the Pro37 Cγ protons shielded by Trp11 and N26 Cβ proton shielded by Phe25 respectively, interactions characteristic of the folded state (Fig S5). The E-Pin and O-Pin variants, eliminating one H-bond in the hydrophobic core, are less stable than V22F/Y23F Pin as indicated by the melting temperature (Tm), and the folding free energy (ΔGf) derived from chaotrope-induced denaturation curves (Table 1, Fig S3). O-Pin exhibits a 0.8 kcal/mol decrease in stability, while E-Pin is 1.3 kcal/mol less stable than the V22F/Y23F variant.
The strength of the proposed O-O repulsion (ΔGO-Orep, Fig 1A) can be estimated by the following equation (for a detailed derivation, see the supporting information):
where, ΔΔGf, E-Pin and ΔΔGf, O-Pin represent the folding free energy difference for E-Pin and O-Pin compared with Pin WW domain variant V22F/Y23F; ΔGt,COO and ΔGt,-CHCH-represent the desolvation energy of the ester bond and E-olefin bond respectively. The desolvation energies can be estimated from the water-to-octanol transfer free energies, with octanol mimicking a protein interior. 8 To measure the relevant transfer free energies in the context of the F22F23 substructure in the Pin WW domain, a Phe-Phe dipeptide derivative, FF-A (Fig 1C), as well as the corresponding ester (FF-E) and E-olefin (FF-O) isosteres, were synthesized. The molecules include a short PEG fragment attached to the core structure to ensure sufficient water solubility so that their partitioning between water and octanol can be accurately evaluated. Measured partition coefficients of FF-A, FF-E and FF-O remained constant over a broad concentration range (10 μM to 1 mM), indicating that aggregation was not occurring. The transfer free energies, calculated from their partition coefficients, are -1.4, - 1.9 and -2.4 kcal/mol for FF-A, FF-E and FF-O respectively. Based on these data, the term (ΔGt,COO- ΔGt,-CHCH-) is calculated to be 0.5 kcal/mol. Therefore, the equation for ΔGO-Orep is solvable, affording a value of 0.3 kcal/mol, which allows us to calculate the perturbed H-bond strength to be 1.3 kcal/mol.1d
The determined value of the O-O repulsion here is small, consistent with some previously reported data allowing a rough estimate of ΔGO-Orep.9 However, a larger value of 2.6 kcal/mol for the O-O repulsion free energy has been reported by comparing the binding affinity of vancomycin for two peptidomimetic compounds, where an amide bond is replaced by an ester and ketomethylene.10 2.6 kcal/mol may be an overestimate of the repulsion energy because it was not corrected for solvation/desolvation energies. In addition, the ketomethylene moiety is more flexible than an amide bond; therefore, conformational alterations are possible. It is also possible that the O-O interaction in a folded β-sheet protein might be different from that observed in vancomycin/peptidomimetic complexes.
In summary, we report backbone perturbations in a β-sheet protein employing A-to-E and A-to-O mutations. Both mutations, deleting the same H-bond in the hydrophobic core, lead to a pronounced decrease in protein stability. The folding free energies of the ester and olefin mutants, together with the transfer free energies measured on relevant model compounds, afford an estimation of 0.3 kcal/mol for the O-O electrostatic repulsion term in the context of a β-sheet H-bond network. The determined value of ΔGO-Orep should enable more accurate H-bond strength measurements utilizing amide-to-ester mutations. From this data, the H-bond between F23 and R14 in the Pin WW domain is determined to be worth 1.3 kcal/mol.
Supplementary Material
ACKNOWLEDGMENT
We gratefully acknowledge financial support from the NIH (GM 51105), the Lita Annenberg Hazen Foundation and the Bundy Foundation.
References
- (1)(a).Dill KA. Biochemistry. 1990;29:7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]; (b) Gruebele M. Curr. Opin. Struct. Biol. 2002;12:161–168. doi: 10.1016/s0959-440x(02)00304-4. [DOI] [PubMed] [Google Scholar]; (c) Ferguson N, Pires JR, Toepert F, Johnson CM, Pan YP, Volkmer-Engert R, Schneider-Mergener J, Daggett V, Oschkinat H, Fersht A. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13008–13013. doi: 10.1073/pnas.221467398. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Powers ET, Deechongkit S, Kelly JW. Adv. Protein. Chem. 2006;72:39–78. doi: 10.1016/S0065-3233(05)72002-7. [DOI] [PubMed] [Google Scholar]; (e) Cheng RP, Gellman SH, Degrado WF. Chem. Rev. 2001;101:3219–3232. doi: 10.1021/cr000045i. [DOI] [PubMed] [Google Scholar]
- (2)(a).Blankenship JW, Balambika R, Dawson PE. Biochemistry. 2002;41:15676–15684. doi: 10.1021/bi026862p. [DOI] [PubMed] [Google Scholar]; (b) Beligere GS, Dawson PE. J. Am. Chem. Soc. 2000;122:12079–12082. [Google Scholar]; (c) Deechongkit S, Nguyen H, Powers ET, Dawson PE, Gruebele M, Kelly JW. Nature. 2004;430:101–105. doi: 10.1038/nature02611. [DOI] [PubMed] [Google Scholar]; (d) Deechongkit S, Dawson PE, Kelly JW. J. Am. Chem. Soc. 2004;126:16762–16771. doi: 10.1021/ja045934s. [DOI] [PubMed] [Google Scholar]; (e) Nakhle BM, Silinski P, Fitzgerald MC. J. Am. Chem. Soc. 2000;122:8105–8111. [Google Scholar]; (f) Koh JT, Cornish VW, Schultz PG. Biochemistry. 1997;36:11314–11322. doi: 10.1021/bi9707685. [DOI] [PubMed] [Google Scholar]
- (3)(a).Hart SA, Sabat M, Etzkorn FA. J. Org. Chem. 1998;63:7580–7581. [Google Scholar]; (b) Gardner RR, Liang G-B, Gellman SH. J. Am. Chem. Soc. 1999;121:1806–1816. [Google Scholar]; (c) Jenkins CL, Vasbinder MM, Miller SJ, Raines RT.Org. Lett 200139–12.11429880 [Google Scholar]
- (4).Fu Y, Bieschke J, Kelly JW. J. Am. Chem. Soc. 2005;127:15366–15367. doi: 10.1021/ja0551382. [DOI] [PubMed] [Google Scholar]
- (5)(a).Deechongkit S, Kelly JW. J. Am. Chem. Soc. 2002;124:4980–4986. doi: 10.1021/ja0123608. [DOI] [PubMed] [Google Scholar]; (b) Ferguson N, Johnson CM, Macias M, Oschkinat H, Fersht A. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13002–13007. doi: 10.1073/pnas.221467198. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Deechongkit S, Nguyen H, Jager M, Powers ET, Gruebele M, Kelly JW. Curr. Opin. Struct. Biol. 2006;16:94–101. doi: 10.1016/j.sbi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- (6).Jager M, Nguyen H, Crane JC, Kelly JW, Gruebele M. J. Mol. Biol. 2001;311:373–393. doi: 10.1006/jmbi.2001.4873. [DOI] [PubMed] [Google Scholar]
- (7)(a).Kowalski JA, Liu K, Kelly JW. Biopolymers. 2002;63:111–121. doi: 10.1002/bip.10020. [DOI] [PubMed] [Google Scholar]; (b) Ranganathan R, Lu K, Hunter T, Noel JP. Cell. 1997;89:875–886. doi: 10.1016/s0092-8674(00)80273-1. [DOI] [PubMed] [Google Scholar]
- (8)(a).Wimley WC, Creamer TP, White SH. Bochemistry. 1996;35:5109–5124. doi: 10.1021/bi9600153. [DOI] [PubMed] [Google Scholar]; Abraham DJ, Kellogg GE, Holt JM, Ackers GK. J. Mol. Biol. 1997;272:613–632. doi: 10.1006/jmbi.1997.1249. [DOI] [PubMed] [Google Scholar]
- (9)(a).Groeger C, Wenzel HR, Tschesche H. Int. J. Pept. Protein Res. 1994;44:166–172. doi: 10.1111/j.1399-3011.1994.tb00572.x. [DOI] [PubMed] [Google Scholar]; (b) Berti PJ, Faerman CH, Storer AC. Biochemistry. 1991;30:1394–1402. doi: 10.1021/bi00219a033. [DOI] [PubMed] [Google Scholar]
- (10).McComas CC, Crowley BM, Boger DL. J. Am. Chem. Soc. 2003;125:9314–9315. doi: 10.1021/ja035901x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


