Abstract
Background
Antimalarial drug resistance is now well established in both Plasmodium falciparum and Plasmodium vivax. In southern Papua, Indonesia, where both strains of plasmodia coexist, we have been conducting a series of studies to optimize treatment strategies.
Methods
We conducted a randomized trial that compared the efficacy and safety of dihydroartemisinin-piperaquine (DHP) with artesunate-amodiaquine (AAQ). The primary end point was the overall cumulative parasitological failure rate at day 42.
Results
Of the 334 patients in the evaluable patient population, 185 were infected with P. falciparum, 80 were infected with P. vivax, and 69 were infected with both species. The overall parasitological failure rate at day 42 was 45% (95% confidence interval [CI], 36%–53%) for AAQ and 13% (95% CI, 7.2%–19%) for DHP (hazard ratio [HR], 4.3; 95% CI, 2.5–7.2; P < .001). Rates of both recrudescence of P. falciparum infection and recurrence of P. vivax infection were significantly higher after receipt of AAQ than after receipt of DHP (HR, 3.4 [95% CI, 1.2–9.4] and 4.3 [95% CI, 2.2–8.2], respectively; P < .001). By the end of the study, AAQ recipients were 2.95-fold (95% CI, 1.2- to 4.9-fold) more likely to be anemic and 14.5-fold (95% CI, 3.4- to 61-fold) more likely to have carried P. vivax gametocytes.
Conclusions
DHP was more effective and better tolerated than AAQ against multidrug-resistant P. falciparum and P. vivax infections. The prolonged therapeutic effect of piperaquine delayed the time to P. falciparum reinfection, decreased the rate of recurrence of P. vivax infection, and reduced the risk of P. vivax gametocyte carriage and anemia.
Antimalarial drug resistance poses a significant threat to communities where malaria is endemic. Although chloroquine- and sulfadoxine-pyrimethamine–resistant strains of Plasmodium falciparum emerged more than 50 years ago, chloroquine resistance in P. vivax was described relatively recently [1, 2]. Chloroquine-resistant strains of P. vivax have now been documented across Asia [3-7] and in South America [8, 9]. Despite these reports, few studies have addressed suitable treatment regimens for infections with such resistant isolates [10]. In Indonesia, the efficacy of amodiaquine is superior to that of chloroquine, but in regions where high levels of chloroquine resistance predominate, day 28 failure rates exceed 25% [11]. Alternative agents, such as mefloquine, halofantrine, and atovaquone-proguanil, are effective but expensive, and they may be poorly tolerated, particularly among young children [10, 12].
Artemisinin combination therapy (ACT) is now widely advocated for the treatment of P. falciparum infection, and in accordance with this policy, the Indonesian Ministry of Health has started the transition to ACT deployment as part of an integrated malaria-control strategy. Although regimens of amodiaquine plus artesunate (AAQ) have been deployed as first-line therapy for P. falciparum infection, their efficacy against chloroquine-resistant P. vivax infection has not been established.
In Papua, Indonesia, where resistance has emerged among both P. falciparum and P. vivax, we have been conducting a series of drug trials to optimize treatment strategies [3, 11, 13]. In the present study, we compare the safety and efficacy of dihydroartemisinin-piperaquine (DHP) with those of AAQ for the treatment of patients with malaria who presented to rural clinics with P. falciparum and/or P. vivax infection.
MATERIALS AND METHODS
Study site
The study was performed at 2 rural clinics west of the Timika in southern Papua, Indonesia. This lowland region is partly forested, with Anopheles koliensis, Anopheles farauti, and Anopheles punctulatus responsible for unstable malaria transmission [14, 15] The annual incidence of malaria in the region is 938 cases per 1000 persons per year (ratio of P. falciparum infections to P. vivax infections, 57:43; unpublished data). Local protocols recommend that all patients with patent parasitemia at any level should be given antimalarial therapy.
Study design
The study was a prospective, open-label, randomized comparison of AAQ with DHP for the treatment of uncomplicated symptomatic malaria. The study was based on the 2001 World Health Organization (WHO) in vivo antimalarial drug susceptibility protocol [16] that was modified to include mixed infections and any level of parasitemia. Patients were observed for 42 days.
Patients
Patients with slide-confirmed malaria (due to P. falciparum, P. vivax, or both) and fever or a history of fever during the preceding 48 h who presented to the outpatient clinic were eligible for enrollment. Pregnant or lactating women and children aged <1 year or who weighed <5 kg were excluded, as were patients with WHO-specified danger signs or signs of severity [17], patients with a parasite load >4%, or patients with concomitant disease that required hospital admission.
Study procedures
After screening and confirmation of eligibility, patients were randomized to receive either AAQ or DHP. A randomization list was generated in blocks of 20 by an independent statistician, with each treatment allocation concealed in an opaque, sealed envelope that was opened once the patient had been enrolled in the study. Demographic information, details of symptoms and their duration, history of previous antimalarial treatment, and clinical examination findings were recorded on a standardized data sheet. Venous blood specimens were obtained for blood film examination and determination of the hematocrit and WBC count.
Parasite counts were determined on Giemsa-stained thick films as the number of parasites per 200 WBCs, and peripheral parasite loads were calculated using the recorded WBC count. Slide examination results were considered to be negative after examination of 200 high-power fields. A thin smear was also examined to confirm parasite species and was used for quantification if the parasite load was >200 parasites per 200 WBCs. All slides were read by a certified microscopist who was blinded to treatment allocation, and findings were cross-checked by a second experienced microscopist. In cases in which readings were discordant, the slides were reexamined by a third microscopist, and a consensus was reached.
Patients were examined daily until they became afebrile and aparasitemic, and they were then seen weekly for 6 weeks. At each clinic appointment, a complete physical examination was performed, the symptom questionnaire was completed, and a blood sample was obtained for determination of the parasite count. Hemoglobin levels were measured at enrollment, on day 7, and on day 28 using a battery-operated portable photometer (Hb201+; HemoCue). Blood spots on filter paper (Whatman BFC 1802) were also assessed on day 0 and the day of treatment failure.
Treatment
AAQ was dispensed as separate tablets of artesunate (Arsumax; Guilin Pharmaceuticals) and amodiaquine (Flavoquine; Aventis) and was administered on the basis of weight, with a target of a total artesunate dose of 12 mg/kg and a total amodiaquine dose of 30 mg/kg. The target total dose of DHP (Artekin [Holley Pharmaceutical], which contains 40 mg of dihydroartemisinin and 320 mg of piperaquine) was 6.75 and 54 mg/kg of dihydroartemisinin and piperaquine, respectively. Doses were rounded up to the nearest half-tablet, and administration was supervised at the time of admission and after 24 and 48 h. If vomiting occurred within 60 min after use, administration of the full dose was repeated. Patients who vomited more than twice were removed from the study. All patients with either P. vivax infection or mixed infection were offered an unsupervised course of primaquine (0.3 mg of base/kg of body weight for 14 days) immediately after completion of the study regimen.
Patients for whom therapy failed were offered an alternative to their primary treatment and were observed for an additional 42 days. Patients who refused further follow-up received unsupervised re-treatment, in accordance with local policy, with quinine (10 mg of salt per kg of body weight orally 3 times per day for 7 days) and with additional doxycycline (100 mg per day for 7 days) if they were >8 years of age and not pregnant.
End points
The analysis of efficacy was conducted using a modified intention-to-treat analysis that included all patients who fulfilled the enrollment criteria. The primary end point was the overall risk of reappearance of any parasitemia during the 42-day follow-up period. Secondary end points included the risk of reappearance of P. falciparum (further divided into the risks of recrudescence and reinfection) and the recurrence of P. vivax infection. Other secondary end points assessed were the proportion of parasitemic patients on days 1, 2, and 3; posttreatment gametocyte carriage; and hematological recovery.
Sample size
The original sample size of 400 patients had 80% power and 95% confidence to detect a 12% difference in the primary end point. An interim analysis was performed by an independent data safety monitoring committee after 3 months, and in view of the statistical difference in efficacy between treatment groups, the committee recommended that patient recruitment be stopped. At this stage, 340 patients had been enrolled.
Statistical analysis
Data were double-entered and validated using EpiData software, version 3.02 (EpiData Association), and analysis was performed using SPSS for Windows, version 14 (SPSS). The Mann-Whitney U test was used for nonparametric comparisons, and Student's t test or 1-way analysis of variance was used for parametric comparisons. Proportions were examined by χ2 test with Yates' correction or by Fisher's exact test.
Efficacy end points were assessed by survival analysis, in which the cumulative risk of failure was calculated by the Kaplan Meier product limit formula and compared by the Mantel-Haenszel log rank test. In addition, treatments were compared, and the hazard ratio (HR) was calculated after stratifying for the initial infecting parasite species using Cox's proportional hazards model. Data were censored for patients who were lost to follow-up or, for secondary end points, represented a different outcome, and were regarded as not experiencing treatment failure. Patients with recurrent vomiting or adverse drug effects who required early termination of treatment and the administration of rescue therapy were regarded as having experienced therapeutic failure.
In patients with P. falciparum (alone or mixed) in both the initial and recurrent parasitemia, reinfections and recrudescent infections were determined by PCR on the basis of polymorphisms in MSP-1, MSP-2, and GLURP, as described elsewhere [18]. Of the 18 P. falciparum paired treatment failures, there were 4 cases (22%) in which PCR was unavailable or the results were indeterminate. In these cases, the cure rates were adjusted on the basis of temporal probabilities of recrudescence versus reinfection, as determined by comparison with patients with complete data.
Gametocyte carriage was assessed by calculating person-gametocyte week rates as a measure of transmission potential [19]. The adverse events reported were commonly associated with acute malaria; thus, comparison between treatment groups was made for patients who did not have the symptom at the time of admission and who developed the symptom after commencement of antimalarial treatment.
Ethics
The study was approved by the ethics committees of the National Institute of Health Research and Development, Indonesian Ministry of Health (Jakarta, Indonesia), and of the Menzies School of Health Research (Darwin, Australia). Written informed consent was obtained from adult patients and from parents of enrolled children. The trial was registered with the clinical trials Web site (http://www.clinicaltrials.gov/ct) as NCT 00157885.
RESULTS
During the period from July 2005 through December 2005, a total of 1310 patients with uncomplicated malaria were treated at the recruitment clinics. Six protocol violations (4 in the AAQ arm and 2 in the DHP arm) were identified within 24 h; patients were offered alternative treatment and excluded from further analysis (figure 1). Of the remaining 334 patients, 185 patients (55%) had P. falciparum infection alone, 114 (34%) had P. vivax infection alone, and both species were present in 35 (10%). Baseline characteristics were similar between treatment groups (table 1). Follow-up to day 42 or to the day of treatment failure was achieved for 134 (81%) of the 166 patients treated with AAQ and for 132 (79%) of the 168 treated with DHP.
Figure 1.
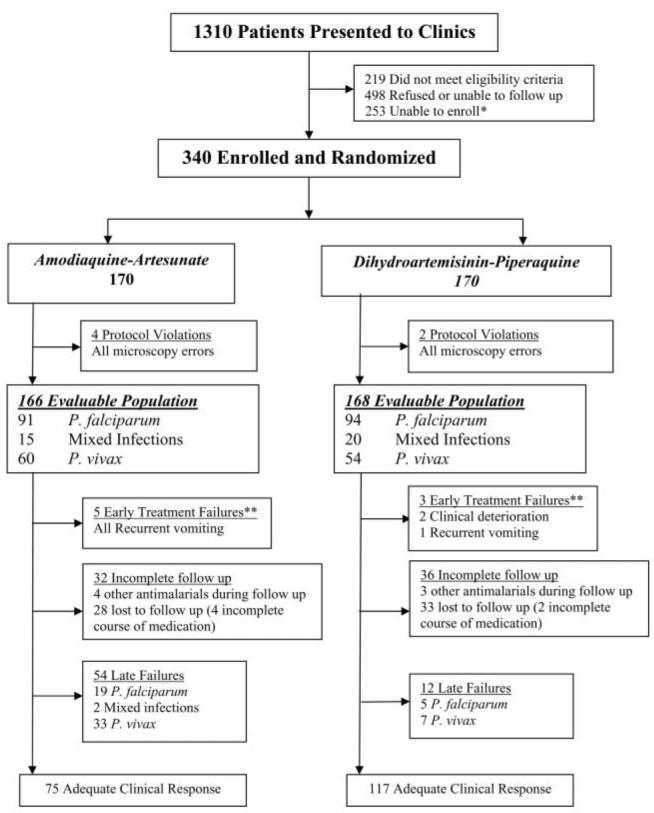
Profile of a study of dihydroartemisinin-piperaquine versus artesunate-amodiaquine as posttreatment prophylaxis against multidrug-resistant Plasmodium falciparum and Plasmodium vivax infection. *A maximum of 5 patients were enrolled per clinic on each day. **Defined on the basis of World Health Organization criteria [17] or as recurrent vomiting or adverse event warranting rescue therapy.
Table 1.
Patient characteristics at baseline in a study of dihydroartemisinin-piperaquine (DHP) versus artesunate-amodiaquine (AAQ) as posttreatment prophylaxis against multidrug-resistant Plasmodium falciparum and Plasmodium vivax infection.
| Characteristic | AAQ arm | DHP arm |
|---|---|---|
| No. of patients in the evaluable population | 166 | 168 |
| Infecting species at time of study enrollment | ||
| P. falciparum | ||
| No. (%) of patients | 91 (55) | 94 (56) |
| Geometric mean parasite load per μL (95% CI) | 4867 (3430–6908) | 5514 (4117–7386) |
| Parasite load >1000 parasites/μL−1 | 75 (82) | 83 (88) |
| P. vivax | ||
| No. (%) of patients | 60 (36) | 54 (32) |
| Geometric mean parasite load per μL (95% CI) | 2575 (1669–3973) | 1504 (979–2308) |
| Parasite load >400 parasites/μL−1 | 52 (87) | 40 (76) |
| Mixed infection | 15 (9) | 20 (12) |
| Geometric mean parasite load per μL (95% CI) | 4072 (2103–7885) | 4764 (2393–9483) |
| Parasite load >400 parasites/μL−1 | 15 (100) | 19 (95) |
| Male sex | 92 (55) | 99 (59) |
| Weight, median kg (range) | 43 (6.6–72) | 46 (8–85) |
| Age | ||
| Median years (range) | 15 (1–60) | 17 (1–56) |
| <5 years | 37 (22) | 30 (18) |
| 5–14 years | 46 (28) | 46 (27) |
| >14 years | 83 (50) | 92 (55) |
| Temperature >37.5°C | 50 (30) | 57 (34) |
| History of malaria in previous month | 27 (16) | 31 (19) |
| Hemoglobin concentration, mean g/dL ± SD | 10.8 ± 2.6 | 11.1 ± 2.6 |
| Splenomegaly | 111 (67) | 123 (73) |
NOTE. Data are no. (%) of patients, unless otherwise indicated.
Early therapeutic response
Five patients (3%) in the AAQ arm and 1 patient (0.6%) in the DHP arm were unable to tolerate their medication because of recurrent vomiting and were given oral or intravenous quinine. Early clinical deterioration that required hospitalization was observed in 2 patients within 4 h after receipt of DHP; both were transferred to the hospital, treated with intravenous quinine, and made unremarkable recoveries. Of the remaining patients, there were no differences between the treatment groups with regard to the rate of parasite or fever clearance time. Within 48 h, 318 (99%) of 321 patients were aparasitemic, and 318 (99%) were afebrile.
Late therapeutic response
In total, 66 patients had a recurrent parasitemia during the follow-up period (figure 1); 29 (46%) of 63 of these patients were symptomatic, 5 (8%) of 63 had documented fever, and 11 (32%) of 34 were anemic (defined as a hemoglobin concentration <10 g/dL). There were no significant differences in these rates between treatment groups or between parasite species.
The cumulative risk of overall parasitological failure by day 42 was 45% (95% CI, 36%–53%) after AAQ treatment, compared with 13% (95% CI, 7.2%–19%) after DHP treatment (HR, 4.3; 95% CI, 2.5–7.2; P < .001). Compared with AAQ recipients, patients treated with DHP had a lower risk of failure for all of the secondary end points assessed (table 2 and figure 2).
Table 2.
Cumulative risk of failure at day 42 after enrollment.
| AAQ arm |
DHP arm |
|||||
|---|---|---|---|---|---|---|
| Variable | No. of patients |
Percentage of patients (95% CI) |
No. of patients |
Percentage of patients (95% CI) |
HR (95% CI) | P |
| Parasitological failure with any species: initial infection with any speciesa |
166 | 45 (36–53) | 168 | 13 (7.2–19) | 4.3 (2.5–7.2)b | <.001 |
| Parasitological failure with Plasmodium falciparum | ||||||
| Initial infection with any speciesc |
166 | 22 (15–29) | 168 | 6.8 (2.7–11) | 3.4 (1.7–7.0)b | <.001 |
| True recrudescence of P. falciparum infectionc,d |
106 | 16 (8.5–23) | 114 | 4.8 (0.7–8.9) | 3.4 (1.2–9.4) | .018 |
| Reinfection with P. falciparumc |
166 | 11 (5.2–17) | 168 | 2.9 (0.2–5.6) | 3.8 (1.2–11.6)b | .02 |
| Parasitological failure with Plasmodium vivax | ||||||
| Initial infection with any speciesc |
166 | 33 (25–42) | 168 | 9.1 (4.2–14) | 4.3 (2.2–8.2)b | <.001 |
| Initial infection with P. vivax (alone or mixed) |
75 | 48 (35–61) | 74 | 16 (6.3–26) | 3.8 (1.8–8.1) | <.001 |
NOTE. AAQ, amodiaquine-artesunate; DHP, dihydroartemisinin-piperaquine; HR, hazard ratio.
Primary end point.
The HR was calculated using a Cox proportional hazards model after stratifying for the initial species of infection.
Secondary end point.
After correction by PCR genotyping, patients with P. falciparum (alone or mixed) infection initially and at the time of treatment failure.
Figure 2.
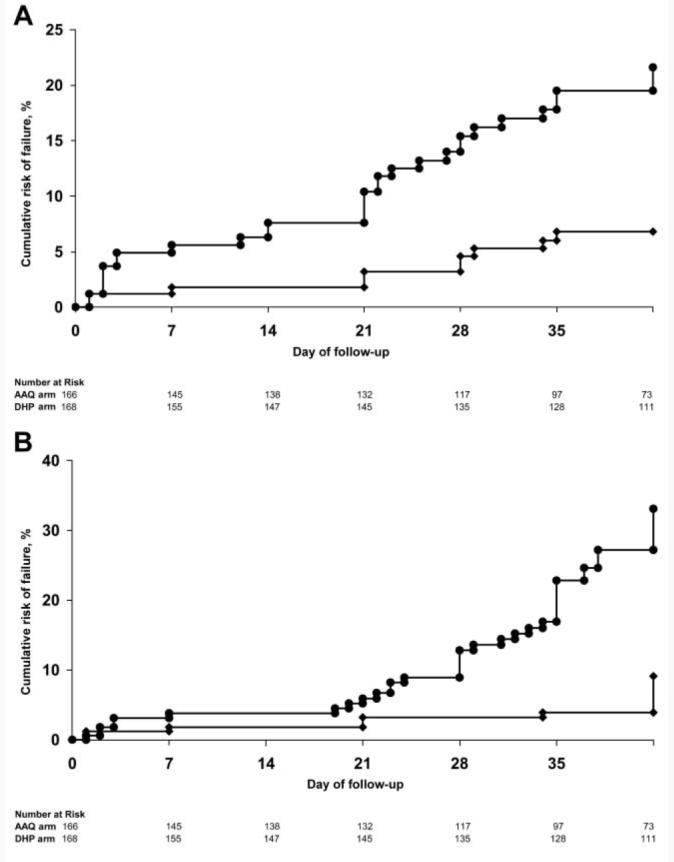
A, Cumulative risk of recurrent Plasmodium falciparum parasitemia (alone or with Plasmodium vivax coinfection). P < .001 for overall difference between treatment groups at day 42. B, Cumulative risk of recurrent P. vivax parasitemia (alone or with P. falciparum coinfection). P < .001 for overall difference between treatment groups at day 42. Circles, artesunate-amodiaquine; diamonds, dihydroartemisinin-piperaquine.
Gametocyte carriage after treatment
At the time of admission, P. falciparum gametocytes were present in 23 (10%) of 220 patients with P. falciparum infection or mixed infection, with no differences in subsequent carriage rates between treatment groups (overall rate, 12.1 cases per 1000-person weeks). P. vivax gametocytes at the time of admission were present in 113 (76%) of 149 patients with P. vivax infection (alone or mixed). During follow-up, P. vivax gametocytemia was always associated with the recurrence of P. vivax asexual stages and was significantly less likely to occur among DHP recipients (2.5 cases per 1000-patient weeks) than among AAQ recipients (36.5 cases per 1000-patients weeks; rate ratio, 14.5; 95% CI, 3.4–61; P < .001).
Hematological recovery
Although there was no significant difference in hemoglobin levels between treatment groups at the time of admission, the rates of anemia at days 7 and 28 were significantly higher in AAQ recipients (figure 3). After stratifying by the presence of anemia at the time of admission, the relative risk (RR) of anemia associated with AAQ at day 7 was 1.65 (95% CI, 1.04–1.85; P = .04), and at day 28, it was 2.95 (95% CI, 1.2–4.9; P = .019).
Figure 3.
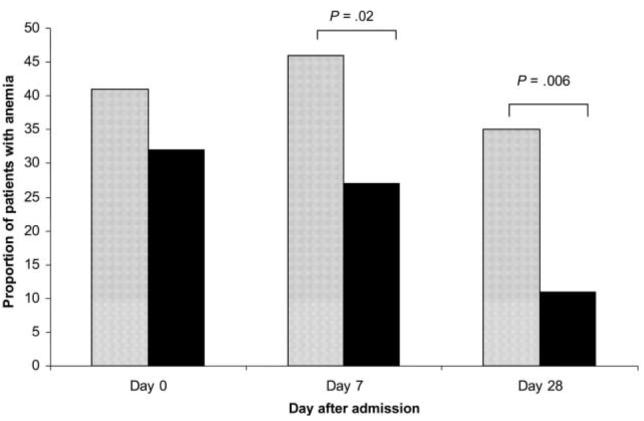
Proportion of patients with anemia. Anemia was defined as a hemoglobin concentration of <10 g/dL. Dark bars, dihydroartemisinin-piperaquine; light bars, artesunate-amodiaquine.
Tolerability
In total, 13 (7.8%) of 166 patients who received AAQ vomited at least 1 dose of medication, compared with 7 (4.2%) of 168 patients who received DHP (P = .2). On days 1 and 2, AAQ recipients were more likely than DHP recipients to report nausea (24 [27%] of 90 vs. 7 [8.6%] of 81; P = .004), vomiting (27 [23%] of 117 vs. 11 [10.7%] of 103; P = .02), and anorexia (20 [36%] of 55 vs. 7 [13%] of 56; P = .007). By day 7 and thereafter, there were no reported differences between groups in symptoms elicited.
Serious adverse events were noted in 3 patients. Two adults developed recurrent vomiting on day 3 after completion of a course of AAQ, requiring admission to hospital and administration of intravenous fluids and antiemetics. Both made a full recovery and were discharged from the hospital within 24 h. A 29-year-old man with P. falciparum infection who was treated with AAQ developed bilateral cerebellar signs (truncal ataxia and intention tremor) on day 7. Neurological examination findings were otherwise unremarkable, and the patient's symptoms resolved over the subsequent 8 days. On day 21, he had a recurrence of P. falciparum infection, which was re-treated with DHP, without further recurrence of infection, symptoms, or cerebellar signs over the subsequent 42 days.
Re-treatment
In total, 66 patients had recurrent parasitemia during follow-up, of whom 14 refused additional follow-up and received unsupervised re-treatment with quinine, with or without doxycycline. Of the remaining patients, 46 were re-treated with DHP (26 patients with P. vivax infection, 14 with P. falciparum infection, and 6 with mixed infection). The overall cumulative failure rate among these patients was 29% (95% CI, 9.9%–49%), with 5 patients having recurrent P. vivax infection and 1 patient having reinfection with P. falciparum. Six patients were re-treated with AAQ, of whom 1 was lost to follow-up on day 21; the rest of these patients had an additional recurrence of parasitemia (3 cases were due to P. vivax, and 2 were mixed infections).
DISCUSSION
In the past decade, an increasing awareness of the extent and impact of antimalarial drug resistance has stimulated renewed interest in comparative drug trials to rationalize treatment policies. Most of these studies have focussed on P. falciparum infection, with virtually no comparative efficacy data addressing the management of chloroquine-resistant P. vivax infection. The importance and threat of the latter infection has been under-estimated [20], with P. vivax malaria posing a significant threat to the 250 million people infected each year [21].
The WHO recommends the use of ACTs for uncomplicated P. falciparum malaria to improve antimalarial efficacy and minimize the risk of selection of drug-resistant parasites. In practice, the infecting species is only correctly identified in a minority of infections before the commencement of treatment; thus, in Asia and South America, where P. vivax often accounts for >50% of cases of malaria, other species will be frequently treated with ACT. The efficacy of ACT against P. vivax—in particular, against chloroquine-resistant strains—is, therefore, an important consideration when optimizing antimalarial protocols. In the present study, we have attempted to mirror local primary health care prescription practices, and we enrolled patients of all ages with all degrees of parasitemia and combinations of species.
The early therapeutic response following both treatments was rapid, and within 48 h, almost all patients had cleared their peripheral parasitemia and were afebrile. During subsequent follow-up, recurrent parasitemia occurred in only 13% of patients treated with DHP, whereas it occurred in 45% of those treated with AAQ (P < .001). Although the risk of true recrudescence with P. falciparum was <5% after receipt of DHP—similar to that found in other areas where multidrug-resistant P. falciparum is common [22-27]—the rates of recrudescence and reinfection involving P. falciparum were significantly higher after receipt of AAQ treatment (adjusted HR, 4 and 3.8, respectively).
The difference in the risk of recurrence was even more marked for P. vivax infection (adjusted HR, 4.3). Such recurrences can be attributed to either recrudescence, reinfection, or relapse from dormant liver-stage infection [28], although we were unable to distinguish between these causes. In Papua and equatorial regions, relapse of P. vivax infection occurs in up to 60% of patients [29], with the first relapse occurring at ∼21 days [29]. Because neither artemisinin, piperaquine, nor amodiaquine is active against the hypnozoite stages of P. vivax [30], the discrepancy between AAQ and DHP regimens is likely to have arisen as a result of a combination of the reduced efficacy of amodiaquine against asexual parasites and its shorter terminal elimination half-life (∼18 days), compared with the half-life of piperaquine (28–35 days) [31, 32]. The posttreatment prophylaxis associated with the long half-life of piperaquine reduces both relapses of P. vivax infection and reinfections with either species. It is possible that a higher dosage of primaquine (0.5 mg/kg per day) could have further reduced the risk of relapse of P. vivax infection; however, in reality, patients rarely adhere to a 14-day regimen. Posttreatment prophylaxis, therefore, provides the only practical means currently available for delaying these relapses. The delays in relapse and reinfection conferred by DHP gave patients a longer period free from symptomatic malaria, allowing for a greater time for hematological recovery and significantly reducing gametocyte carriage and the transmission potential to the mosquito vector.
A major concern with deploying antimalarial drugs that have a long half-life is that they will facilitate the selection of drug-resistant parasites. Although it is hoped that combination with an artemisinin derivative will reduce the risk of de novo selective transmission, once drug-resistant strains do emerge, selective transmission in the prolonged subtherapeutic tail of piperaquine is likely to encourage the spread of resistance. Therefore, careful monitoring of in vivo and in vitro antimalarial efficacy must remain a priority.
There were also significant differences in the tolerability between the treatment regimens. Patients treated with AAQ were 2–3-fold more likely to develop nausea, vomiting, and anorexia while receiving treatment, compared with DHP recipients. One patient developed ataxia and an intention tremor 7 days after commencing treatment with AAQ. Cerebellar dysfunction, although rare, is a well-recognized complication of malaria [33, 34], and although cases of cerebellar dysfunction have been attributed to artemisinin neurotoxicity [35], the clinical scenario and body of evidence to date suggest that postmalaria neurological syndrome is a more plausible explanation for what occurred in our patient [33, 34, 36].
In conclusion, DHP was more efficacious and better tolerated than AAQ for the treatment of multidrug-resistant P. falciparum and P. vivax infections in Papua, Indonesia. Its coformulation, cost (US$2 per adult), and posttreatment prophylactic effect offer significant benefits over other available ACTs, making it a prime option for the management of uncomplicated malaria in this region.
Acknowledgments
We are grateful to the staff of PT Freeport Indonesia Public Health & Malaria Control Department, International SOS, and Lembaga Pengembangan Masyarakat Amungme Kamoro for support and technical assistance. We thank Mauritz Okeseray, Rosmini, Buhari, Alan Brockman, Kim Piera, Ferryanto Chalfein, and Budi Prasetyorini for their support and technical assistance. We are also grateful to Morrison Bethea and PT Freeport Indonesia for their ongoing support. Pascal Ringwald (WHO) kindly provided the amodiaquine tablets. The members of the data safety monitoring committee were Allen Cheng, Liliana Kurniawan, Julie Simpson, and Bob Taylor.
Financial support. Wellcome Trust–National Health and Medical Research Council (NHMRC; Wellcome Trust International Collaborative Research Grant GR071614MA–NHMRC ICRG ID 283321). N.A. is supported by an NHMRC Practitioner Fellowship. R.P. is funded by a Wellcome Trust Career Development Award, affiliated with the Wellcome Trust–Mahidol University–Oxford Tropical Medicine Research Programme (074637).
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Rieckmann KH, Davis DR, Hutton DC. Plasmodium vivax resistance to chloroquine? Lancet. 1989;2:1183–4. doi: 10.1016/s0140-6736(89)91792-3. [DOI] [PubMed] [Google Scholar]
- 2.Baird JK, Basri H, et al. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am J Trop Med Hyg. 1991;44:547–52. doi: 10.4269/ajtmh.1991.44.547. [DOI] [PubMed] [Google Scholar]
- 3.Tjitra E, Baker J, Suprianto S, Cheng Q, Anstey NM. Therapeutic efficacies of artesunate-sulfadoxine-pyrimethamine and chloroquine-sulfadoxine-pyrimethamine in vivax malaria pilot studies: relationship to Plasmodium vivax dhfr mutations. Antimicrob Agents Chemother. 2002;46:3947–53. doi: 10.1128/AAC.46.12.3947-3953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baird JK, Sustriayu Nalim MF, Basri H, et al. Survey of resistance to chloroquine by Plasmodium vivax in Indonesia. Trans R Soc Trop Med Hyg. 1996;90:409–11. doi: 10.1016/s0035-9203(96)90526-x. [DOI] [PubMed] [Google Scholar]
- 5.Marlar T, Myat Phone K, Aye Yu S, Khaing Khaing G, Ma S, Myint O. Development of resistance to chloroquine by Plasmodium vivax in Myanmar. Trans R Soc Trop Med Hyg. 1995;89:307–8. doi: 10.1016/0035-9203(95)90556-1. [DOI] [PubMed] [Google Scholar]
- 6.Phan GT, de Vries PJ, Tran BQ, et al. Artemisinin or chloroquine for blood stage Plasmodium vivax malaria in Vietnam. Trop Med Int Health. 2002;7:858–64. doi: 10.1046/j.1365-3156.2002.00948.x. [DOI] [PubMed] [Google Scholar]
- 7.Kurcer MA, Simsek Z, Zeyrek FY, et al. Efficacy of chloroquine in the treatment of Plasmodium vivax malaria in Turkey. Ann Trop Med Parasitol. 2004;98:447–51. doi: 10.1179/000349804225021343. [DOI] [PubMed] [Google Scholar]
- 8.Soto J, Toledo J, Gutierrez P, et al. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am J Trop Med Hyg. 2001;65:90–3. doi: 10.4269/ajtmh.2001.65.90. [DOI] [PubMed] [Google Scholar]
- 9.Phillips EJ, Keystone JS, Kain KC. Failure of combined chloroquine and high-dose primaquine therapy for Plasmodium vivax malaria acquired in Guyana, South America. Clin Infect Dis. 1996;23:1171–3. doi: 10.1093/clinids/23.5.1171. [DOI] [PubMed] [Google Scholar]
- 10.Baird JK. Chloroquine resistance in Plasmodium vivax. Antimicrob Agents Chemother. 2004;48:4075–83. doi: 10.1128/AAC.48.11.4075-4083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratcliff A, Siswantoro H, Kenangalem E, et al. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia. Trans R Soc Trop Med Hyg. 2007;101:351–9. doi: 10.1016/j.trstmh.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maguire JD, Krisin, Marwoto H, Richie TL, Fryauff DJ, Baird JK. Mefloquine is highly efficacious against chloroquine-resistant Plasmodium vivax malaria and Plasmodium falciparum malaria in Papua, Indonesia. Clin Infect Dis. 2006;42:1067–72. doi: 10.1086/501357. [DOI] [PubMed] [Google Scholar]
- 13.Ratcliff A, Siswantoro H, Kenangalem E, et al. Comparison of two fixed dosed artemisinin combinations for multi-drug resistant falciparum and vivax malaria in Papua, Indonesia. Lancet. doi: 10.1016/S0140-6736(07)60160-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee VH, Atmosoedjono S, Aep S, Swaine CD. Vector studies and epidemiology of malaria in Irian Jaya, Indonesia. Southeast Asian J Trop Med Public Health. 1980;11:341–7. [PubMed] [Google Scholar]
- 15.Pribadi W, Sutanto I, Atmosoedjono S, Rasidi R, Surya LK, Susanto L. Malaria situation in several villages around Timika, south central Irian Jaya, Indonesia. Southeast Asian J Trop Med Public Health. 1998;29:228–35. [PubMed] [Google Scholar]
- 16.World Health Organization . Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. Geneva: World Health Organization; 2003. (Document WHO/HTM/RBM2003.50). [Google Scholar]
- 17.World Health Organization, Communicable Diseases Cluster Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 18.Brockman A, Paul RE, Anderson TJ, et al. Application of genetic markers to the identification of recrudescent Plasmodium falciparum infections on the northwestern border of Thailand. Am J Trop Med Hyg. 1999;60:14–21. doi: 10.4269/ajtmh.1999.60.14. [DOI] [PubMed] [Google Scholar]
- 19.Price RN, Nosten F, Luxemburger C, et al. Effects of artemisinin derivatives on malaria transmissibility. Lancet. 1996;347:1654–8. doi: 10.1016/s0140-6736(96)91488-9. [DOI] [PubMed] [Google Scholar]
- 20.Price RN, Tjitra E, Guerra CA, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. in press. [PMC free article] [PubMed] [Google Scholar]
- 21.Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis. 2004;4:327–36. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denis MB, Davis TM, Hewitt S, et al. Efficacy and safety of dihydroartemisinin-piperaquine (Artekin) in Cambodian children and adults with uncomplicated falciparum malaria. Clin Infect Dis. 2002;35:1469–76. doi: 10.1086/344647. [DOI] [PubMed] [Google Scholar]
- 23.Ashley EA, Krudsood S, Phaiphun L, et al. Randomized, controlled dose-optimization studies of dihydroartemisinin-piperaquine for the treatment of uncomplicated multidrug-resistant falciparum malaria in Thailand. J Infect Dis. 2004;190:1773–82. doi: 10.1086/425015. [DOI] [PubMed] [Google Scholar]
- 24.Tran TH, Dolecek C, Pham PM, et al. Dihydroartemisinin-piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomised clinical trial. Lancet. 2004;363:18–22. doi: 10.1016/s0140-6736(03)15163-x. [DOI] [PubMed] [Google Scholar]
- 25.Smithuis F, Kyaw MK, Phe O, et al. Efficacy and effectiveness of dihydroartemisinin-piperaquine versus artesunate-mefloquine in falciparum malaria: an open-label randomised comparison. Lancet. 2006;367:2075–85. doi: 10.1016/S0140-6736(06)68931-9. [DOI] [PubMed] [Google Scholar]
- 26.Mayxay M, Thongpraseuth V, Khanthavong M, et al. An open, randomized comparison of artesunate plus mefloquine vs. dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in the Lao People's Democratic Republic (Laos) Trop Med Int Health. 2006;11:1157–65. doi: 10.1111/j.1365-3156.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 27.Karema C, Fanello CI, Van Overmeir C, et al. Safety and efficacy of dihydroartemisinin/piperaquine (Artekin) for the treatment of uncomplicated Plasmodium falciparum malaria in Rwandan children. Trans R Soc Trop Med Hyg. 2006;100:1105–11. doi: 10.1016/j.trstmh.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 28.White NJ. The assessment of antimalarial drug efficacy. Trends Parasitol. 2002;18:458–64. doi: 10.1016/s1471-4922(02)02373-5. [DOI] [PubMed] [Google Scholar]
- 29.Baird JK, Leksana B, Masbar S, et al. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am J Trop Med Hyg. 1997;56:621–6. doi: 10.4269/ajtmh.1997.56.621. [DOI] [PubMed] [Google Scholar]
- 30.Corcoran KD, Hansukjariya P, Sattabongkot J, et al. Causal prophylactic and radical curative activity of WR182393 (a guanylhydrazone) against Plasmodium cynomolgi in Macaca mulatta. Am J Trop Med Hyg. 1993;49:473–7. doi: 10.4269/ajtmh.1993.49.473. [DOI] [PubMed] [Google Scholar]
- 31.Hung TY, Davis TM, Ilett KF, et al. Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria. Br J Clin Pharmacol. 2004;57:253–62. doi: 10.1046/j.1365-2125.2003.02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarning J, Lindegardh N, Annerberg A, et al. Pitfalls in estimating piperaquine elimination. Antimicrob Agents Chemother. 2005;49:5127–8. doi: 10.1128/AAC.49.12.5127-5128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senanayake N, de Silva HJ. Delayed cerebellar ataxia complicating falciparum malaria: a clinical study of 74 patients. J Neurol. 1994;241:456–9. doi: 10.1007/BF00900965. [DOI] [PubMed] [Google Scholar]
- 34.George IA, Varghese L, Mathews PK. Hemiparesis and cerebellar dysfunction complicating mixed malarial infection with falciparum and vivax malaria. Indian J Med Sci. 2006;60:296–7. [PubMed] [Google Scholar]
- 35.Panossian LA, Garga NI, Pelletier D. Toxic brainstem encephalopathy after artemisinin treatment for breast cancer. Ann Neurol. 2005;58:812–3. doi: 10.1002/ana.20620. [DOI] [PubMed] [Google Scholar]
- 36.Newton PN, Day NP, White NJ. Misattribution of central nervous system dysfunction to artesunate [letter] Clin Infect Dis. 2005;41:1687–8. doi: 10.1086/498033. [DOI] [PubMed] [Google Scholar]


