Abstract
The cytoadherence-linked asexual gene 9 (clag 9) of Plasmodium falciparum has been implicated in the cytoadherence of infected erythrocytes. To determine the immunogenicity of the clag 9 gene product (CLAG 9 protein) in humans, we measured antibody responses to 11 synthetic CLAG 9 peptides in a group of 177 asymptomatic children and adults subject to intense malaria exposure in Madang, Papua New Guinea. The CLAG 9 peptides were immunogenic in adults and children. Antibody responses to peptides 4 and 10 were high across all age groups and detectable in a majority of children less than five years of age. While CLAG 9 peptides are immunogenic in humans, longitudinal studies will be required to determine the longevity of antibody responses to CLAG 9 and their role in protection from disease.
INTRODUCTION
A central process in the pathophysiology of Plasmodium falciparum malaria is the cytoadherence of infected erythrocytes to endothelial cells lining the capillaries and venules of bodily organs, resulting in microvascular ischemia and localized immunopathology.1 The resulting microvascular sequestration of parasites is also thought to contribute to evasion of immune responses, enabling parasitized erythrocytes to avoid splenic clearance. Cytoadherence thus contributes both directly and indirectly to the pathology of severe malaria.
Ongoing exposure to malaria infection leads to the development of natural immunity, and consequently, older children and adults living in endemic areas are relatively protected from the severe clinical consequences of infection with P. falciparum. The effector mechanisms that mediate naturally acquired immunity to malaria are not completely understood, but antibodies to the asexual blood stage parasites2-4 and to purified proteins5,6 are associated with protection from disease and are thought to be important. Since the cytoadherence of infected erythrocytes to post-capillary venular endothelium is thought to contribute to virulence and immune evasion, generation of immune responses that prevent cytoadherence is an important consideration in vaccine development.
A number of parasite adhesion molecules have been identified.7-13 One of these, P. falciparum erythrocyte membrane protein 1 (PfEMP1), has been the focus of intense research. Antibodies directed against PfEMP1 are thought to be a key element in the development of immunity to P. falciparum14-16 and can block the adhesion of mature parasitized erythrocytes to specific receptors.17 However, despite strong evidence supporting a role for PfEMP1 alone in cytoadherence, we have previously shown by targeted gene disruption and antisense RNA inhibition that the cytoadherence-linked asexual gene 9 (clag 9) of P. falciparum plays a role, either directly or indirectly, in the cytoadherence of P. falciparum-infected erythrocytes to CD36.18,19
CLAG 9 is a one member of a gene family of rhoptry protein(s) that may be involved in erythrocyte invasion by merozoites.20 The sequence of clag 9 is highly conserved, with > 98% identity between isolates.21 Transcription has been shown to occur at a late stage of parasite development.19 and it appears that CLAG 9, like other members of this gene family, is initially trafficked to the rhoptries of P. falciparum.22 There is evidence that some rhoptry proteins are inserted into the red blood cell membrane immediately after the merozoite invades a new red blood cell.23,24 The synthesis of mature stage parasite adhesion molecules such as PfEMP1 begins very early in the ring stage of the parasite and they are inserted into the red blood cell membrane by the time the trophozoite stage is reached.25 Thus, it is possible that CLAG 9 is involved in trafficking of these proteins or in the initial remodeling of the host red blood cell so that these proteins can be trafficked to the correct location.
The mechanism by which the loss of clag 9 leads to loss of cytoadherence is presently unknown and it is unclear whether CLAG 9 (as a potential anti-adherence vaccine target) is naturally immunogenic in humans. However, the high level of conservation seen in clag 9, combined with its implication in cytoadherence, indicates that further investigation of this molecule is warranted. We hypothesized that a malaria-exposed population would recognize and produce antibodies to CLAG 9, and that antibody production would increase with age in parallel with increasing anti-parasitic immunity/decreasing risk of severe clinical malaria. Furthermore, we hypothesized that antibody blocking of cytoadherence would result in enhanced splenic clearance of infected erythrocytes, and therefore that aparasitemic malaria-exposed subjects would have higher levels of antibodies than those with P. falciparum parasitemia. We tested these hypotheses by measuring antibodies to the two most reactive epitopes of CLAG 9 (derived from a set of 11 synthetic peptides derived from a proposed external domain) in heavily malaria-exposed children and adults from Madang, Papua New Guinea. The population chosen was asymptomatic such that antibody responses would not be influenced by acute disease.
MATERIALS AND METHODS
The study was conducted in Madang Province of Papua New Guinea between February and May 2000. Ethical approval was obtained from the Papua New Guinea Medical Research Advisory Committee, the Health Research Ethics Committee of Menzies School of Heath Research, and the Queensland Institute of Medical Research Human Ethics Committee.
Study site and population
The study site and the malaria epidemiology of the region have been previously described in detail.26-28 Briefly, asymptomatic residents were recruited from two neighboring coastal villages located approximately 20 km north of Madang Township in Papaua New Guinea. The region is characterized by infection with four human malaria species, and with little seasonal variation in parasitemia rates.27 Residents of these particular villages have been estimated to receive on average approximately one infective bite per day,29 with transmission highest during the wet season from October to May.
Following informed consent, non-pregnant adults and children who were ≥ 1 year of age were screened at a baseline survey by administration of a clinical questionnaire, measurement of axillary temperature, and examination of a finger prick blood smear for malaria parasites. A second peripheral blood smear was taken at the time of venous blood collection 24 hours after initial screening to maximize detection by accounting for periodic fluctuation of P. falciparum density.30,31 The combined readings were used to categorize the parasite species present.
Enrollment was confined to asymptomatic subjects, with the selective aim of including subjects who were parasite positive and parasite negative on microscopy across a variety of age groups. Participants were excluded from enrollment if they were febrile (axillary temperature ≥ 37.5°C) at screening or on two subsequent occasions over the next 24 hours; had taken antimalarials within the preceding week; or had a clinical history (fever, chills, sweats, headache, or myalgia) suggesting recent (≤ 1 week) malaria infection.
Specimen collection and processing
The sample collection and processing procedures have been previously reported.26 Briefly, thick and thin blood smears from all screened and enrolled subjects were treated with a 4% Giemsa stain. Smears were defined as negative if no parasites were seen in 100 high-power (magnification × 1,000) oil-immersion fields. Positive slides with scanty parasitemias (≤ 5 parasites/200 leukocytes) and a random 10% of all slides from enrolled subjects were cross-checked by a second microscopist and discrepant slides were reviewed by both microscopists to arrive at a final result. Venous blood from each enrolled subject was collected into sterile heparinized tubes from which a manual leukocyte count was performed for calculation of parasite densities. Plasma was separated by centrifugation and stored at -70°C. Plasma specimens obtained from 26 Australian adults from Brisbane with no previous history of malaria exposure were used as a control.
Peptides
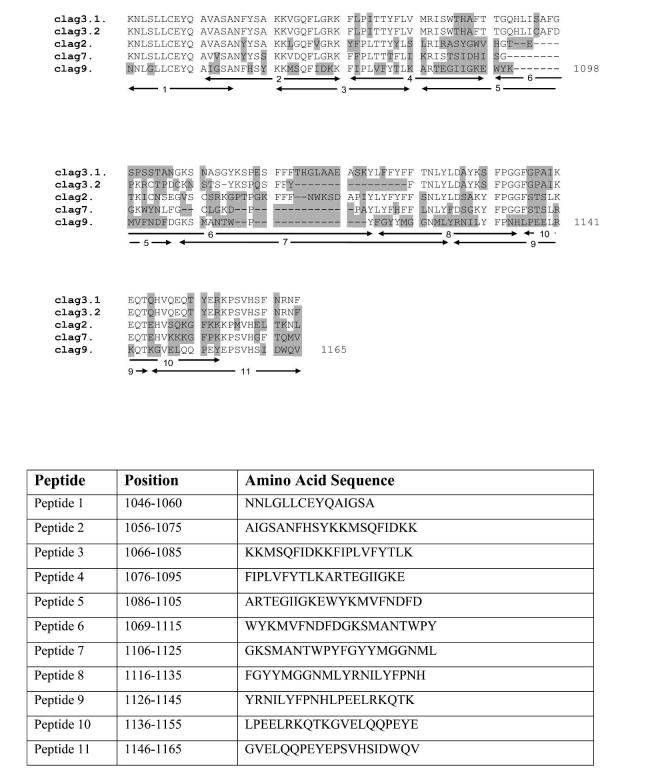
Peptides were obtained from Mimotopes (Clayton, Victoria, Australia). Eleven peptides derived from the published sequence of the clag 9 gene of P. falciparum strain 3D7 were synthesized to cover a 119 amino acid region between two putative transmembrane domains unique to clag 9. Peptides 1 and 2 had some overlap with other clag genes, but 3 to 11 were unique to clag 9 (Figure 1). Peptides were 20 amino acids long (with the exception of peptide 1, which was 15 amino acids) with a 10-amino acid overlap. Amino acid sequences of the peptides are shown in Figure 1.
FIGURE 1.
Top, Alignment of peptides used in this study demonstrating lack of overlap with other cytoadherence-linked asexual genes. The numbers 1-11 indicate the position of each peptide. The highlighted regions represent non-conserved amino acid changes. Bottom, Amino acid sequence of peptides used in this study.
Antibody reactivity to synthetic peptides
Peptides were screened to identify the most reactive epitopes. IgG antibodies to CLAG 9 were measured by an enzyme-linked immunosorbent assay. All serum samples were tested in duplicate against the 11 CLAG peptides, P. falciparum 3D7 schizont extract, and a negative control of skim milk in a carbonate/bicarbonate buffer. Assays were repeated at least once. Working solutions of peptides were prepared at concentrations 100 μg/mL in phosphate-buffered saline (PBS) plus 0.1% bovine serum albumin and 0.1% sodium azide and stored at -20°C prior to use. Schizont extract was prepared at a concentration of 108 schizonts/mL and stored at -20°C prior to use.
MaxiSorp plates (Nunc, Roskilde, Denmark) were coated with either 100 μL of peptide at a concentration of 1 μg/mL, schizont extract at a concentration of 106 schizonts/mL, or 5% skim milk in carbonate/bicarbonate buffer (pH 9.6) for six hours at 37°C. The coating solution was discarded and 100 μL of methanol added to all wells (to improve antigen presentation32), and the plates incubated at room temperature for 20 minutes. The plates were then washed five times in PBS plus 0.05% Tween 20 (PBST) and 100 μL of PBS plus 5% skim milk plus 0.05% Tween 20 was then added to all wells. After overnight incubation at 4°C, the plates were washed five times in PBST, and 100 μL of serum diluted 1/500 in PBST plus 0.5% skim milk was then added. The plates were incubated for one hour at 37°C, washed five times in PBST, and 100 μL of goat anti-human IgG (heavy plus light chains conjugated to horseradish peroxidase; Bio-Rad Laboratories, Hercules, CA) diluted to 1/5,000 in PBST plus 0.5% skim milk was then added to all wells. Following a one-hour incubation at 37°C, the plates were again washed five times in PBST. Optical densities (ODs) were read at a wavelength of 450 nm after 30 minutes of incubation with 100 μL of o-phenylenediamine dihydrochloride substrate (Sigma, St. Louis, MO).
Data analysis
For each serum sample, the background OD from uncoated wells was subtracted from the peptide reading on each plate. The resulting density difference was averaged from the duplicates; this is the value used to represent the OD of the samples. Positive sera were defined as those that had an OD greater than the mean + 2 SD of OD values obtained from the control sera. The non-parametric Mann-Whitney test was used to compare the distributions of OD responses between samples from asymptomatic individuals and control sera for each of the 11 peptides. Due to the overlapping nature of the peptides, and the correspondingly high correlation between peptide responses, only responses to the two most frequently recognized non-overlapping peptides (numbers 4 and 10) were subjected to statistical analysis. Since the OD values for peptides 4 and 10 were highly skewed and not readily transformable to normality using standard transformations, a Box-Cox transformation33 was applied with λ = −9.4 and −12.2, respectively. In both instances, a value of 1.5 was added to the raw OD values prior to transformation to ensure that all values were positive. Multivariate analysis using Pillai’s Trace criterion was conducted to test for differences in the mean OD responses of each peptide from samples obtained from individuals in different age groups (1-4, 5-9, 10-14, 15-19, and ≥ 20 years) and also those samples with and without a current P. falciparum infection on microscopy. The effect of age on peptide response was assessed using regression analysis, while Spearman’s rank correlation was used to investigate the relationship between OD response and P. falciparum parasite density for infected individuals. Statistical analysis was performed using SPSS for Windows version 9.0.1 (SPSS Inc., Chicago, IL).
RESULTS
Baseline characteristics
The baseline and parasitologic characteristics of the 177 individuals seen at the baseline survey in whom IgG antibody responses to CLAG 9 peptides were measured are provided in Table 1. The characteristics of the study group have been previously described in detail.26 Volunteers ranged from 1 to 70 years of age (mean = 17 years and 5 months, sex = 46% male). Overall, 69% of the subjects were parasitemic for one or more parasites species by microscopy, and 54% of the subjects were positive for P. falciparum.
TABLE 1.
Baseline characteristics of subjects included in the study group*
| Age group (years) | n | % Male | Pf | Pf/Pv | Pf/Pm | Pf/Pv/Pm | Pf/Pv/Po | Pv | Pv/Pm | Pm | Po | Neg (%) | Pf† no. (%) | Pf/μL‡ (95% CI) | Parasites/μL§ (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-4 | 17 | 24 | 8 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 (18) | 11 (65) | 917 (303-2,774) | 1,235 (552-2,765) |
| 5-9 | 34 | 53 | 13 | 7 | 3 | 2 | 0 | 4 | 0 | 0 | 0 | 5 (15) | 25 (74) | 431 (209-892) | 457 (256-817) |
| 10-14 | 37 | 54 | 10 | 6 | 4 | 1 | 1 | 4 | 1 | 3 | 0 | 7 (19) | 22 (59) | 281 (160-493) | 233 (145-373) |
| 15-19 | 23 | 39 | 11 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 7 (30) | 16 (70) | 163 (90-295) | 191 (111-327) |
| ≥ 20 | 66 | 50 | 20 | 3 | 0 | 1 | 0 | 9 | 2 | 1 | 1 | 29 (44) | 24 (36) | 103 (65-165) | 93 (67-130) |
| Total | 177 | 47 | 62 | 21 | 8 | 5 | 2 | 20 | 3 | 4 | 1 | 51 (29) | 98 (55) | 256 (188-349) | 244 (188-316) |
Number of subjects with each species (or combined species) of parasite on examination of two consecutive daily blood smears. Pf = P. falciparum; Pv = P. vivax; Pm = P. malariae; Po = P. ovale; Neg = negative for parasites; CI = confidence interval.
Number and percentage of subjects with P. falciparum parasitemia either as the sole infecting parasite or in combination with other parasites.
Geometric mean of the highest-density P. falciparum parasitemia measured from two consecutive daily blood smears.
Geometric mean of the highest densities of combined species (summed) measured from two consecutive daily blood smears.
Antibody reactivity to all 11 CLAG 9 peptides, as determined by OD value, was significantly higher among the Papua New Guinean subjects than the Brisbane controls (P < 0.001). Since these results were highly significant, statistical correction for multiple comparisons was deemed unnecessary. The percentage of individual serum samples that had a positive response (i.e., greater than the control mean + 2 SD) was greatest for peptides 4 and 10 (89% and 75% positive, respectively) and lowest for peptide 3 (22% positive) across all age groups (Figure 2). Antibody reactivity to peptides 4 and 10 was therefore subjected to statistical analysis. Subject age group and current P. falciparum status on microscopy were assessed for their effect on peptide OD. For each peptide, neither current P. falciparum status nor the interaction term of age and current P. falciparum status significantly affected peptide response (P > 0.2). However, a significant difference was detected between the peptide response for different age groups for peptide 4 (P = 0.001), while peptide 10 showed a similar but non-significant relationship (P = 0.056; Figure 3). There was no correlation between the OD response for either peptide 4 or 10 and the density of P. falciparum parasites in infected individuals (P > 0.4).
FIGURE 2.
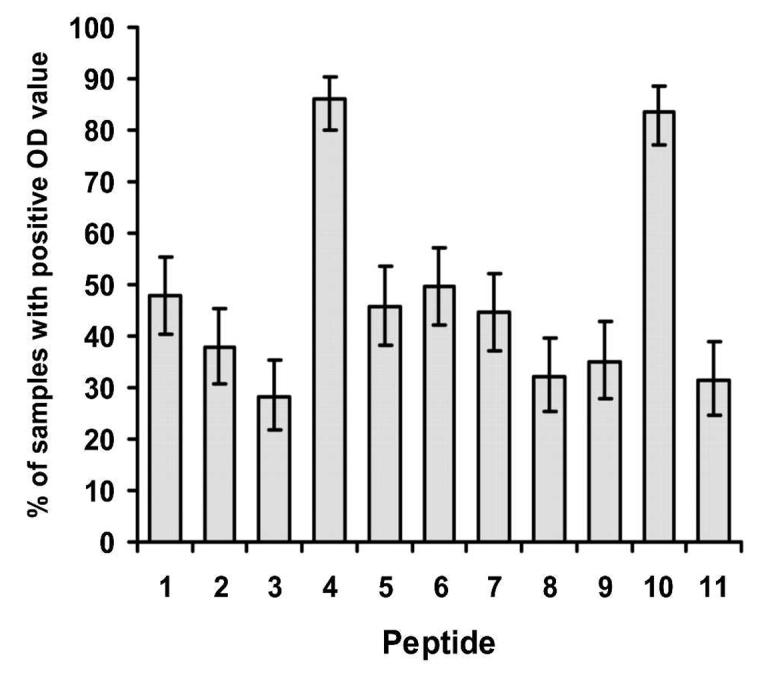
Percent of samples with a positive optical density (OD) value for each of the 11 peptides tested. IgG antibodies to cytoadherence-linked asexual gene 9 peptides were measured by an enzyme-linked immunosorbent assay in 177 asymptomatic children and adults subjected to intense malaria exposure in Madang, Papua New Guinea. Positive sera were defined as those with an OD greater than the mean + 2 SD of OD values obtained from non-malaria-exposed control sera. Error bars show the mean ± SEM.
FIGURE 3.
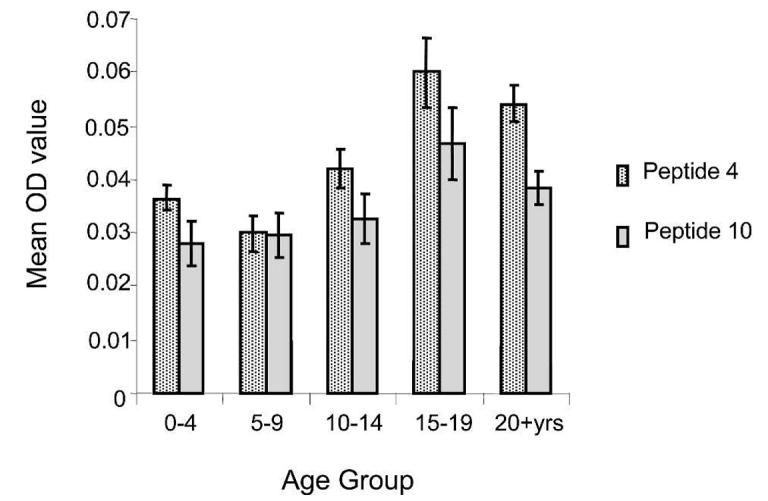
Age-related changes in antibody reactivity to cytoadherence-linked asexual gene (CLAG) peptides 4 and 10 (mean optical density [OD] ± SEM) as measured by an enzyme-linked immunosorbent assay. Of the 11 CLAG 9 peptides tested, peptides 4 and 10 were the two most frequently recognized non-overlapping peptides.
To further investigate the effect of age on peptide response, a number of regression models were fitted using transformed peptide responses as the dependent variable and subject age in years (rather than grouped as above) as the independent variable. For peptide 4, both linear and logarithmic regression models fitted the data equally well, producing significant models in both instances (P < 0.001). For peptide 10, the logarithmic model fitted the data best (P = 0.064) with a rapid increase in peptide response up to five years of age, and a slower continual increase thereafter.
A post hoc analysis of peptide 4 responses showed that individuals with only P. vivax infections (i.e., microscopically negative for all other species) had significantly lower OD values than individuals who had only P. falciparum infections (i.e., microscopically negative for all other species) after controlling for the effect of age (P = 0.019).
DISCUSSION
We have demonstrated that CLAG 9 is immunogenic in humans by showing that antibody reactivity to CLAG 9 peptides (determined by OD value) was significantly higher amongst the malaria-exposed Papua New Guinea study group than the Brisbane controls. Antibody responses to the peptides as determined by OD value were relatively low compared with those of schizont antigens (Figure 4), but this was not surprising since the CLAG responses were to individual peptides only 20 amino acids in length.
FIGURE 4.
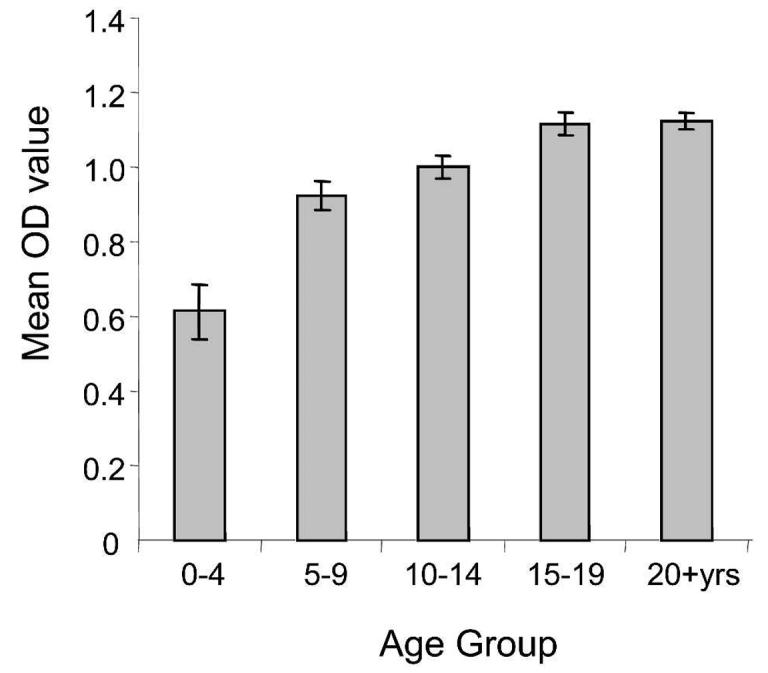
Age-related changes in antibody reactivity to schizont antigen (mean optical density [OD] ± SEM). Antibodies to whole schizont antigen were measured by an enzyme-linked immunosorbent assay.
Peptides 4 and 10 were the most immunogenic of 11 CLAG 9 peptides, with antibody levels to most of the peptides increasing with age. This is likely to reflect age-related maturation of the immune response, given the intensity of malaria exposure in this population. It is well documented that responses to numerous malaria antigens, including vaccine candidate molecules such as erythrocyte binding antigen 175, glycosylphosphatidylinositols, and merozoite surface protein26,34,35 are frequently lower in children than in adults. It is therefore noteworthy that antibody responses to peptides 4 and 10 are high across all age groups and detectable in a majority of children less than five years of age. Of the 11 peptides tested, only peptides 1 and 2 showed any homology to other clag genes, so the high level of reactivity to peptides 4 and 10 is not likely to represent cross-reactivity to other members of the clag gene family. Given that severe malarial disease and mortality is largely restricted to early childhood in highly endemic areas,27 these observations raise the possibility that inclusion of CLAG 9 epitopes in a vaccine for use in this target group may be particularly advantageous should antibodies to CLAG 9 subsequently be shown to protect against disease. Longitudinal studies will be useful in defining what role, if any, CLAG 9 epitopes may play in this regard.
Studies of thick blood film-negative residents in the same population clearly show that parasites can be detected by a polymerase chain reaction in a high proportion of both adults and children in the Madang region.36 It is therefore conceivable that our inability to discern a difference in anti-CLAG 9 antibody levels in microscopically positive P. falciparum subjects in our study was confounded by submicroscopic levels of parasitemia in the thick blood film-negative group. If our initial hypothesis was correct (i.e., that antibody blocking of cytoadherence would result in enhanced splenic clearance of infected erythrocytes and reduced risk of parasitemia), then antibody levels in the microscopically parasite-negative group may have been biased upward by submicroscopic P. falciparum parasitemia.
Our post-hoc finding that subjects with only P. vivax infections appeared to have significantly lower levels of antibodies to CLAG 9 than subjects with only P. falciparum infections is a potentially informative one. Since P. vivax is thought to have only one clag gene, and its degree of homology with clag 9 is not high,37 it is unlikely that antibody reactivity in the P. vivax only group was influenced by cross-reactive antibodies. It is more likely that the P. vivax only response represents decaying antibody reactivity to recent P. falciparum infection, and that subjects infected with P. vivax are less likely to have submicroscopic P. falciparum infection than microscopically negative subjects. Although speculative, this is consistent with previous data from the Madang region showing that species-transcending, density-dependent regulation acts within hosts to produce sequential, rather than concurrent, P. vivax /P. falciparum infections.38 Longitudinal studies may better define the longevity of antibody responses to CLAG 9 and whether higher antibody levels lead to faster clearance of P. falciparum parasitemia.
Immunity to malaria is unlikely to be mediated by responses to any single protein, but is likely to be due to antibodies to multiple parasite proteins, one of which may be CLAG 9. This study has demonstrated that sera collected from individuals resident in an area with intense year round transmission of malaria recognize CLAG 9-specific peptides. Longitudinal studies will be required to determine if such antibodies mediate protection from clinical disease. However, if antibodies to CLAG 9 do subsequently prove to be protective, then the immunogenicity of peptides 4 and 10 that we have demonstrated in children less than five years of age suggests that these peptides may be suitable candidates for multivalent vaccines targeting this high-risk age group.
Footnotes
Financial support: This work was supported by Australian National Health and Medical Research Council (NHMRC) grant no. 199608 to David J. Kemp, Katharine R. Trenholme, and Donald L. Gardiner, and by a generous donation from Mark Nicholson, Alice Hill and the Tudor Foundation. Craig S. Boutlis was supported by an NHMRC postgraduate scholarship and Nicholas M. Anstey was supported by an NHMRC Practitioner Fellowship. Michelle L. Gatton was supported by a University of Queensland post-doctoral research fellowship.
Authors’ addresses: Katharine R. Trenholme, Rachel Kuns, Michelle L. Gatton, David J. Kemp, Michael F. Good, and Donald L. Gardiner. Division of Infectious Diseases and Immunology, The Australian Centre for International and Tropical Health and Nutrition, The Queensland Institute of Medical Research, 300 Herston Road, Brisbane, Queensland, Australia, 4006, Telephone: 61-7-3362-0222, Fax: 61-7-3362-0111. Craig S. Boutlis and Nicholas M. Anstey, Department of Tropical Medicine and International Health, Menzies School of Health Research, PO Box 41096, Casuarina, Darwin, Northern Territory, Australia 0811, Telephone: 61-8-8922-8196, Fax: 61-8-8927-5187. Moses Lagog and Moses J. Bockarie, Papua New Guinea Institute of Medical Research, PO Box 378, Madang, Papua New Guinea, Telephone: 675-852-2909, Fax: 675-852-3289.
REFERENCES
- 1.Miller LH, Good MF, Milon G. Malaria pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nauchni Tr Vissh Med Inst Sofiia. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S, Butcher GA. Properties of protective malarial antibody. Nature. 1970;225:732–734. doi: 10.1038/225732a0. [DOI] [PubMed] [Google Scholar]
- 4.Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, Foucault C, Chongsuphajaisiddhi T, Druilhe P. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 5.Collins WE, Pye D, Crewther PE, Vandenberg KL, Galland GG, Sulzer AJ, Kemp DJ, Edwards SJ, Coppel RL, Sullivan JS. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am J Trop Med Hyg. 1994;51:711–719. doi: 10.4269/ajtmh.1994.51.711. [DOI] [PubMed] [Google Scholar]
- 6.Inselburg J, Bzik DJ, Li WB, Green KM, Kansopon J, Hahm BK, Bathurst IC, Barr PJ, Rossan RN. Protective immunity induced in Aotus monkeys by recombinant SERA proteins of Plasmodium falciparum. Infect Immun. 1991;59:1247–1250. doi: 10.1128/iai.59.4.1247-1250.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, Taraschi TF, Howard RJ. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez V, Hommel M, Chen Q, Hagblom P, Wahlgren M. Small, clonally variant antigens expressed on the surface of the Plasmodium falciparum-infected erythrocyte are encoded by the rif gene family and are the target of human immune responses. J Exp Med. 1999;190:1393–1404. doi: 10.1084/jem.190.10.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyes SA, Rowe JA, Kriek N, Newbold CI. Rifins: a second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc Natl Acad Sci U S A. 1999;96:9333–9338. doi: 10.1073/pnas.96.16.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ockenhouse CF, Klotz FW, Tandon NN, Jamieson GA. Sequestrin, a CD36 recognition protein on Plasmodium falciparum malaria-infected erythrocytes identified by anti-idiotype antibodies. Proc Natl Acad Sci U S A. 1991;88:3175–3179. doi: 10.1073/pnas.88.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pouvelle B, Buffet PA, Lepolard C, Scherf A, Gysin J. Cytoadhesion of Plasmodium falciparum ring-stage-infected erythrocytes. Nat Med. 2000;6:1264–1268. doi: 10.1038/81374. [DOI] [PubMed] [Google Scholar]
- 12.Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, Peterson DS, Pinches R, Newbold CI, Miller LH. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su XZ, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, Peterson DS, Ravetch JA, Wellems TE. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 14.Dodoo D, Staalsoe T, Giha H, Kurtzhals JA, Akanmori BD, Koram K, Dunyo S, Nkrumah FK, Hviid L, Theander TG. Antibodies to variant antigens on the surfaces of infected erythrocytes are associated with protection from malaria in Ghanaian children. Infect Immun. 2001;69:3713–3718. doi: 10.1128/IAI.69.6.3713-3718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bull PC, Marsh K. The role of antibodies to Plasmodium falciparum-infected-erythrocyte surface antigens in naturally acquired immunity to malaria. Trends Microbiol. 2002;10:55–58. doi: 10.1016/s0966-842x(01)02278-8. [DOI] [PubMed] [Google Scholar]
- 16.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 17.Smith JD, Craig AG, Kriek N, Hudson-Taylor D, Kyes S, Fagen T, Pinches R, Baruch DI, Newbold CI, Miller LH. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: a parasite adhesion trait implicated in cerebral malaria. Proc Natl Acad Sci U S A. 2000;97:1766–1771. doi: 10.1073/pnas.040545897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardiner DL, Holt DC, Thomas EA, Kemp DJ, Trenholme KR. Inhibition of Plasmodium falciparum clag9 gene function by antisense RNA. Mol Biochem Parasitol. 2000;110:33–41. doi: 10.1016/s0166-6851(00)00254-1. [DOI] [PubMed] [Google Scholar]
- 19.Trenholme KR, Gardiner DL, Holt DC, Thomas EA, Cowman AF, Kemp DJ. clag9: A cytoadherence gene in Plasmodium falciparum essential for binding of parasitized erythrocytes to CD36. Proc Natl Acad Sci U S A. 2000;97:4029–4033. doi: 10.1073/pnas.040561197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneko O, Tsuboi T, Ling IT, Howell S, Shirano M, Tachibana M, Cao YM, Holder AA, Torii M. The high molecular mass rhoptry protein, RhopH1, is encoded by members of the clag multigene family in Plasmodium falciparum and Plasmodium yoelii. Mol Biochem Parasitol. 2001;118:223–231. doi: 10.1016/s0166-6851(01)00391-7. [DOI] [PubMed] [Google Scholar]
- 21.Manski-Nankervis JA, Gardiner DL, Hawthorne P, Holt DC, Edwards M, Kemp DJ, Trenholme KR. The sequence of clag 9, a subtelomeric gene of Plasmodium falciparum is highly conserved. Mol Biochem Parasitol. 2000;111:437–440. doi: 10.1016/s0166-6851(00)00323-6. [DOI] [PubMed] [Google Scholar]
- 22.Gardiner DL, Spielmann T, Dixon MW, Hawthorne PL, Ortega MR, Anderson KL, Skinner-Adams TS, Kemp DJ, Trenholme KR. CLAG 9 is located in the rhoptries of Plasmodium falciparum. Parasitol Res. 2004;93:64–67. doi: 10.1007/s00436-004-1098-4. [DOI] [PubMed] [Google Scholar]
- 23.Sam-Yellowe TY, Shio H, Perkins ME. Secretion of Plasmodium falciparum rhoptry protein into the plasma membrane of host erythrocytes. J Cell Biol. 1988;106:1507–1513. doi: 10.1083/jcb.106.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douki JB, Sterkers Y, Lepolard C, Traore B, Costa FT, Scherf A, Gysin J. Adhesion of normal and Plasmodium falciparum ring-infected erythrocytes to endothelial cells and the placenta involves the rhoptry-derived ring surface protein-2. Blood. 2003;101:5025–5032. doi: 10.1182/blood-2002-12-3710. [DOI] [PubMed] [Google Scholar]
- 25.Kriek N, Tilley L, Horrocks P, Pinches R, Elford BC, Ferguson DJ, Lingelbach K, Newbold CI. Characterization of the pathway for transport of the cytoadherence-mediating protein, PfEMP1, to the host cell surface in malaria parasite-infected erythrocytes. Mol Microbiol. 2003;50:1215–1227. doi: 10.1046/j.1365-2958.2003.03784.x. [DOI] [PubMed] [Google Scholar]
- 26.Boutlis CS, Gowda DC, Naik RS, Maguire GP, Mgone CS, Bockarie MJ, Lagog M, Ibam E, Lorry K, Anstey NM. Antibodies to Plasmodium falciparum glycosylphosphatidylinositols: inverse association with tolerance of parasitemia in Papua New Guinean children and adults. Infect Immun. 2002;70:5052–5057. doi: 10.1128/IAI.70.9.5052-5057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cattani JA, Tulloch JL, Vrbova H, Jolley D, Gibson FD, Moir JS, Heywood PF, Alpers MP, Stevenson A, Clancy R. The epidemiology of malaria in a population surrounding Madang, Papua New Guinea. Am J Trop Med Hyg. 1986;35:3–15. doi: 10.4269/ajtmh.1986.35.3. [DOI] [PubMed] [Google Scholar]
- 28.Cox MJ, Kum DE, Tavul L, Narara A, Raiko A, Baisor M, Alpers MP, Medley GF, Day KP. Dynamics of malaria parasitaemia associated with febrile illness in children from a rural area of Madang, Papua New Guinea. Trans R Soc Trop Med Hyg. 1994;88:191–197. doi: 10.1016/0035-9203(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 29.Burkot TR, Graves PM, Cattan JA, Wirtz RA, Gibson FD. The efficiency of sporozoite transmission in the human malarias, Plasmodium falciparum and P. vivax. Bull World Health Organ. 1987;65:375–380. [PMC free article] [PubMed] [Google Scholar]
- 30.Bruce MC, Donnelly CA, Packer M, Lagog M, Gibson N, Narara A, Walliker D, Alpers MP, Day KP. Age- and species-specific duration of infection in asymptomatic malaria infections in Papua New Guinea. Parasitology. 2000;121:247–256. doi: 10.1017/s0031182099006344. [DOI] [PubMed] [Google Scholar]
- 31.Delley V, Bouvier P, Breslow N, Doumbo O, Sagara I, Diakite M, Mauris A, Dolo A, Rougemont A. What does a single determination of malaria parasite density mean? A longitudinal survey in Mali. Trop Med Int Health. 2000;5:404–412. doi: 10.1046/j.1365-3156.2000.00566.x. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt R, Steinhilber W, Ruppert C, Daum C, Grimminger F, Seeger W, Gunther A. An ELISA technique for quantification of surfactant apoprotein (SP)-C in bronchoalveolar lavage fluid. Am J Respir Crit Care Med. 2002;165:470–474. doi: 10.1164/ajrccm.165.4.2102080. [DOI] [PubMed] [Google Scholar]
- 33.Box GEP, Cox DR. An analysis of transformations (with discussion) J R Stat Soc. 1964;26:211–252. [Google Scholar]
- 34.al Yaman F, Genton B, Kramer KJ, Chang SP, Hui GS, Baisor M, Alpers MP. Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. Am J Trop Med Hyg. 1996;54:443–448. doi: 10.4269/ajtmh.1996.54.443. [DOI] [PubMed] [Google Scholar]
- 35.Okenu DM, Riley EM, Bickle QD, Agomo PU, Barbosa A, Daugherty JR, Lanar DE, Conway DJ. Analysis of human antibodies to erythrocyte binding antigen 175 of Plasmodium falciparum. Infect Immun. 2000;68:5559–5566. doi: 10.1128/iai.68.10.5559-5566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Felger I, Tavul L, Narara A, Genton B, Alpers M, Beck HP. The use of the polymerase chain reaction for more sensitive detection of Plasmodium falciparum. P N G Med J. 1995;38:52–56. [PubMed] [Google Scholar]
- 37.Holt DC, Fischer K, Tchavtchitch M, Wilson DW, Hauquitz NE, Hawthorne PL, Gardiner DL, Trenholme KR, Kemp DJ. Clags in Plasmodium falciparum and other species of Plasmodium. Mol Biochem Parasitol. 2001;118:259–263. doi: 10.1016/s0166-6851(01)00378-4. [DOI] [PubMed] [Google Scholar]
- 38.Bruce MC, Donnelly CA, Alpers MP, Galinski MR, Barnwell JW, Walliker D, Day KP. Cross-species interactions between malaria parasites in humans. Science. 2000;287:845–848. doi: 10.1126/science.287.5454.845. [DOI] [PubMed] [Google Scholar]