There has been longstanding recognition that life stress in its myriad forms can influence health and the development of disease. However, the mechanisms of these effects of stress, especially in humans, have remained somewhat elusive, and, although rarely articulated, lie at the core of our understanding of how neuroendocrine and immune pathways interact during stress to ultimately contribute to disease. One clue that has received increasing attention is the notion that chronic stress is associated with activation of innate immune, inflammatory responses. Indeed, stress-induced activation of innate immune responses and inflammatory signaling pathways is posited to be an important link between stress and illness, especially given that inflammation is increasingly recognized as a common mechanism of disease in a number of disorders, including cardiovascular disease, diabetes, and cancer (1,2). Glucocorticoids, the end product of activation of stress-induced neuroendocrine pathways and the hypothalamic-pituitary-adrenal (HPA) axis, are some of the most potent anti-inflammatory hormones in the body and thus serve to restrain innate immune responses during infection and other forms of immune activation (3). Nevertheless, much has been written regarding the potentially damaging effects of excessive glucocorticoids in the context of chronic stress (4), thus presenting a conundrum regarding the potential role of inflammation versus glucocorticoids as purveyors of pathology in the contributions of life stress to disease development and progression. Although some investigators have suggested that glucocorticoids may be pro-inflammatory under some circumstances and in some tissues (e.g. the brain) (5), it seems unlikely that an inflammatory response can prevail in an environment in which glucocorticoid signaling is intact (or even excessive).
Some critical insights into the relative balance between glucocorticoids and the inflammatory response during chronic stress are provided in the study by Miller et al., which appears in the current issue of Biological Psychiatry. Using genome wide microarrays and promoter-based bioinformatics techniques, the expression of transcripts bearing response elements for glucocorticoids and nuclear factor kappa B (NF-kB), a lynchpin in the inflammatory response, were examined in peripheral blood monocytes isolated from a small sample of individuals experiencing the stress of caring for a family member suffering from brain cancer (n=11). Results were compared to an age, sex and race-matched control group with no major life stressors over the preceding year (n=10). Monocytes were chosen for analysis because they are a critical immune cell type responsible for initiation of innate immune responses to pathogens in large part through activation of pattern recognition receptors, called toll-like receptors (TLRs), which are linked to inflammatory signaling molecules including NF-kB. In addition, assessments were made of peripheral blood biomarkers of inflammation including c-reactive protein (CRP), interleukin (IL)-6, and IL-1 receptor antagonist (IL-1ra) as well as measures of salivary cortisol production as determined immediately upon awakening and across the diurnal cycle over a 3 day period. Interestingly, chronic caregiving stress was associated with a significant downregulation of genes expressing one or more glucocorticoid response elements and an upregulation in genes expressing response elements for NF-kB. Results for selected genes were confirmed using real time RT-PCR. Moreover, evaluation of gene expression for surface markers differentiating resident versus activated monocytes revealed no differences in the relative populations of these cells between caregivers and controls. Consistent with the increased expression of genes bearing NF-kB response elements, caregivers also exhibited increased serum concentrations of IL-1ra as well as CRP, whose mean concentration in caregivers fell into the high risk (high inflammation) category for the development of cardiovascular disease according to the guidelines of the American Heart Association and the Centers for Disease Control and Prevention (i.e. CRP>3 mg/L) (6). Of note, salivary cortisol output and glucocorticoid receptor (GR) mRNA were no different between the groups. The authors acknowledge that the sample size was small, and the mRNA examined was exclusively derived from a small subset of mononuclear cells of immune origin. Therefore, generalization of these results to other populations of individuals experiencing significant life stress or other cell types or tissues in the body (including the brain) remains to be determined. Nevertheless, the results provide intriguing data to support the notion that at least at the level of the immune system and more specifically peripheral blood monocytes, chronic stress is associated with decreased glucocorticoid signaling coupled with an increase in activity in inflammatory signaling pathways, which in turn were associated with increases in peripheral inflammatory markers of a sufficient degree to pose significant disease risk.
There is a rich literature detailing protein-protein interactions between the GR and NF-kB. Indeed, in addition to glucocorticoid induction of Inhibitor of kappa B (IkB) (which stabilizes NF-kB in the cytosol, thereby preventing its nuclear translocation), nuclear GR-NF-kB protein-protein interactions are believed to play a primary role in the capacity of glucocorticoids to inhibit NF-kB DNA binding and thus inflammatory responses (3). The relative presence of increased expression of transcripts bearing response elements for NF-kB coupled with the decrease in expression of transcripts bearing response elements for the GR in the absence of decreased GR mRNA expression or cortisol output in the Miller et al. study, suggest that there is a disruption of GR function leading to reduced glucocorticoid sensitivity of monocytes and thus increased NF-kB gene expression.
Glucocorticoid resistance in the context of stress has been previously described in mice subjected to social disruption and may provide further insights into the mechanisms of the blunted glucocorticoid and increased NF-kB signaling found in stressed cancer caregivers. Monocytes isolated from the spleen of mice repeatedly exposed to an aggressive, intruder mouse have been found to exhibit decreased sensitivity to glucocorticoids in vitro that is associated with an exaggerated pulmonary immune response to influenza infection, which leads to increased mortality (7). Glucocorticoid resistance in splenic monocytes from mice exposed to social disruption has been shown to be a function of decreased GR translocation from cytoplasm to nucleus with associated decreased inhibition of NF-kB (8). Moreover, social disruption is accompanied by increased monocyte bactericidal activity; an effect that is mediated in part by increased expression of TLRs, which as noted above, are intimately linked to activation of NF-kB (9). Splenic monocytes from IL-1 knock out mice subjected to social disruption fail to exhibit changes in glucocorticoid sensitivity, suggesting that IL-1 plays a central role in reduced GR sensitivity and function (10). Interestingly, in vitro studies also have shown that IL-1 as well as other innate immune cytokines can disrupt GR nuclear translocation and DNA binding, an effect that is mediated by activation of cytokine signaling pathways including p38 mitogen activated protein kinase (11). Cytokines have also been shown to induce GR beta, an inert isoform of the GR (11). Taken together, these data suggest that the increased NF-kB activity in stressed caregivers observed by Miller et al. may be a combination of both upregulation of relevant TLR- linked NF-kB signaling pathways as well as altered GR translocation, GR-DNA binding and/or GR beta expression, possibly secondary to interactions between the GR and innate immune signaling pathways. Of note, glucocorticoid resistance in asthma, a major clinical challenge, is also believed to result in part from cytokine effects on GR.
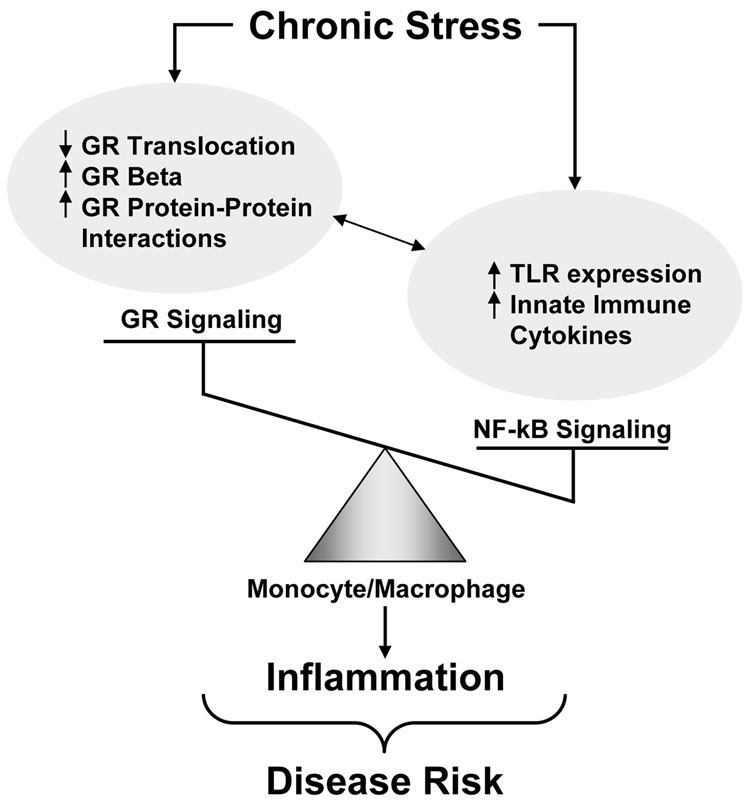
In the context of this discourse, it is convenient to speculate that during chronic stress in humans the balance of neuroendocrine and immune forces that contribute to pathology are tipped in favor of increased inflammation and decreased glucocorticoid signaling (Figure 1). Consistent with this notion is the glucocorticoid resistance seen in stress-related disorders including depression, where nonsuppression of HPA axis function following administration of dexamethasone is believed to contribute to hypersecretion of corticotropin releasing hormone (11). Nevertheless, it should be noted that in socially stressed mice, only monocytes from the spleen exhibited glucocorticoid resistance, whereas monocytes from other tissues remained unaffected (12). Thus glucocorticoid resistance may be a cell or tissue specific phenomenon, leaving other tissues vulnerable to the consequences of glucocorticoid excess. Moreover, although subjects in the Miller et al. study were chronically stressed, it is not clear that these individuals were suffering from any major psychiatric disorders. Transition from chronic stress to frank psychiatric disease may be triggered by failures in adaptation that usher in vulnerabilities to multiple neuroendocrine and immune pathologies. Future studies are clearly warranted to further wrestle with the relative contribution of glucocorticoid and inflammatory signaling to disease development and progression in both psychiatric and other disorders. Indeed, it is conceivable that neuroendocrine and immune contributions to disease may differ among patients, thus highlighting the need for individualized treatments. Nevertheless, the treatment ramifications are not trivial, given that antagonizing glucocorticoid receptors for example, may exacerbate inflammatory contributions to disease. Alternatively, broader spectrum approaches addressing both neuroendocrine and immune components may be indicated.
Figure 1. Tipping the Balance Toward NF-kB Signaling and Inflammation During Chronic Stress.
Increased inflammatory [nuclear factor kappa B (NFkB)] signaling during chronic stress may be a function of increased expression of NF-kB-linked toll-like receptors (TLRs) and/or increased production of innate immune cytokines associated with a decrease in glucocorticoid signaling secondary to decreased glucocorticoid receptor (GR) translocation, GR protein-protein interactions with other inflammatory signaling molecules or increased expression of the inert GR isoform, GR beta.
Footnotes
Financial Disclosure:
Dr. Miller reports being a consultant for Shering-Plough Corporation and has received research grant support from Johnson and Johnson (Janssen/Centocor) and GlaxoSmithKline.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J. 2002;23:831–834. doi: 10.1053/euhj.2001.3052. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 3.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 4.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 5.Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 7.Sheridan JF, Stark JL, Avitsur R, Padgett DA. Social disruption, immunity, and susceptibility to viral infection. Role of glucocorticoid insensitivity and NGF. Ann N Y Acad Sci. 2000;917:894–905. doi: 10.1111/j.1749-6632.2000.tb05455.x. [DOI] [PubMed] [Google Scholar]
- 8.Quan N, Avitsur R, Stark JL, He L, Lai W, Dhabhar F, et al. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J Neuroimmunol. 2003;137:51–58. doi: 10.1016/s0165-5728(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 9.Bailey MT, Engler H, Powell ND, Padgett DA, Sheridan JF. Repeated social defeat increases the bactericidal activity of splenic macrophages through a Toll-like receptor-dependent pathway. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1180–R1190. doi: 10.1152/ajpregu.00307.2007. [DOI] [PubMed] [Google Scholar]
- 10.Engler H, Bailey MT, Engler A, Stiner-Jones LM, Quan N, Sheridan JF. Interleukin-1 receptor type 1-deficient mice fail to develop social stress-associated glucocorticoid resistance in the spleen. Psychoneuroendocrinology. 2008;33:108–117. doi: 10.1016/j.psyneuen.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avitsur R, Stark JL, Dhabhar FS, Padgett DA, Sheridan JF. Social disruption-induced glucocorticoid resistance: kinetics and site specificity. J Neuroimmunol. 2002;124:54–61. doi: 10.1016/s0165-5728(02)00010-3. [DOI] [PubMed] [Google Scholar]