Abstract
Objectives
The purpose of this study was to investigate whether mechanisms of azole resistance in Candida albicans contribute to reduced micafungin activity in vitro.
Methods
MICs were determined for a collection of strains with well-characterized mechanisms of azole resistance obtained from systemic, oral and vaginal infections. This collection of strains includes those with resistance-associated phenotypes. All known molecular mechanisms of azole resistance are included in this set of isolates (alone or in combination). Micafungin activity was further investigated for a subset of isolates by agar dilution.
Results
There was no correlation between any of the azole resistance mechanisms or resistance phenotypes and micafungin activity as determined by MIC, even in isolates with cross-resistance to multiple azole drugs. Overexpression of the ABC transporter CDR2 has been suggested to contribute to reduced echinocandin activity in agar dilution studies. By broth microdilution, there was no difference in MIC between the pump overexpressors and the collection as a whole. However, azole-resistant isolates from matched strains exhibited a small increase in their micafungin MICs relative to their susceptible controls. By agar dilution analysis, multiple CDR2-overexpressing strains exhibited reduced growth in the presence of micafungin relative to the laboratory strain SC5314.
Conclusions
Azole resistance mechanisms do not contribute to increased micafungin MIC as determined by broth microdilution. However, within sets of matched isolates, strains overexpressing CDR2 had a slight increase in micafungin MIC. Changes in micafungin susceptibility are associated with CDR2 overexpression in agar dilution tests.
Keywords: echinocandins, antifungals, XTT
Introduction
Candida albicans is the most common opportunistic fungal pathogen of humans and the fourth leading cause of nosocomial infection in the USA.1 The successful treatment of candidiasis by azoles (particularly fluconazole) has been impaired by the emergence of drug-resistant strains in patients undergoing long-term or prophylactic treatment.
The echinocandins are an emerging class of low-toxicity antifungal drugs effective against many pathogenic fungi. Micafungin is a water soluble, semi-synthetic echinocandin with in vitro and in vivo activity against many fungal species.2–7 Caspofungin and anidulafungin, the two other currently approved echinocandins, exhibit paradoxical attenuated activity at high concentrations.8,9 While this paradoxical attenuation has been described for micafungin, it is considerably more rare and has not yet been described in C. albicans.10 As such, micafungin is poised to become a clinically important member of the antifungal armamentarium.
Unlike the azoles, echinocandins act by specific, non-competitive inhibition of the β-(1,3)-d-glucan synthase enzyme complex that catalyses glucan polymers, a major component of fungal cell walls.11 The reduced activity of echinocandin drugs has been observed in C. albicans strains containing point mutations in FKS1, which encodes a subunit of the glucan synthase complex.12–14 There are no orthologues in humans. Owing to the different sites of action of azoles and echinocandins, it is speculated that azole resistance mechanisms will not contribute to reduced activity of micafungin.
Resistance to azole drugs can be achieved through mutation of the drug target gene (ERG11) or increased expression of ERG11 or of genes involved in drug efflux (CDR1, CDR2 and MDR1).15 A large number of azole-resistant C. albicans strains have increased expression of CDR1 and CDR2, which encode two ABC transporters, and MDR1, which encodes a major facilitator pump. By up-regulating expression of these genes, alone or in combination, azole levels in the cell are kept low through efflux. All previous studies of micafungin activity against azole-resistant C. albicans have been performed on clinical isolates with uncharacterized mechanisms of azole resistance.16–21 This study is the first to evaluate micafungin activity in isolates with known mechanisms of azole resistance.
In addition to contributing to azole resistance, overexpression of CDR2 may play a role in reduced activity of echinocandins.22–24 Agar dilution studies have suggested a correlation between CDR2 overexpression and reduced caspofungin activity. This is not observed in broth microdilution studies. To investigate this possibility for micafungin and for the strains in this collection, agar dilution was performed on the CDR2 overexpressors and on matched azole-susceptible isolates.
In addition to determining micafungin activity in isolates with well-characterized genotypic mechanisms of azole resistance, micafungin activity was determined for C. albicans with resistance-associated phenotypes including mating-type linked (MTL) resistance, trailing resistance, heterogeneous resistance and inducible resistance.25–27 Isolates with MTL resistance have an azole resistance phenotype that correlates with homozygosity at the mating locus possibly associated with alteration in the TAC1 transcription factor.2 Residual growth in a high concentration of the drug is a characteristic of a trailing phenotype.3 A small number of colonies of heterogeneous-resistant (HET-R) isolates will grow in high drug concentrations, but the cells from these colonies are not intrinsically resistant; even cells growing on high concentrations of the drug will remain azole-susceptible.4 In strains with inducible resistance, unstable resistance can be generated through serial passage in the presence of azoles, but is lost in the absence of azoles.4
This study is the first to characterize micafungin activity in a collection of isolates with defined azole resistance mechanisms. Micafungin MICs are determined by turbidity and XTT reduction for this collection. XTT reduction is a quantitative surrogate determination of cell growth.28–31 Isolates from multiple biological sites (oral, systemic and vaginal) and encompassing all known mechanisms of azole resistance (alone or in combination) including resistance-associated phenotypes are represented. This collection includes four matched sets of azole-susceptible and -resistant isolates, three from bone marrow transplant patients32,33 and one from an HIV patient.33–35 In addition to broth-based MIC determinations, to investigate whether CDR2 overexpression plays a role in reduced micafungin activity in agar-based studies, agar dilution was performed on 22 CDR2 overexpressors and on the matched azole-susceptible isolates.
Materials and methods
Organisms and media
A total of 75 isolates were used in this study.14,18,32,36 These isolates have been characterized as C. albicans by their hospital of origin and in this lab by growth on ChromAgar (DRG International, Mountainside, NJ, USA). For long-term storage, cultures were maintained at −80°C in yeast peptone dextrose (YPD; 20 g of dextrose, 20 g of Bacto peptone and 10 g of Difco yeast extract per litre) containing 10% glycerol. Prior to micafungin testing, the isolates were subcultured on YPD at 30°C to ensure growth and purity. Overnight cultures were started from single colonies inoculated into YPD liquid medium and grown at 30°C, 180 rpm.
Chemicals
Micafungin (FK-463, Astellas Pharma, Osaka, Japan) was prepared as a 1.6 mg/mL stock solution in water, filtered through a 0.22 µm filter, aliquotted and frozen at −20°C until use. Owing to its poor solubility in water or RPMI, XTT (X6493, Molecular Probes) was prepared as a 1 mg/mL solution in Ringer’s lactate (Phoenix Pharmaceutical, St Joseph, MO, USA) and filtered through a 0.22 µm filter. XTT solution was made immediately prior to use. Menadione (M5625, Sigma-Aldrich, St Louis, MO, USA) was dissolved in acetone as a 10 mM stock solution and stored at room temperature until use.
Turbidity assay
MICs were ascertained in accordance with the CLSI reference method M27-A2 using RPMI 1640 medium with shaking to provide increased aeration and reduce clumping that is more problematic with micafungin. Briefly, a micafungin solution was diluted 2-fold in 96-well microdilution plates with a total well volume of 100 µL per well. Wells were inoculated with 100 µL of culture diluted to OD600 = 0.00005 and incubated in a moist chamber at 35°C with shaking for 24 or 48 h. Final micafungin concentrations ranged from 0.002 to 1.00 mg/L. Endpoints were determined by comparing OD540 of cells grown in the absence and presence of the drug. The MIC80 was defined as the lowest concentration of the drug that inhibited 80% of a strain’s growth at 48 h relative to the no-drug control. For each plate of isolates tested, controls were performed using C. albicans FKS1 mutants: S20, S22 or S25 kindly provided by David Perlin, Newark, NJ, USA12 and C. albicans ATCC strain 90028.
XTT reduction assay
MIC plates were prepared and inoculated as per the turbidity assay described above. After 24 or 48 h of growth, 4 µL of menadione stock solution was added to 8 mL of XTT solution. Fifty microlitres of this solution was added to each well of the 96-well plate. The plates were incubated for 3 h at 35°C at 180 rpm and then centrifuged to pellet the yeast. Endpoints were determined by comparing the OD492 of supernatants from cells grown in the absence and presence of the drug. The MIC50 and MIC80 were defined as the lowest concentration of the drug that inhibited 50% of XTT reduction at 24 h or 80% of XTT reduction at 48 h, respectively. Control strains were the same as for the turbidity assay.
Agar dilution assay
Overnight cultures of cells were diluted to OD600 of 0.2 in 0.85% NaCl. An aliquot of 2.5 µL of each of three serial 10-fold dilutions was spotted onto an YPD agar plate containing 0.075 mg/L micafungin. Plates were incubated for 24 h at 30°C, the standard temperature for agar dilution. Photographs were taken with a FlouroChem 8900 (Alpha Innotech Corp., San Leandro, CA, USA) and analysed using AlphaEaseFC software. Interpretation of agar dilution is subjective and not quantitative. It is not possible to determine an endpoint, only to compare how well a strain grows in the absence and presence of the drug when compared with control strains.
Results
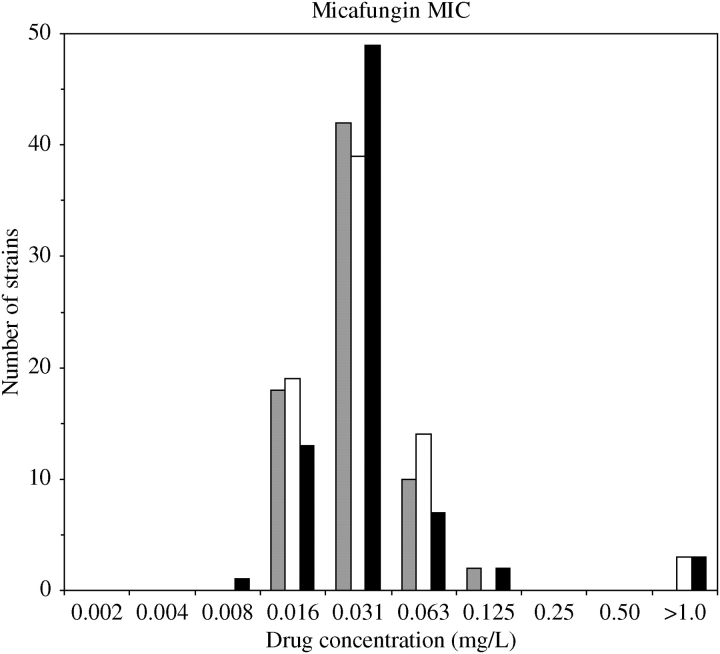
Broth microdilution as defined by the CLSI is the clinically accepted standard method for determining MIC. In order to investigate whether any azole resistance mechanism(s) contributes to a reduction in micafungin activity, micafungin MIC was determined by broth microdilution for a collection of 75 isolates with defined azole resistance mechanisms. MIC80 based on turbidity ranged from 0.016 to 0.125 mg/L micafungin (grey bars in Figure 1). Under these conditions, the three FKS1 mutant strains, S20, S22 and S25, formed hyphal mats that could not be resuspended in broth culture. As a result, their MIC80 could not be determined accurately. MIC80 at 48 h was determined by turbidity for all isolates excluding the FKS1 mutants. Of the 72 isolates for which MIC80 could be determined, 25% had MIC80 ≤ 0.016 mg/L, 83% had MIC80 ≤ 0.031 mg/L, 97% had MIC80 ≤ 0.063 mg/L and 100% had MIC80 ≤ 0.125 mg/L.
Figure 1.
Micafungin MIC values for a collection of azole-resistant C. albicans isolates. MIC80s determined at 48 h by turbidity and XTT reduction are shown as grey and white bars, respectively. MIC50s determined at 24 h by XTT reduction are shown as black bars. Strains with XTT MICs > 1.0 mg/L are FKS1 mutant control strains that could not be analysed by turbidity due to excessive hyphal growth (no grey bar >1.0 mg/L).
When analysis based on turbidity is impractical, surrogate determinations of cell growth must be implemented. C. albicans reduces the tetrazolium salt, XTT, in the presence of an electron-coupling agent (such as menadione) to yield a water soluble, coloured formazan. The intensity of the colour change directly correlates with the number of metabolically active (live) yeast and, as such, is a quantitative surrogate determination of cell growth.28–31 MIC80 at 48 h and MIC50 at 24 h were determined by the CLSI broth microdilution method using XTT reduction as an indicator of cell viability. The MIC80 at 48 h is the standard for most drugs but, because echinocandins are fungicidal, high levels of cellular debris can confound spectrophotometric analysis.37 Thus, for echinocandins, determining the MIC50 at 24 h yields more consistent inter-laboratory results.
MIC80 as determined by XTT reduction ranged from 0.016 to 0.063 mg/L micafungin excluding FKS1 mutant controls (white bars in Figure 1). The three FKS1 mutant strains, S20, S22 and S25, had MIC80 > 1.0 mg/L. Cumulatively, of the 72 test isolates, 26% had MIC80 ≤ 0.016 mg/L, 81% had MIC80 ≤ 0.031 mg/L and 100% had MIC80 ≤ 0.063 mg/L. The only strains that do not fall within this range are the FKS1 mutant controls.
Inter-laboratory differences in MIC80 testing have been reported for the echinocandins. A multicentre study with caspofungin suggested that MIC50 measured at 24 h yields more consistent results between laboratories.37 MIC50s as determined by XTT reduction were evaluated at 24 h and ranged from 0.008 to 0.125 mg/L micafungin excluding FKS1 mutant control strains (black bars in Figure 1). The three control strains, S20, S22 and S25, had MIC50 > 1.0 mg/L as determined by relative growth, or XTT reduction, at 24 h. Cumulatively, of the 72 test isolates, 19% had MIC50 ≤ 0.016 mg/L, 88% had MIC50 ≤ 0.031 mg/L, 97% had MIC50 ≤ 0.063 mg/L and 100% had MIC50 ≤ 0.125 mg/L.
For the majority of strains tested, MIC values (MIC80 determined by turbidity, MIC80 determined by XTT reduction and MIC50 determined by XTT reduction) were identical. Compared with turbidity, the MIC80 determined by XTT reduction for all isolates differed by 2-fold or less, with the majority of isolates having no difference in MIC80, regardless of testing method. By XTT reduction, 47 isolates had identical MIC50 and MIC80, 26 isolates differed by 2-fold and 2 isolates differed by 4-fold. A 4-fold difference in MIC is the allowable variation in independent tests. Thus, all methods of determining MIC yielded values that were similar, if not the same.
The micafungin MIC50s as determined by XTT reduction for this collection are summarized in Table 1 and grouped into subcategories based on: biological origin (rows 2–3), azole resistance profiles (rows 4–10), mechanisms of azole resistance (rows 11–16) and resistance-associated phenotypes (rows 17–20). In this collection, there was no correlation between micafungin MIC80 and the biological origin of the isolate, its azole resistance mechanisms, combination of mechanisms or resistance-associated phenotypes. Even in isolates cross-resistant to multiple azoles, there was no correlation between any azole resistance mechanism and/or resistant phenotype and micafungin activity.
Table 1.
Micafungin MIC50 for azole-resistant isolates by XTT reduction
| MIC50 (mg/L) | ||||
|---|---|---|---|---|
| ≤0.016 | 0.031 | ≥0.063 | Total | |
| Total | 14 | 48 | 10 | 72 |
| Origin | ||||
| oral | 2 | 13 | 5 | 20 |
| systemic | 6 | 22 | 4 | 32 |
| Azole resistance | ||||
| suseptiblea | 3 | 9 | 1 | 13 |
| resistantb | 11 | 39 | 9 | 59 |
| cross-resistantc | 3 | 16 | 5 | 24 |
| fluconazole | 6 | 22 | 8 | 36 |
| itraconazole | 4 | 16 | 4 | 24 |
| ketoconazole | 3 | 13 | 5 | 21 |
| clotrimazole | 2 | 12 | 4 | 18 |
| Mechanism of resistance | ||||
| CDR overexpression | 1 | 14 | 9 | 24 |
| MDR1 overexpression | 0 | 5 | 2 | 7 |
| FLU1 overexpression | 1 | 4 | 1 | 6 |
| ERG11 overexpression | 0 | 6 | 2 | 8 |
| ERG11 mutation | 0 | 5 | 1 | 6 |
| unknownd | 6 | 18 | 0 | 24 |
| Resistance-associated phenotype | ||||
| MTL | 2 | 9 | 3 | 14 |
| HET-R | 1 | 2 | 0 | 3 |
| inducing | 1 | 1 | 1 | 3 |
| trailing | 3 | 5 | 0 | 8 |
aSusceptible isolates represent both susceptible and susceptible dose-dependent isolates.
bIsolates resistant to at least one of the azoles listed.
cIsolates resistant to more than one of the azole drugs listed.
dIsolates that have been characterized, but for which no known resistance-associated gene is overexpressed or mutated.
Overexpression of CDR1 or CDR2 may correlate with a slightly higher MIC when compared with other mechanisms of resistance (compare row 11 with the total trend in Table 1). Regardless of the micafungin MIC determination method, the MICs for CDR2 overexpressors were at least 8-fold lower than the FKS1 mutants and similar to the other isolates tested. Likewise, the micafungin MIC50 for strain DSY1050 that has deletions of MDR1, CDR1 and CDR2 was identical to that for control strain 90028, indicating that the loss of MDR1, CDR1 or CDR2 does not affect micafungin MIC50 (Table 2).
Table 2.
MICs (mg/L) of multiple antifungals for FKS1 mutants, 90028 and DSY1050 (CDR2−/−)
| Strains | MFNa | CAS | FLC | KTC | ITC | VRC | AMB |
|---|---|---|---|---|---|---|---|
| S20 | >1.0 | 3 | 0.5 | 0.006 | 0.016 | 0.008 | 0.047 |
| S22 | >1.0 | 3 | 0.5 | 0.004 | 0.016 | 0.008 | 0.047 |
| S25 | >1.0 | 4 | 0.5 | 0.006 | 0.016 | 0.008 | 0.047 |
| 90028 | 0.016 | 0.064 | 0.5 | 0.008 | 0.023 | 0.012 | 0.094 |
| DSY1050 | 0.016 | 0.023 | 0.047 | <0.002 | <0.002 | 0.002 | 0.032 |
MFN, micafungin; CAS, caspofungin; FLC, fluconazole; KTC, ketoconazole; ITC, itraconazole; VRC, voriconazole; AMB, amphotericin B.
aMIC50 was determined by broth microdilution. All other MICs were determined by Etest.
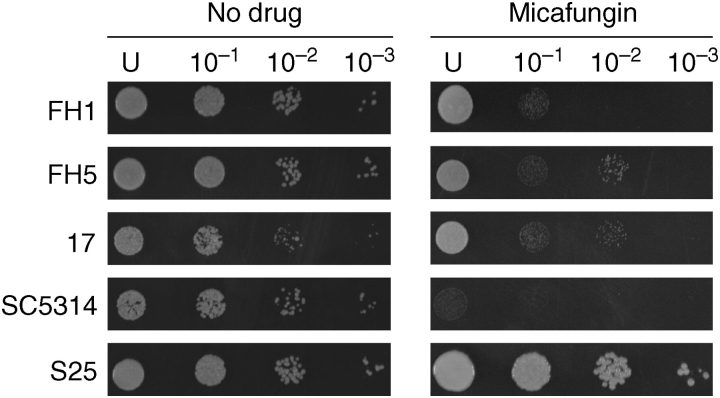
Previous studies using agar dilution methods suggest that CDR2 may play a role in reduced echinocandin activity.22,23 To investigate this possibility for micafungin and for the strains in this collection, agar dilution was performed on 22 CDR2 overexpressors (alone or in combination with CDR1, MDR1 or ERG11), matched azole-susceptible isolates, as well as the common laboratory strain SC5314, and the FKS1 mutant S25. By agar dilution, many CDR2-overexpressing strains were able to grow in the presence of micafungin (Figure 2). When compared with SC5314, 3 isolates had equivalent growth, 7 grew at levels one dilution factor greater, 10 grew better by two dilution factors and 2 had growth equivalent to the FKS1 mutant in the presence of 0.075 mg/L micafungin.
Figure 2.
Agar dilution of CDR2 overexpressors. Serial 10-fold dilutions of exponentially growing cultures were spotted onto YPD plates containing 0.075 mg/L micafungin and incubated at 30°C for 48 h. Strains are: parental azole-susceptible isolate FH1, matched CDR2 overexpressor azole-resistant isolate FH5, azole-resistant CDR2 overexpressor 17, common laboratory strain SC5314 and FKS1 mutant S25. U indicates undiluted.
Fluconazole resistance was not predictive of changes in micafungin activity for the strains tested. The three micafungin-resistant FKS1 mutants, control strain 90028 and DSY1050 (CDR2−/−) were tested for their susceptibility to multiple antifungals (including azole antifungals). By Etest, FKS1 mutant strains had MIC similar to the control strain 90028 for all the antifungals tested: fluconazole, ketoconazole, itraconazole, voriconazole and amphotericin B except for the echinocandins micafungin and caspofungin (Table 2).
For the 22 CDR2-overexpressing isolates tested by agar microdilution, there was no correlation between MICs as determined by the broth microdilution methods (Table 1) and growth on agar (Figure 2). For example, the two isolates that grew as well as the FKS1 mutant by agar dilution have MICs within one dilution factor of SC5314 by all other testing methods. One isolate identical to SC5314 by all MIC tests differed by two dilution factors by agar dilution. While there is a strong correlation within MIC determinations, MIC determination by broth microdilution does not correlate with growth on agar in the presence of the drug.
Three sets of matched azole-susceptible and -resistant isolates (overexpressing CDR2) were further tested by agar dilution at micafungin concentrations of 0.01, 0.03 and 0.075 mg/L (Table 3). For the matched isolates #1 and #17, all three MIC determinations and all three agar dilutions demonstrate reduced growth for the susceptible isolate in the presence of micafungin when compared with the resistant partner. Likewise, in matched isolates FH1, FH5 and FH8, MIC values increase with CDR2 overexpression by all three methods. However, by agar dilution, all three strains have identical growth in the presence of micafungin at any concentration. In matched isolates FHB1 and FHB3, there is an increase in MIC for two of the three MIC determinations. By agar dilution, growth is identical for both strains at one concentration of micafungin, and for the remaining two drug concentrations, the azole-susceptible strain not overexpressing CDR2 grows better in the presence of micafungin. Within these sets of matched isolates, strains overexpressing CDR2 had a slight increase in the MIC of micafungin, while agar dilution results are variable (Table 3).
Table 3.
Micafungin MICs in matched isolates
| Agar dilution | |||||||
|---|---|---|---|---|---|---|---|
| Strain | MIC80 | MIC80, XTT | MIC50 | 0.01 | 0.03 | 0.075 | Notes |
| #1 | 0.031 | 0.031 | 0.031 | 3 | 0 | 0 | fluconazole-susceptible |
| #17 | 0.063 | 0.063 | 0.063 | 4 | 2 | 1 | overexpresses MDR1, CDR1 and CDR2 |
| FH1 | 0.031 | 0.031 | 0.031 | 4 | 2 | 1 | fluconazole-susceptible |
| FH5 | 0.125 | 0.063 | 0.063 | 4 | 2 | 1 | overexpresses CDR1 and CDR2 |
| FH8 | 0.063 | 0.063 | 0.063 | 4 | 2 | 1 | overexpresses CDR1 and CDR2 |
| FHB1 | 0.031 | 0.031 | 0.016 | 5 | 3 | 2 | fluconazole-susceptible |
| FHB3 | 0.031 | 0.063 | 0.031 | 4 | 3 | 1 | overexpresses CDR1 and CDR2 |
| 90028 | 0.016 | 0.016 | 0.016 | 4 | 2 | 1 | control laboratory strain |
| S25 | >10 | >10 | >10 | 5 | 5 | 4 | FKS1 mutant |
All drug concentrations are expressed in mg/L.
On agar dilution plates, each strain was plated at five different cell concentrations. Numbers represent the number of 10-fold dilutions that were able to grow.
Discussion
In this collection of azole-resistant C. albicans isolates, micafungin activity is unaffected by any known mechanism of azole resistance, alone or in combination with other mechanisms. Likewise, isolates with resistance-associated phenotypes did not have MICs significantly different from the ATCC strain 90028. This is not surprising given previous findings that azole-resistant isolates have no change in susceptibility to caspofungin.23 This study is the first to determine that no azole resistance mechanism, set of mechanisms or resistant phenotypes contribute to reduced micafungin activity.
While interpretive criteria for micafungin have not been established, strains with point mutations in FKS1, the gene encoding a subunit of the glucan synthase complex, had MICs that were >8-fold higher than the highest MIC for the azole-resistant strains. Interestingly, the FKS1 mutant strains S20, S22 and S25 were all susceptible to azoles and other antifungals by broth microdilution, suggesting that no known mechanism confers cross-resistance to both azoles and micafungin.
The standard method of determining MIC relies on the turbidity of cultures grown in the absence and presence of the drug. The MIC50 and MIC80 as defined by the CLSI are the drug concentrations at which growth of cells exposed to the drug is inhibited 50% and 80%, respectively, when compared with the same isolate grown in the absence of the drug on the same microtitre plate. The MIC80 at 48 h is the standard for most drugs but, for echinocandins, the MIC50 at 24 h yields more consistent inter-laboratory results.37,38
Relative growth of FKS1 mutants was difficult to characterize by turbidity because they formed hyphal mats in broth culture with or without drug and could not be resuspended. XTT reduction was used as a quantitative surrogate determination of cell growth. MIC80 determined by XTT reduction and turbidity differed by 2-fold or less for all strains tested.
The site of isolation may effect the development of resistance as demonstrated by the paucity of azole-resistant vaginal isolates, despite decades of long- and short-term use of azole drugs to treat vaginal yeast infections.39 In previous studies of azole susceptibility testing, the majority of isolates were collected from the oral cavity. In addition to oral isolates, this collection contains isolates from vaginal and bloodstream infections. There was no correlation between biological site of infection and micafungin MIC (Table 1).
In addition to up-regulation or mutation of resistance-associated genes, resistance to azole drugs can be associated with several drug resistance phenotypes: MTL, HET-R, inducible resistance and trailing resistance (described in the Introduction section and in detail in references 25–27). There was no significant difference in MIC between the ATCC strain 90028 and isolates with inducible resistance, homozygosity at the mating locus or heterogeneous resistance. Inducible resistance can occur after serial passage in the presence of the drug so prolonged passage in the presence of micafungin might lead to transient changes in micafungin MICs though this has not, as yet, been tested. Trailing resistance, characterized by a low level of residual growth in the drug, has been described only for fungistatic drugs. Because micafungin is fungicidal, it is not surprising that there was no significant difference between MIC for the trailing azole-resistant isolates and laboratory control strain 90028.
Of the azole-resistant isolates tested in this study that overexpress a resistance-associated gene, the majority (24/26) express more than one resistance-associated gene in combination. Although multiple azole resistance mechanisms are active in these isolates, there is no indication of reduced micafungin activity by MIC, confirming that micafungin activity is separate from the ergosterol biosynthetic pathway and probably not a substrate for the CDR1, CDR2 and MDR1 pumps.
Twenty-four isolates in this collection are resistant to azole antifungals, but the mechanism of resistance is unknown (i.e. these isolates do not show overexpression or point mutation of resistance-associated genes). Even against these isolates, which may represent entirely new mechanisms of azole resistance, micafungin is effective.
Overexpression of CDR2 contributes to reduced caspofungin activity in agar dilution studies with a limited number of strains.22,23 In the current study, CDR2 overexpressors were tested for their susceptibility to micafungin by broth microdilution methods and by agar microdilution. By broth microdilution, CDR2 overexpressors did not have MICs significantly different from the ATCC strain 90028. In addition, a laboratory-derived CDR2 deletion strain had an unaltered micafungin MIC. However, in sets of matched isolates, strains that overexpressed CDR2 had MICs that were equal to, or more often higher than, their azole-susceptible partners, suggesting that CDR2 may have a small effect when monitored in accordance with the CLSI. Using agar dilution, 2 of the CDR2 overexpressors had equivalent growth to an FKS1 mutant, 3 had growth equivalent to SC5314 and the remaining 17 strains had intermediate growth, demonstrating that the phenotype is common but variable. In agar dilution studies of matched isolates, CDR2 overexpression in an identical strain background does not correlate with an ability to grow on plates containing higher micafungin concentrations (Table 3).
There are a variety of differences between MIC and agar dilution studies such as differences in oxygen availability, oxygen tension, media, temperature of incubation and time of incubation (for MIC80). Additionally, these tests monitor different growth capacities. In broth microdilution, one concentration of cells is immersed in different concentrations of the drug in broth culture. In agar dilution, different concentrations of cells are grown on agar containing one concentration of the drug. Broth microdilution is currently the standard as defined by the CLSI for the determination of the drug activity in clinical diagnostic laboratories and, in the current study, broth microdilution produced consistent results between tests within individual strains. It is unclear whether broth or agar dilution studies most closely represent the infection in the host. Each study may model a different stage of infection.
There was no correlation between any of the azole resistance mechanisms or resistant phenotypes and micafungin activity as determined by MIC, even in isolates with cross-resistance to multiple azole drugs. However, it is difficult to assess the role of CDR2 overexpression with regard to micafungin. By broth microdilution, there was no difference in MIC between the pump overexpressors and the collection as a whole. Although azole-resistant, matched isolates exhibit higher micafungin MICs relative to their susceptible counterparts, the difference is not significant, yet it is reproducible. By agar dilution, many CDR2-overexpressing isolates display intermediate growth in the presence of micafungin. Isolation, culture and more detailed analysis of micafungin breakthrough Candida isolates may provide insight into the role CDR2 overexpression in echinocandin susceptibility.
Funding
This research was supported by a Preclinical Research Agreement from Astellas Healthcare, Inc. to T. C. W. and by NIH NIDCR grants R01 DE11367, R01 DE14161 and R01 DE17078. T. S. R. was supported in part by NIH training grant T32 AI07509.
Transparency declarations
None to declare.
Acknowledgements
We thank David Perlin (Public Health Research Institute, Newark, NJ, USA), Michael Pfaller (University of Iowa, Iowa City, IA, USA), David Stevens (Stanford University, Stanford, CA, USA), Kieren Marr (Oregon Health & Science University, Portland, OR, USA) and Luis Ostrosky-Zeichner (University of Texas Health Science Center, Houston, TX, USA) for providing us with some of the isolates used in this study.
References
- 1.Marr KA. Invasive Candida infections: the changing epidemiology. Oncology (Williston Park) 2004;18:9–14. [PubMed] [Google Scholar]
- 2.de Wet N, Llanos-Cuentas A, Suleiman J, et al. A randomized, double-blind, parallel-group, dose-response study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV-positive patients. Clin Infect Dis. 2004;39:842–9. doi: 10.1086/423377. [DOI] [PubMed] [Google Scholar]
- 3.de Wet NT, Bester AJ, Viljoen JJ, et al. A randomized, double blind, comparative trial of micafungin (FK463) vs. fluconazole for the treatment of oesophageal candidiasis. Aliment Pharmacol Ther. 2005;21:899–907. doi: 10.1111/j.1365-2036.2005.02427.x. [DOI] [PubMed] [Google Scholar]
- 4.Ernst EJ, Roling EE, Petzold CR, et al. In vitro activity of micafungin (FK-463) against Candida spp.: microdilution, time-kill, and postantifungal-effect studies. Antimicrob Agents Chemother. 2002;46:3846–53. doi: 10.1128/AAC.46.12.3846-3853.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groll AH, Stergiopoulou T, Roilides E, et al. Micafungin: pharmacology, experimental therapeutics and clinical applications. Expert Opin Investig Drugs. 2005;14:489–509. doi: 10.1517/13543784.14.4.489. [DOI] [PubMed] [Google Scholar]
- 6.Hiemenz J, Cagnoni P, Simpson D, et al. Pharmacokinetic and maximum tolerated dose study of micafungin in combination with fluconazole versus fluconazole alone for prophylaxis of fungal infections in adult patients undergoing a bone marrow or peripheral stem cell transplant. Antimicrob Agents Chemother. 2005;49:1331–6. doi: 10.1128/AAC.49.4.1331-1336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tawara S, Ikeda F, Maki K, et al. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob Agents Chemother. 2000;44:57–62. doi: 10.1128/aac.44.1.57-62.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens DA, Espiritu M, Parmar R. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob Agents Chemother. 2004;48:3407–11. doi: 10.1128/AAC.48.9.3407-3411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens DA, White TC, Perlin DS, et al. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn Microbiol Infect Dis. 2005;51:173–8. doi: 10.1016/j.diagmicrobio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Chamilos G, Lewis RE, Albert N, et al. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and Candida species-related differences. Antimicrob Agents Chemother. 2007;51:2257–9. doi: 10.1128/AAC.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denning DW. Echinocandin antifungal drugs. Lancet. 2003;362:1142–51. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 12.Balashov SV, Park S, Perlin DS. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob Agents Chemother. 2006;50:2058–63. doi: 10.1128/AAC.01653-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas CM, D’Ippolito JA, Shei GJ, et al. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob Agents Chemother. 1997;41:2471–9. doi: 10.1128/aac.41.11.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S, Kelly R, Kahn JN, et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother. 2005;49:3264–73. doi: 10.1128/AAC.49.8.3264-3273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowen LE, Anderson JB, Kohn LM. Evolution of drug resistance in Candida albicans. Annu Rev Microbiol. 2002;56:139–65. doi: 10.1146/annurev.micro.56.012302.160907. [DOI] [PubMed] [Google Scholar]
- 16.Messer SA, Diekema DJ, Boyken L, et al. Activities of micafungin against 315 invasive clinical isolates of fluconazole-resistant Candida spp. J Clin Microbiol. 2006;44:324–6. doi: 10.1128/JCM.44.2.324-326.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espinel-Ingroff A. In vitro antifungal activities of anidulafungin and micafungin, licensed agents and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature. Rev Iberoam Micol. 2003;20:121–36. [PubMed] [Google Scholar]
- 18.Ostrosky-Zeichner L, Rex JH, Pappas PG, et al. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob Agents Chemother. 2003;47:3149–54. doi: 10.1128/AAC.47.10.3149-3154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung-Tomc JC, White TC, Minassian B, et al. In vitro antifungal activity of BMS-207147 and itraconazole against yeast strains that are non-susceptible to fluconazole. Diagn Microbiol Infect Dis. 1999;35:163–7. doi: 10.1016/s0732-8893(99)00063-2. [DOI] [PubMed] [Google Scholar]
- 20.Takakura S, Fujihara N, Saito T, et al. National surveillance of species distribution in blood isolates of Candida species in Japan and their susceptibility to six antifungal agents including voriconazole and micafungin. J Antimicrob Chemother. 2004;53:283–9. doi: 10.1093/jac/dkh053. [DOI] [PubMed] [Google Scholar]
- 21.van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39:1407–16. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 22.Niimi K, Maki K, Ikeda F, et al. Overexpression of Candida albicans CDR1, CDR2, or MDR1 does not produce significant changes in echinocandin susceptibility. Antimicrob Agents Chemother. 2006;50:1148–55. doi: 10.1128/AAC.50.4.1148-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuetzer-Muehlbauer M, Willinger B, Krapf G, et al. The Candida albicans Cdr2p ATP-binding cassette (ABC) transporter confers resistance to caspofungin. Mol Microbiol. 2003;48:225–35. doi: 10.1046/j.1365-2958.2003.03430.x. [DOI] [PubMed] [Google Scholar]
- 24.Silver PM, Oliver BG, White TC. Characterization of caspofungin susceptibilities by broth and agar in Candida albicans clinical isolates with characterized mechanisms of azole resistance. Med Mycol. 2008;46:231–9. doi: 10.1080/13693780701816557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rustad TR, Stevens DA, Pfaller MA, et al. Homozygosity at the Candida albicans MTL locus associated with azole resistance. Microbiology. 2002;148:1061–72. doi: 10.1099/00221287-148-4-1061. [DOI] [PubMed] [Google Scholar]
- 26.Marr KA, Rustad TR, Rex JH, et al. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob Agents Chemother. 1999;43:1383–6. doi: 10.1128/aac.43.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marr KA, Lyons CN, Ha K, et al. Inducible azole resistance associated with a heterogeneous phenotype in Candida albicans. Antimicrob Agents Chemother. 2001;45:52–9. doi: 10.1128/AAC.45.1.52-59.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manavathu EK, Cutright J, Chandrasekar PH. Comparative study of susceptibilities of germinated and ungerminated conidia of Aspergillus fumigatus to various antifungal agents. J Clin Microbiol. 1999;37:858–61. doi: 10.1128/jcm.37.3.858-861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhn DM, Balkis M, Chandra J, et al. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J Clin Microbiol. 2003;41:506–8. doi: 10.1128/JCM.41.1.506-508.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honraet K, Goetghebeur E, Nelis HJ. Comparison of three assays for the quantification of Candida biomass in suspension and CDC reactor grown biofilms. J Microbiol Methods. 2005;63:287–95. doi: 10.1016/j.mimet.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Tellier R, Krajden M, Grigoriew GA, et al. Innovative endpoint determination system for antifungal susceptibility testing of yeasts. Antimicrob Agents Chemother. 1992;36:1619–25. doi: 10.1128/aac.36.8.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marr KA, White TC, van Burik JA, et al. Development of fluconazole resistance in Candida albicans causing disseminated infection in a patient undergoing marrow transplantation. Clin Infect Dis. 1997;25:908–10. doi: 10.1086/515553. [DOI] [PubMed] [Google Scholar]
- 33.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White TC. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–7. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White TC, Pfaller MA, Rinaldi MG, et al. Stable azole drug resistance associated with a substrain of Candida albicans from an HIV-infected patient. Oral Dis. 1997;3(Suppl 1):S102–9. doi: 10.1111/j.1601-0825.1997.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 36.White TC, Holleman S, Dy F, et al. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother. 2002;46:1704–13. doi: 10.1128/AAC.46.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odds FC, Motyl M, Andrade R, et al. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J Clin Microbiol. 2004;42:3475–82. doi: 10.1128/JCM.42.8.3475-3482.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfaller MA, Messer SA, Boyken L, et al. Further standardization of broth microdilution methodology for in vitro susceptibility testing of caspofungin against Candida species by use of an international collection of more than 3,000 clinical isolates. J Clin Microbiol. 2004;42:3117–9. doi: 10.1128/JCM.42.7.3117-3119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sobel JD, Zervos M, Reed BD, et al. Fluconazole susceptibility of vaginal isolates obtained from women with complicated Candida vaginitis: clinical implications. Antimicrob Agents Chemother. 2003;47:34–8. doi: 10.1128/AAC.47.1.34-38.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]