Abstract
Since the first Wnt gene was identified in 1982, the functions and mechanisms of Wnt signaling have been extensively studied. Wnt signaling is conserved from invertebrates to vertebrates and regulates early embryonic development as well as the homeostasis of adult tissues. In addition, both embryonic stem cells and adult stem cells are regulated by Wnt signaling. Deregulation of Wnt signaling is associated with many human diseases, particularly cancer. In this review, we will discuss in detail the functions of many components involved in the Wnt signal transduction pathway. Then, we will explore what is known about the role of Wnt signaling in stem cells and cancer.
Keywords: Wnt, β-catenin, cancer, stem cell
The orchestration of proliferation and differentiation of each cell in a specific spatial and temporal manner is critical in the development of any multicellular organism. To this effect, multiple signaling pathways have evolved to help coordinate these events and facilitate between cells. The Wnt signaling pathway is exploited in wide variety of contexts to achieve these aims. Wnt signaling controls many events in early development including axis determination, patterning of organs, and cell fate. In the adult organism, Wnt signaling is critically involved in the homeostasis of many tissues, including the intestine, skin, bone and hematopoietic system [1,2]. In addition, recent research suggests that Wnt signaling is also essential in stem cell self-renewal [1,2]. Moreover, the Wnt pathway as a whole and the majority of its components are preserved in many organisms used in biological research, including Drosophila melanogaster, Caenorhabditis elegans, Xenopus laevis, and Mus musculus.
The Wnt-1 gene was first identified as a preferential insertion site for the Murine Mammary Tumor Virus, resulting in overexpression of the Wnt-1 ligand and the formation of mammary tumors [3]. Wnt-1 was originally called int-1. Its Drosophila homolog, Wingless (Wg), controls segment polarity in early development [4], whereas injection of Wnt1 mRNA into ventral side of a Xenopus embryo induces body axis duplication [5]. Wnt proteins activate three different downstream pathways: the canonical pathway, the planar cell polarity (PCP) pathway and the Wnt/Ca2+ pathway. This review will focus on the canonical pathway, which regulates cellular responses through β-catenin. Readers interested in the PCP pathway and Ca2+ pathway are directed to several excellent reviews already written on the subject [6–9].
Deregulated Wnt signaling has been implicated in many hereditary diseases and cancers. Constitutive activation of Wnt signaling is the initiating event in both colorectal cancer and hepatocellular carcinoma, whereas mutations that result in decreased or absent Wnt signaling have been found in several disorders as well. For example, Osteoporosis-pseudoglioma syndrome, which causes defects in bone density, and Familial Exudative Vitreoretinopathy, which results in defective vasculogenesis of the retina [1,2]. A comprehensive resource of information about Wnt signaling can be found on the web at http://www.stanford.edu/~rnusse/Wntwindow.html.
Major Components of the Wnt Pathway
Wnt genes have been identified in many organisms, including insects, nematodes, Cnidaria and vertebrates [10]. In both human and mouse genomes nineteen Wnt genes have been found. Each individual Wnt protein can have drastically different effects on the target. Some activate the canonical pathway, whereas others activate the PCP pathway, and/or the Ca2+ pathway. The latter two pathways are collectively referred to as the “non-canonical” pathways. Thus, Wnt species are generally classified according to which particular pathways they activate [1,2].
In the canonical Wnt pathway, a large number of components work together to transduce an external signal into changes in gene expression within the target cell (Figs. 1 and 2). Wnt is a secreted ligand that binds to its receptor at the cell membrane. The major effect of Wnt binding its ligand is the stabilization of cytoplasmic β-catenin through inhibition of the β-catenin degradation complex. β-catenin is then free to enter the nucleus and activate Wnt-regulated genes through its interaction with TCF (T-cell factor) family transcription factors and concomitant recruitment of co-activators such as p300/CBP, Pygopus, and BCL9/Legless. This section will explore the many different components of the Wnt pathway in more detail.
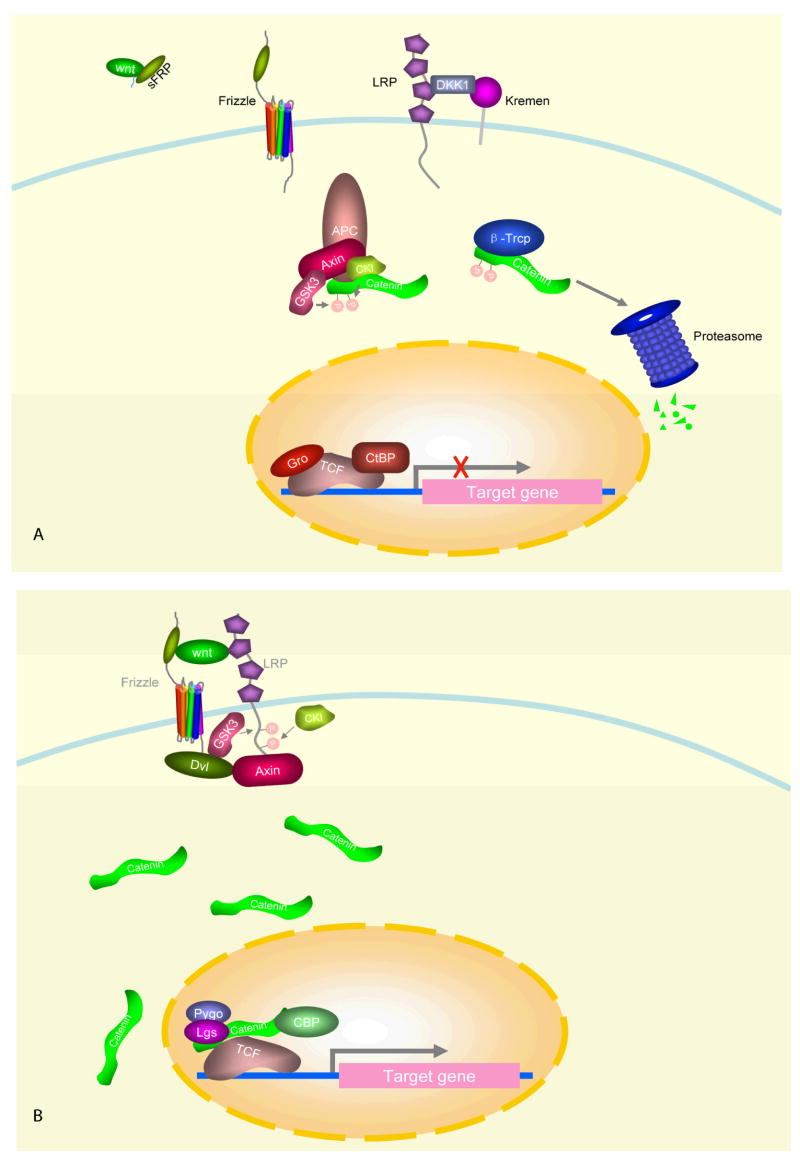
Fig. 1. Secretion of Wnt proteins.
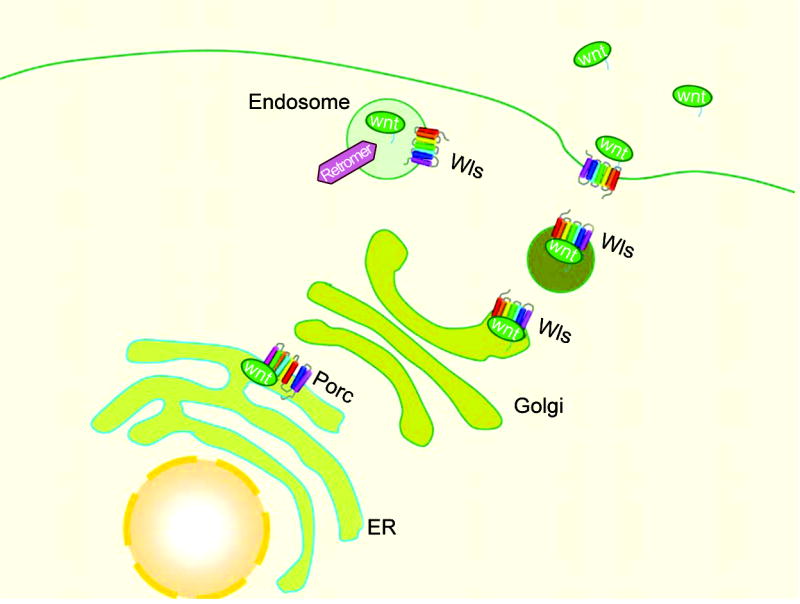
Wnt is synthesized by ribosomes and acylated by Porcupine (Porc) in the ER. Wnt is then transferred to the Golgi. Wntless (WIS) helps transfer Wnt to endosomes, which then fuse with the cell membrane and Wnt is released outside of the cell. However, the secretion of Drosophila WntD does not require lipid modification.
Fig. 2. Wnt signaling transduction pathway.
(A) When Wnt signaling is “turned off”, cytoplasmic β-catenin is degraded by the Axin complex consisting of APC, GSK3 and CKIα. Within this complex, CKIα and GSK3 phosphorylate β-catenin, phosphorylated β-catenin is recognized by β-Trcp and degraded via the ubiquitination-proteasome pathway. Wnt protein can be inhibited by sFRPs, and LRP5/6 can be inhibited by DKK1. (B) When Wnt signaling is “turned on”, Wnt protein binds its receptor Frizzled and co-receptor LRP5/6 and stimulates LRP5/6 phosphorylation with the help of Dishevelled. Phosphorylated LRP5/6 recruits Axin to the membrane and disrupts the Axin complex. β-catenin accumulates in the cytoplasm and enters nucleus, where it binds TCF/LEF and co-activators, such as pygopus, legless and CBP/p300, and activates gene expression.
Wnt is a secreted ligand
Despite the differences individual Wnts can have on target cells, all Wnt proteins are similar in that they have an N-terminal signal peptide, one or more N-linked glycosylation sites, and 23 conserved cysteine residues [2]. In addition, most Wnt proteins are lipid-modified and are thus hydrophobic in nature, explaining the difficulty many have had in their purification [11]. The acyl-transferase protein Porcupine regulates lipid modification of Wnt proteins in the ER and is critical in the transport and secretion of Wnt (Fig. 1) [11,12]. However, the secretion of Drosophila WntD does not require lipid modification [13].
Wntless (Wls, also known as Evenness Interrupted, EVI, or Sprinter, SRT) is a multi-trans-membrane protein localized in the Golgi apparatus and in the cell membrane that also regulates the secretion of Wnt [14–16]. Moreover, Wntless genes have been found in both worms and mammals, suggesting that it is a conserved member of the Wnt pathway. Wntless directly interacts with Wnt and may act as a receptor for transporting Wnt from the trans-Golgi network to endosomes. In both the Golgi and endosomes, Wntless co-localizes with retromer, a highly conserved multi-protein complex involved in cell sorting. Furthermore, in C. elegans, RNAi-mediated knock-down of a key component of the retromer complex, Vps35, hampers the formation of Wnt gradients [17]. Additional studies in Drosophila and C. elegans suggest that retromer regulates the retrieval of Wntless (mig14 in C. elegans). For example, when Vps35 is inhibited, Wntless is targeted to the lysosome for degradation, compromising the secretion of Wnt [18,19].
The Wnt receptors––Frizzled, and others
After Wnt is secreted from a cell, it diffuses to nearby cells and binds to its receptor, Frizzled (Fz) (Fig. 2). Fz was originally identified as the receptor in Drosophila through biochemical and genetic means [20]. Many vertebrate homologues of Fz were identified soon after [21]. Fz receptors are seven trans-membrane repeat proteins that belong to a family of G-protein coupled receptors. Interestingly, this family also includes Smoothened, a key component in Hedgehog signaling [22]. The extra-cellular N-terminus of Fz contains a cysteine-rich domain (CRD) that directly interacts with Wnt. In vitro, each Wnt can bind to many different Fz receptors [23,24]. In vivo, specificity may be achieved by additional factors or by the restricted temporal expression of both Wnt and Frizzled. The cytoplasmic tail of Frizzled has a conserved KTxxxW motif that interacts with a downstream mediator of Wnt signaling, Dishevelled (Dvl), through its PDZ domain [25,26]. In addition, some Frizzled receptors have an S/TxV motif that may also bind to PDZ domain of Dvl [27]. In addition to signaling through Dvl, it has also been suggested that Frizzled may activate signaling through heterotrimeric G protein [28,29].
Low-density-lipoprotein receptor-related proteins 5 and 6 (Lrp5/6) are co-receptors of Fz, whereas arrow is the Drosophila homolog [30–32]. Genetic deletion of Lrp5/6 in mice results in a phenotype that resembles a Wnt null mutation, suggesting that Lrp5/6 is a critical component of the Wnt pathway [30,32,33]. Lrp5/6 is a single trans-membrane protein. The extracellular domain contains an YWTD β-propeller and an EGF-like domain, and is required for binding Wnt as well as signal transduction [31,34]. On the cytoplasmic side, the C-terminus of Lrp5/6 contains five conserved PPP(S/T)P motifs which are equally indispensable for the transduction of Wnt signaling (See below). The proper expression and localization of Lrp5/6 is itself subject to regulation. Mesd, an ER chaperone protein, is involved the maturation of the Lrp5/6 receptor and its transport to the cell surface. Boca is its Drosophila homologue [35,36].
Additional receptors for Wnt have also been reported. For example, the atypical receptor tyrosine kinase Ryk binds Wnt and regulates neurite outgrowth. Derailed, its Drosophila homologue, similarly mediates axon guidance [37,38]. Wnt5a can also signal through Ror2, a receptor tyrosine kinase, to regulate convergent extension through the activation of PI3K and Cdc42 [39–41].
In addition to Wnt, several other ligands can bind Fz receptors and activate the canonical pathway. For example, Norrin binds Fz4 and this interaction appears to be required for vascular development in eye and ear [42]. In addition, mutations in either Norrin or Fz4 result in the retinal vascular defects found in both Norrie disease and Familial Exudative Vitreoretinopathy (FEVR) [42].
Similarly, R-spondin family members bind Fz8 and Lrp6 and activate expression of Wnt target genes [43–45]. In vertebrates, the R-spondin family contains four members. R-Spondin2 activates Wnt signaling in Xenopus embryo [46], whereas the intestinal epithelium of mice overexpressing R-spondin1 has hyperplasia and elevated β-catenin levels [46]. Additional roles of R-spondin family members have been reported in limb and lung development as well as sex determination [47–49].
Extracellular Wnt antagonists
Many extracellular inhibitors of Wnt signaling have been reported. For example, both Secreted Frizzled-related protein (sFRP) and Wnt-inhibitory factor (WIF) antagonize Wnt signaling by sequestering the Wnt protein in the extracellular matrix. sFRP binds Wnt through its CRD domain, whereas [50,51] WIF proteins binds Wnt through its WIF domain [52].
Using an entirely different mechanism Wise [53], SOST [54,55], and Dickkopf (Dkk) [56] antagonize Wnt through interactions with LRP. In addition, Dkk1 bridges Lrp6 and another transmembrane protein, Kremen, inducing endocytosis of Lrp6. Without Lrp6 available at the cell surface, Wnt signaling is effectively inhibited [57].
Crossing the membrane
The extracellular binding of Wnt to Fz and Lrp5/6 modulates intracellular components of the Wnt pathway through at least two mechanisms (Fig. 2).
First, the binding of Wnt induces structural changes in the receptor that result in recruitment of Dvl to the cytoplasmic tail of Fz through its intracellular KTxxxW motif [25,26,58,59]. Dvl is an important cytoplasmic component of Wnt pathway that is conserved in both flies and vertebrates [60–62], and genetic epistasis studies place Dishevelled downstream of Frizzled, but upstream of GSK3 (Glycogen Synthase Kinase 3) and β-catenin [63]. Wnt also induces phosphorylation of Dvl [64], although the role of the phosphorylation of Dvl is still unclear. However, several kinases phosphorylate Dvl, including casein kinase I (CKI), CKII, and Par-1 [65–67].
Second, the C-terminus of LRP5/6 interacts with Axin, which is an inhibitory downstream component of the Wnt pathway. Recruitment of Axin to the cell membrane inhibits its function, resulting in the stabilization of β-catenin [34]. The binding of Wnt also induces phosphorylation of the co-receptor Lrp5/6 at its PPPSP motif, creating a docking site for Axin [68]. Phosphorylation of the PPPSP motif is mediated by membrane-bound GSK3 and CKI [69]. In response to Wnt stimulation, LRP5/6 is phosphorylated at an additional site, N-terminal to PPPSP motif by the membrane-bound protein casein kinase Iγ (CKIγ). Moreover, phosphorylation at this site is indispensable for Wnt signaling [70].
Although it is thought that the physical proximity of Fz and LRP5/6 induced by Wnt binding is crucial in activating downstream components, surprisingly, Arrow mutant flies were only inefficiently rescued by a Frizzled-arrow fusion protein. To explain this, a two-step signaling mechanism was recently proposed. The initiation step requires both Frizzled and arrow, whereas the amplification step depends only on arrow [71]. In support of this, both Frizzled and Dishevelled are required for phosphorylation of LRP6 [72–74]. Thus, the PPPSP motif of LRP5/6 may function as an amplifier of Wnt signaling. Using live imaging of vertebrate cells, one study found that Wnt induces the organization of phosphorylated LRP6 into aggregates known as signalosomes, in a Dvl-dependent manner [74]. These findings suggest that Fz, LRP5/6, Dvl and Axin must organize into macromolecular complex in order to efficiently mediate Wnt signaling.
In the cytoplasm
β-catenin was originally identified as E-cadherin binding partner and important in cell-cell adhesion, prior to the discovery of its involvement in the Wnt pathway [75]. However, it was found that mutations of the Drosophila homologue of β-catenin, Armadillo (Arm) [76,77], gave a similar phenotype as the Wg mutant [4], suggesting that Arm might be part of the Wg signaling pathway. Further studies found that injection of β-catenin mRNA into ventral side of Xenopus embryos induced a secondary axis, a hallmark of Wnt signaling [78]. Thus, it became clear that β-catenin/Arm functions downstream of Wnt/Wg [79], in addition to its previously characterized role in cell-cell adhesion.
Many of the other components of the Wnt pathway were soon found by a variety of means. In mice, Axin is encoded by the fused locus, and Axis duplication was observed in fused homozygous mutant embryo [80]. Similar results were found in Xenopus embryos, implicating Axin as a negative regulator of Wnt signaling [80]. The Serine/Threonine kinase GSK-3β was initially found as a key regulator of glycogen metabolism, as it can phosphorylate and inactivate glycogen-synthase. However, it was later found to be essential in several signaling pathways as well [81]. Zeste-White 3, the Drosophila homologue of GSK-3β, was found to be a negative regulator of segment polarity downstream of Wg [82]. In the Xenopus embryo, GSK-3β suppresses axis formation induced by Wnt [83].
Adenomatous polyposis coli (APC) is a tumor suppressor protein frequently mutated in colorectal cancer [84,85]. In early attempts to identify the function of APC, it was found that APC could directly interact with β-catenin and decrease levels of cytoplasmic β-catenin [86–88], whereas mutations of APC found in human colorectal cancer lead to accumulation of β-catenin [89,90]. In addition, APC also binds and is phosphorylated by GSK-3β [89].
Thus, it is now clear that the central task of canonical Wnt signaling is to regulate β-catenin stability. The level of cytoplasmic β-catenin is tightly controlled by the cytoplasmic degradation complex (Fig. 2), which contains the scaffold protein Axin, as well as β-catenin, CKI, GSK3 and APC [91–95]. In unstimulated cells, this complex mediates the degradation of cytoplasmic β-catenin through a multistep process. First, β-catenin is phosphorylated at N-terminus by casein kinase Iα (CKIα) [95,96] and GSK-3β [97]. Phosphorylated β-catenin is then ubiquitinated by β-Trcp, a component of an E3 ubiquitin ligase complex [98–102]. Ubiquitinated β-catenin is then rapidly degraded by proteasome.
The structure of part of this destruction complex has been solved [103–112]. The structures of central armadillo repeats as well as the full-length β-catenin have also been solved [109,113]. These studies suggest that β-catenin degradation is regulated by a dynamic protein complex. APC may regulate the assembly of Axin complex. When β-catenin is phosphorylated by CKI and GSK-3β within this complex, APC is also phosphorylated. Phosphorylated APC binds β-catenin with a significantly higher affinity, displacing β-catenin from the Axin complex [108,114,115]. Axin is the least abundant protein among the destruction complex proteins and appears to be the rate-limiting factor [116]. β-catenin can be phosphorylated in colon cancer cell line SW480, which contains truncated APC. However, β-catenin ubiquitination cannot be detected in SW480 cells, suggesting that separate domains of APC are required for phosphorylation and ubiquitination. In addition, overexpression of a functional APC fragment can restore β-catenin ubiquitination and degradation, further suggesting that APC regulates β-catenin phosphorylation and degradation by distinct domains and steps.
As mentioned earlier, Wnt proteins bind Fz and Lrp5/6, resulting in phosphorylation of cytoplasmic tail of Lrp5/6 and recruitment of Dvl [73,74]. Additionally, phosphorylated Lrp5/6 relocates Axin to the cell membrane, inhibiting the cytoplasmic degradation complex through a mechanism that is not completely understood. β-catenin is then free to accumulate and translocate into the nucleus. Moreover, Axin degradation upon Wnt stimulation provides another way to stabilize β-catenin [34 117–119].
Since phosphorylation plays important roles in Wnt signaling, the many associated kinases and phosphatases have been extensively studied. For example, Axin binds the catalytic domain of Protein Phosphatase 2A (PP2A) [120]. However, both positive and negative roles for PP2A in Wnt signaling have been reported [121–128]. The exact role of PP2A in Wnt signaling may depend on the composition of different PP2A regulatory subunits and needs further examination. Protein phosphatase 1 (PPI) has a clear positive role in Wnt signaling [129]. PP1 binds and de-phosphorylates Axin, decreasing its affinity for GSK-3β, therefore leads to stabilization of β-catenin [129].
By tandem-affinity purification, WTX (Wilms Tumor suppressor X chromosome), was found to interact with β-catenin, Axin, APC and β-Trcp. Moreover, WTX promotes β-catenin degradation and ubiquitination in mammalian cells, as well as zebra fish and Xenopus [130]. As WTX is inactivated in one third of Wilms tumors [131], it will be interesting to investigate its role in other types of tumors.
Into the nucleus
β-catenin does not contain a nuclear localization sequence. It has been suggested that β-catenin can directly interact with nuclear pore components, bypassing the importin/karyopherin proteins, in order to enter the nucleus [132,133]. Recently, it was discovered that JNK2 phosphorylates β-catenin at Ser191 and Ser605 in response to Rac1 activation, and that phosphorylation at these two serine controls nuclear translocation of β-catenin [134].
β-catenin contains a nuclear export sequence, consistent with its ability to shuttle in and out of the nucleus in response to changes in Wnt signaling, but how other factors regulate its export is still a contentious issue. One model suggests that Axin [135] or APC [136–138] actively export β-catenin from the nucleus, in addition to their more fully characterized role in β-catenin degradation. An alternate model suggests that these factors do not actively participate in shuttling, but rather as an “anchor” to retain β-catenin within their respective compartments [139]. In this model TCF4, Pygopus, and BCL9 function as nuclear “anchors” [140], and Axin functions as a cytoplasmic “anchor” [141].
In the nucleus β-catenin interacts with TCF family of transcription factors [142,143]. The TCF family includes TCF-1, LEF-1 (Lymphoid enhancer factor-1), TCF-3, and TCF-4. Among them, TCF4 is the primary member of the TCF family that is regulated by β-catenin in response to Wnt signaling in the intestine. In the unbound state, TCF/LEF family members actively recruit co-repressors such as CtBP [144], HDAC1 [145,146], and Groucho/TLE [147–149] to inhibit transcription. Groucho/TLE, in turn, interacts with hypo-acetylated histone H3, presumably to help maintain a repressive chromatin environment [150]. However, once β-catenin enters the nucleus, it binds TCF4 through its central armadillo repeats, displaces Groucho/TLE1 from TCF/LEF [151] and recruits co-activators through its N- and C-terminal transactivation domains (Fig. 2).
The N-terminal transactivation domain of β-catenin, extends from the region just C-terminal to the regulatory region involved in its stability, to the first four Armadillo repeats [152]. This transactivation domain directly associates with BCL9/Legless, which in turn recruits the transcriptional co-activator Pygopus [153–156]. Pygopus contains a Plant Homeodomain (PHD). PHD domain can interact with tri-methylated histone H3, and is thought to regulate epigenetic modifications on target genes [157]. In addition, Pygopus can dimerize through this domain in vitro [110].
The C-terminus of β-catenin contains a strong transactivation domain [142,158,159]. This transactivation domain recruits p300/CBP which is required for Wnt signaling [158,160]. p300 and CBP are paralogous transcriptional co-activators; they acetylate nearby histones, loosening chromatin in order to facilitate binding of other transcription factors [161,162]. In addition, the C-terminal transactivation domain associates with Parafibromin, a component of PAF1 complex, and is recruited after Pygopus. PAF1 is important for the initiation and elongation steps of transcription through its interaction with RNA polymerase II. The association of β-catenin with the PAF1 complex is required for transactivation. Overexpression of Parafibromin compensated for loss of Legless in vivo [163].
Other data suggests that β-catenin can interact with the co-activator FHL2 [164], the basal transcription factor TBP [165], the ATP-dependent chromatin remodeling factors Brg-1/Brahma [166 ] and the ATP-dependent helicase TIP49a/Pontin52 [167,168]. However, these interactions have not been fully characterized.
Wnt Signaling in Stem Cells
The cells of mammalian organisms are highly dynamic. Every day, millions of cells are replaced due to physical, chemical and immunologic injuries. Stem cells are required to maintain the architecture and function of organisms. These cells reside in special microenvironment called a niche and they maintain the proliferative potential of tissues throughout the life of an organism. Key features of stem cells are self-renewal and their ability to give rise to different cell lineages. Wnt signaling is critical in the self-renewal of stem cells in many different tissues, including the skin, intestine, brain and blood. This section will further explore the role of Wnt signaling in this context.
Intestinal stem cells
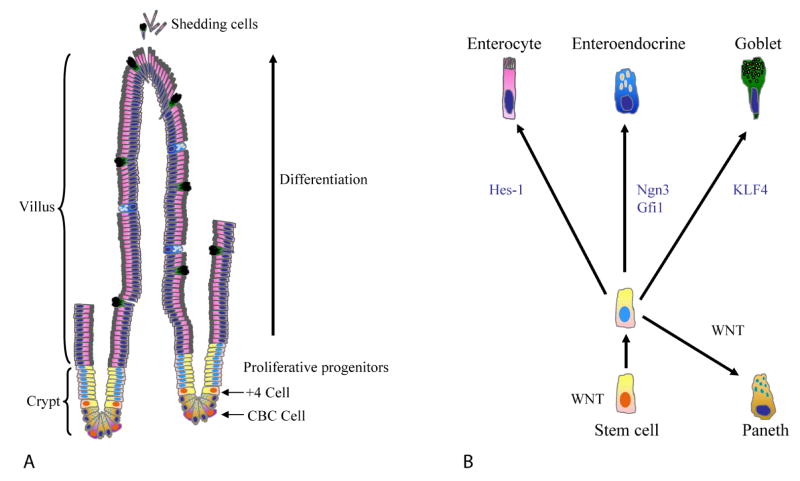
The gut is a tube-like organ that originates from all three germ layers: the endoderm, mesoderm and ectoderm. The luminal surface of the gut is covered by a continuous sheet of epithelial cells derived from endoderm. In the epithelium of the small of intestine, this sheet folds into finger-like protrusions that extend into the lumen, called villi. In between each villus, the epithelial sheet additionally invaginates inward to form the crypts of Lieberkühn [169] (Fig. 3). Notably, no villi are present in the colon and instead the colonic epithelium consists entirely of crypts. Stem cells that replenish the intestinal epithelium are located at the bottom of crypts. Crypt stem cells produce transit-amplifying cells that ultimately differentiate into enterocytes, goblet cells, and enteroendocrine cells (Fig. 3). In the small intestine, transit-amplifying cells additionally differentiate into Paneth cells [169,170]. Enterocytes are the most abundant cell type of the intestine, and perform its primary absorptive function. Goblet cells secrete mucin that protects the luminal surface. Enteroendocrine cells are located throughout the crypt-villus axis and secrete intestinal hormones. Paneth cell are found at the bottom of crypts and release lysozyme as well as other anti-microbial molecules. With the exception of Paneth cells, terminally differentiated cells migrate along the crypt-villi axis and are shed into lumen after 5–7 days. In each crypts, stem cells have to generate around 300 cells per day in order to replenish those lost [170].
Fig. 3.
Structure of the intestine (A) and differentiation of intestinal stem cell (B)
Wnt signaling is critical in the regulation of intestinal homeostasis. TCF4, encoded by Tcf7l2 gene, is a downstream target of Wnt signaling and is highly expressed in the intestinal epithelium. TCF4 knock out mice lack crypts, suggesting that TCF4 is essential for maintenance of epithelial stem cell compartment [171]. Overexpression of Dkk1, a Wnt inhibitor, in intestine causes loss of crypt and secretory cell lineages [172]. These data suggest Wnt is essential for the homeostasis of intestine epithelium. A similar phenotype was observed when Dkk1 was overexpressed using adenoviruses [173]. As mentioned earlier, APC is a negative regulator of β-catenin and a tumor suppressor in colorectal cancer. Targeted deletion of APC in the mouse intestine activates Wnt signaling and results in expansion of the crypts [174]. In addition, Goblet cells are lost, and Paneth cells are mis-positioned throughout the crypts-villus axis [174], resembling loss of the cell-sorting receptor EphB3 [175]. Expression of the Wnt agonist R-spondin1 in mice induces crypt cell proliferation [46]. Crypt epithelial cells consistently produce Wnt3, Wnt6, and Wnt9b [176], suggesting that Wnt might function in a paracrine or autocrine manner. MYC is a well established Wnt target gene [177]. Interestingly, deletion of MYC in APC−/− intestine rescues the defects in proliferation and migration found in APC−/− mice [178]. These data suggest that Wnt is an essential mitogen in the crypt.
Because no specific marker for intestinal stem cells has been found, the exact position of these cells within the crypts is still unclear. However, experiments following the retention of labeled DNA suggest that the stem cell might at the +4 position, just above the crypt base columnar and Paneth cells found at the base of the crypt [170]. In addition crypt base columnar cells have been suggested has stem cell activity [179]. LGR5 is an orphan G-protein coupled receptor first identified as Wnt target gene in micro-array studies [180]. In situ hybridization and reporter knock-in studies suggest that LGR5 is an intestinal stem cell marker and that the crypt columnar base cells are the stem cells of the intestine [181]. However, the relationship between +4 cells and the crypt columnar base cells are remained to be determined.
Recent studies suggest that a small subset of cells in tumors have stem cell-like characteristics. Two groups independently reported the identification of a colorectal cancer intiating cell based on the surface marker CD133 [182,183]. The markers for colon cancer stem cell need to be further characterized. Since various Wnt signaling pathway components, such as APC, Axin or β-catenin, are mutated in more than 90% of colorectal cancer patients, it is of great interest to know what is the role of Wnt signaling in colorectal cancer stem cells.
Hematopoietic stem cells
Hematopoietic stem cells (HSCs) are multi-potent cells that are able to give rise to all blood cell lineages. The fates of HSCs progeny are determined in a step-wise, hierarchical fashion. HSCs give rise to common myeloid progenitor (CMP) and common lymphoid progenitor (CLP) cells. Red blood cells, macrophages, granulocytes and platelets derive from CMP cells, whereas T cells, B cells, dendritic cells and natural killer cells derive from the CLP cells. In the early embryo, HSCs derive from the mesoderm. Hematopoiesis begins in the yolk sac but then quickly shifts to the liver. Later, fetal HSCs home to the bone marrow, where they will reside throughout the life of the adult [184].
HSCs are the best characterized type of stem cell because of the ability to purify them to homogeneity based on cell surface markers. For example, mouse HSCs express cell surface proteins C-kit and Sca-1 but are negative for lineage markers (Lin−Sca-1+c-Kit+, or LSK cells) [185].
Recent studies have begun to uncover the role of Wnt in hematopoiesis. TCF1 and LEF1, transcription factors targeted by the Wnt signaling pathway, and are expressed in a specific pattern, suggests Wnt signaling might be important in hematopoiesis, both in self-renewal and differentiation [186,187]. For example, knock-out TCF1 or LEF1 in HSCs blocks T-cell differentiation [188], whereas in vitro purified Wnt3a stimulates self-renewal of HSCs [11]. Overexpression of activated β-catenin promotes the growth of HSCs and maintains an immature phenotype in long term culture. In addition, these expanded HSCs reconstitute the blood system more efficiently in lethally irradiated mice [189]. Inhibition of Wnt signaling by over expressing Axin leads to slow growth of HSCs and reduced percentage of reconstituted stem cells [189].
However, other reports have found that knocking down expression of β-catenin had no effect on the ability of HSC to reconstitute hematopoiesis in an irradiated host mice [190]. Other study found that simultaneous knock-out β-catenin and γ-catenin did not impair reconstitution [191,192]. Using a synthetic reporter, however, another report found that canonical Wnt signaling still active in these double knock-out cells [192]. Enhancing Wnt signaling by treating lethally irradiated mice with a GSK3 inhibitor increases the likelihood of hematopoietic repopulation [193]. On the other hand, overexpression of β-catenin in transgenic mice blocks lineage differentiation and results in an inability to repopulate irradiated hosts [194,195]. Although completely resolution of these discrepancies will require additional carefully designed experiments, it is likely supra-physiologic levels of β-catenin enforced cell cycling of HSCs and exhausted the long-term stem cell pool [195]. Thus, it appears that maintaining a critical level of β-catenin might be important for the normal function of HSCs.
Additional data suggests that non-canonical Wnt signaling also has role in hematopoiesis. For example, treating HSCs cells with Wnt5a enhances their ability to reconstitute hematopoiesis [196]. In addition, Wnt5a antagonizes canonical Wnt signaling, keeping HSCs in the quiescent, G0 state, and increases the ability of HSCs to repopulate the irradiated hosts [197].
In addition, the bone marrow itself provides cues for HSCs in deciding between self-renewal and differentiation. For example, both osteoblasts and vascular cells have been implicated in maintaining the stem cell niche. Osteoblasts may function to retain HSCs in the bone marrow and regulate their stemness [184]. For example, using a transgene driven by a Collagen1a promoter, targeted overexpression of Dkk1 in osteoblasts results in HSCs that are unable to reconstitute bone marrow in lethally irradiated mice. Since Dkk1 is a Wnt antagonist, this implies that Wnt signaling is required for self-renewal, although interestingly, the transgenic donor mice themselves have a relatively normal hematopoietic cell population. However, analysis of these HSCs by flow cytometry suggests they are not quiescent, suggesting that Wnt signals from neighboring osteoblasts cells may keep HSCs in the quiescent phase and able to maintain their stemness [198].
Skin stem cells
Skin is the largest organ of human body. It separates an organism from outside world, is the first barrier to fight against microbes, and protects the body from chemical and physical injury. To protect itself from permanent injury, the epithelium of the skin rapidly turns over, replacing the entire barrier every four weeks.
Similar to the intestinal epithelium, the skin relies on stem cells to replenish lost cells. Some evidence suggests that the epidermal stem cells are found within the basal cell compartment. Stem cell progeny migrate upward towards the surface as they deposit cytokeratins. Terminally differentiated keratinocytes become enucleated and contain cross-linked cytokeratins. Eventually, these cells are sloughed off at the surface [199].
In addition to the replenishing keratinocytes, the skin must also repopulate the cells that constitute a hair follicle. Hair follicle stem cells reside in bulge region, located in the middle of hair follicles. Bulge cells are quiescent and retain BrdU labeling after administration. Activated bulge stem cells move out of this niche and proliferate to supply the hair regeneration at the beginning of a new hair cycle [199].
The importance of Wnt signaling in the skin homeostasis has long been observed and several Wnt genes are expressed in skin. In addition, LEF1 deficient mice lacked body hair and whiskers [200]. Conditional ablation of β-catenin in the skin blocks placode formation and hair follicle growth [201]. Blocking Wnt signaling by expressing Dkk1 in the skin results in a similar phenotype [202]. Transient or continuous over-expression of a stabilized β-catenin mutant in skin causes excess follicle formation [203–206].
Wnt10a and Wnt10b are specifically expressed in placode [207]. Using a galactosidase reporter in transgenic mice, Wnt signaling appears to be active in the cortical cells of the hair shaft, whereas the bulge region is largely inactive [208]. LEF1 may mediate Wnt activity in cortex by the fact the co-staining of nuclear β-catenin and galactosidase reporter in the precotex [209,210]. TCF3 is expressed in the bulge region and keeps the follicle stem cell in a quiescent state by inhibiting Wnt signaling [209,210]. In studying hair follicle regeneration after wounding the skin, it was found that the regeneration process was enhanced by over-expression Wnt7a whereas it was blocked by express Dkk1 in skin [211]. These data highlight the critical role of Wnt in hair follicle formation.
Neural stem cells
Adult neural stem cells are present in the subventricular zone of the lateral ventricles and in the subgranular zone of the hippocampus. Neurons from subventricular migrate towards olfactory bulb, while neurons from subgranular zone integrate into the existing circuitry [212].
In situ hybridization studies suggest that Wnt3 is expressed close to the subgranular zone [213]. Staining patterns in a transgenic mouse with Wnt signaling reporter demonstrate that Wnt signaling is active in the subgranular zone and dentate granule cell layer [213]. Over-expressing Wnt3 in purified hippocampal stem cells increases neuronal production [213], whereas blocking Wnt signaling with the dominant negative Wnt1 blocks neurogenesis at subgranular zone [213]. In addition, expressing Wnt3 in subgranular zone region by injecting lentiviruses enhances neurogenesis in the hippocampus [213]. Details of the role of Wnt signaling in the adult neural stem cell will be uncovered by further analysis, using tissue specific knock-out and transgenic animals.
Embryonic stem cells
Embryonic stem (ES) cells derive from inner cell mass (ICM) of mouse blastocysts. In contrast to tissue stem cells, embryonic stem cells are pluripotent. When injected into an embryo, an ES cell is able to give rise to all cell lineages in the adult. Pluripotency of ES cell is controlled by an intricate signaling network [214].
Individual APC mutations have marked differences in their ability to regulate the level of β-catenin in ES cells. In addition, these ES cells show different levels of Wnt signaling, as demonstrated by activity of the TOPFlash reporter. Differentiation patterns of teratomas generated by APC1638T/1638T ES cell are indistinguishable from wild type teratomas, whereas APC1638N/1638N ES cells, which have low level of APC expression, have differentiation defects in the neuroectodermal, dorsal mesodermal and endodermal lineages. ES cells from APCMin/Min, on the other hand mice could not form teratomas at all. Interestingly, ES cells with a stabilizing mutation in β-catenin are able to form teratomas with only limited differentiation capabilities [215].
Activation of Wnt signaling by a GSK3 inhibitor maintains the undifferentiated state of ES cells [216]. Small molecule IQ-1 targets PR72/130 subunit of PP2A. It increases β-catenin/CBP mediated expression at the expense of β-catenin/p300 mediated expression. Through this mechanism it helps maintain the pluripotency of embryonic stem cell [217]. Oct4 and Nanog are two important transcriptional factors control ES cell pluripotency. In ES cells, TCF3, Oct4, and Nanog co-occupy the promoters of many genes throughout the genome [218]. Knockdown TCF3 expression or treatment with Wnt-conditioned medium stimulates Oct4 and Nanog expression and facilitates the maintenance of pluripotency [218]. Thus, the function of TCF3 may be to balance self-renewal and differentiation in ES cells. These data suggest that the Wnt signaling levels are critical in regulation of ES cell differentiation and self-renewal.
Wnt Signaling in Cancers
As a central pathway in both development and homeostasis, the Wnt pathway regulates cell growth, survival and movement, and uncontrolled activation of this pathway can result in neoplasia and cancer. Mutations of β-catenin, APC, and Axin have been found in many cancers, including colon, liver, ovary, brain, prostate, uterus, and the skin cancer [219].
Colorectal cancer (CRC)
Aberrant Wnt signaling was first linked to cancer by the observation that Familial Adenomatous polyposis (FAP) patients had a mutation in the APC gene [220–222]. In addition, aberrations in Wnt signaling have been identified in 90% of sporadic CRCs [223]. The absence of functional APC protein results in chronic activation of Wnt signaling, resulting in the formation of adenomas that ultimately progress to adeno-carcinomas. Genetic studies using the APCmin/+ mouse model clearly demonstrate the role of mutant APC in initiating the formation of tumors in the intestine [224,225]. These mice are heterozygous for a C-terminal truncated form of the APC gene. Loss-of-heterozygosity of the wild-type allele leaves only mutant APC, which is deficient to participate in the cytoplasmic degradation complex. This allows β-catenin to accumulate and results in constitutively active Wnt signaling [90,226]. Moreover, conditional deletion of the APC gene in the mouse adult intestine results in a “crypt progenitor-like” phenotype with altered patterns of proliferation and differentiation [174,227], and eventually leads to the formation of tumors [228].
In sporadic colorectal tumors that retain wild-type APC, mutations are frequently found in the β-catenin gene (CTNNB1) [226,229] or Axin2 [230]. Moreover, targeted deletion of the N-terminus of β-catenin in the intestinal epithelium of mice produces thousands of adenomatous polyps within weeks [231]. Finally, in a mouse model of colitis-associated colorectal carcinoma, using 1,2-dimethylhydrazine and dextran sulfate sodium, mice develop dysplastic lesions and invasive colorectal cancer that strongly stains for β-catenin in the nuclei [232].
Although, APC and β-catenin mutations are the initiating step of colonic tumorigenesis [85], down-regulation of other tumor suppressor genes may also contribute to the development of colon cancer. For example, Krüppel like factor 4 (KLF4), interacts with β-catenin, repressing Wnt signaling and inhibiting tumor growth [233]. KLF+/−/APCMin/+ mice developed, on average, 59% more intestinal adenomas than ApcMin/+ mice [234]. It is important to further analyze the cross-talk between Wnt signaling and other signaling pathways, such as PTEN/Akt, Notch, BMP and Hedgehog in the tumorigenesis of colon cancer as well as other cancers.
Prostate cancer
Prostate cancer is the most commonly diagnosed malignancy in American males. The prostate gland is an organ dependent on androgen. Androgen, via the androgen receptor (AR), controls the initial growth of prostatic tumor. Androgen ablation therapy causes tumor regression in the early stages of prostate cancer [235,236], clearly highlighting the dependence of tumor growth on androgens. In prostate cells, the binding of androgen hormones to AR allows AR to interact with β-catenin and stimulate AR-mediated transcriptional activity [237–244]. In prostate cancer, β-catenin similarly binds AR and activates AR target gene expression [245]. On the other hand, AR can promote β-catenin nuclear translocation in prostate cells [238]. Mutations in components of the Wnt pathway have also been found in prostate cancer. In stark contrast to colorectal cancer, mutations in APC are rarely detected [246], and instead N-terminal stabilizing mutations of β-catenin are much more frequent [245,247]. Mutant β-catenin induces hyperplasia, squamous cell trans-differentiation and prostate intraepithelial neoplasia (PIN) in mice, suggesting that β-catenin can induce neoplastic transformation in the prostate [248,249]. β-catenin activity is also regulated by other molecules in prostate cancer. For example, growth factors such as IGF and HGF activate β-catenin [250,251]. The tumor suppressor PTEN, which is frequently mutated in prostate cancer, inhibits β-catenin signaling [250]. This suggests some degree of cross-talk between the Wnt and PI3-Kinase pathways in the context of prostate cancer.
Liver cancer
Wnt signaling also plays a central role in regulating liver cell proliferation during development [252–254] and in governing essential functions of the adult liver [255–257]. Moreover, aberrant reactivation of Wnt signaling due to accumulation of β-catenin is evident in many different tumors of the liver [258]. Mutations in the β-catenin and Axin genes that lead to constitutive activation of β-catenin have been found in hepatocellular carcinoma (HCC) and hepatoblastoma. In addition, frequent overexpression of the Wnt receptor Frizzled-7 is a common early event in hepatocarcinogenesis [259,260]. Genotype-phenotype correlation analysis in hepatocellular adenoma showed that mutation of β-catenin occurs in only 12% of adenomas but in 46% of these adenomas progressed to HCC [261], suggesting a role for β-catenin in the progression of pre-cancerous lesions to HCC. Furthermore, simultaneous mutation of β-catenin and H-ras leads to 100% incidence of HCC in mice [262]. These findings suggest that the aberrant Wnt signaling is important in the progression of HCC.
Skin cancer
Wnt signaling regulates hair morphogenesis. Mice expressing a truncated form of β-catenin have abnormal hair follicle morphogenesis [203]. Pilomatricoma is a common benign skin adnexal tumor showing differentiation towards the matrix cells of the hair follicle. About 75% of pilomatricomas have an activating mutation in β-catenin at the N-terminal phosphorylation site, which results in cytoplasmic accumulation and nuclear translocation of β-catenin, resulting in transcriptional activation of many target genes, such as c-Myc, cyclin D1 [263].
Isolation of CD34+/K14+ cells from early mouse epidermal tumors results in a population of cells that are more than 100-fold more potent in initiating secondary tumors than the original heterogeneous mixture of cells isolated from the tumor. These cells express many markers of bulge skin stem cells, suggesting that the CD34+/K14+ cells might be a type of cancer stem cell. Nuclear β-catenin and high expression level of Axin2, both hallmarks of Wnt signaling, are also evident in these skin tumors. Moreover, their tumor-initiating ability depends on β-catenin signaling as loss of β-catenin results in tumor regression [264].
Research from last two decades strongly emphasizes the importance of Wnt/β-catenin signaling in stem cell and cancers. Whether Wnt signaling has a general role in cancer stem cells is not yet known. However, clearly a deeper understanding of the molecular mechanisms of Wnt signaling in human cancers will lead to translational research regarding novel methods in cancer diagnosis and treatment.
Acknowledgments
PME is supported by a Multidisciplinary Training in Cancer Research pre-doctoral training grant from the Sealy Center for Cancer Cell Biology and the National Institutes of Health Grant T32CA117834; CL is supported by the grants from the National Institutes of Health
References
- 1.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 3.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 4.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 5.McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989;58:1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- 6.Seifert JR, Mlodzik M. Frizzled/PCP signaling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 7.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of β-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 8.Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 9.Kohn AD, Moon RT. Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Guder C, Philipp I, Lengfeld T, Watanabe H, Hobmayer B, Holstein TW. The Wnt code: cnidarians signal the way. Oncogene. 2006;25:7450–7460. doi: 10.1038/sj.onc.1210052. [DOI] [PubMed] [Google Scholar]
- 11.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 12.Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Ching W, Hang HC, Nusse R. Lipid-independent secretion of a Drosophila Wnt protein. J Biol Chem. 2008 Apr 22; doi: 10.1074/jbc.M802059200. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 15.Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Goodman RM, Thombre S, Firtina Z, Gray D, Betts D, Roebuck J, Spana EP, et al. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- 17.Coudreuse DY, Roel G, Betist MC, Destree O, Korswagen HC. Wnt gradient formation requires retromer function in Wnt-producing cells. Science. 2006;312:921–924. doi: 10.1126/science.1124856. [DOI] [PubMed] [Google Scholar]
- 18.Port F, Kuster M, Herr P, Furger E, Banziger C, Hausmann G, Basler K. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol. 2008;10:178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- 19.Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell. 2008;14:140–147. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 21.Yang-Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996;6:1302–1306. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- 22.Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin JP, Davenport AP, Spedding M, et al. International Union of Pharmacology. XLVI G protein-coupled receptor list. Pharmacol Rev. 2005;57:279–288. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- 23.Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ. Insights into Wnt binding and signaling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh JC, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc Natl Acad Sci USA. 1999;96:3546–3551. doi: 10.1073/pnas.96.7.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umbhauer M, Djiane A, Goisset C, Penzo-Mendez A, Riou JF, Boucaut JC, Shi DL. The C-terminal cytoplasmic Lys-Thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/β-catenin signaling. EMBO J. 2000;19:4944–4954. doi: 10.1093/emboj/19.18.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, et al. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulte G, Bryja V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:518–525. doi: 10.1016/j.tips.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Rubin JS, Kimmel AR. Rapid, Wnt-induced changes in GSK3β associations that regulate β-catenin stabilization are mediated by Gα proteins. Curr Biol. 2005;15:1989–1997. doi: 10.1016/j.cub.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 30.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signaling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 31.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 32.Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, et al. Arrow encodes an LDL-receptor-related protein essential for Wingless signaling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- 33.Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- 34.Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 35.Culi J, Mann RS. Boca, an endoplasmic reticulum protein required for wingless signaling and trafficking of LDL receptor family members in Drosophila. Cell. 2003;112:343–354. doi: 10.1016/s0092-8674(02)01279-5. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh JC, Lee L, Zhang L, Wefer S, Brown K, DeRossi C, Wines ME, et al. Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell. 2003;112:355–367. doi: 10.1016/s0092-8674(03)00045-x. [DOI] [PubMed] [Google Scholar]
- 37.Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Yoshikawa S, McKinnon RD, Kokel M, Thomas JB. Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature. 2003;422:583–588. doi: 10.1038/nature01522. [DOI] [PubMed] [Google Scholar]
- 39.Hikasa H, Shibata M, Hiratani I, Taira M. The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development. 2002;129:5227–5239. doi: 10.1242/dev.129.22.5227. [DOI] [PubMed] [Google Scholar]
- 40.Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25:7461–7468. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- 41.Schambony A, Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell. 2007;12:779–792. doi: 10.1016/j.devcel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 42.Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 43.Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate β-catenin-dependent gene expression. J Biol Chem. 2006;281:13247–13257. doi: 10.1074/jbc.M508324200. [DOI] [PubMed] [Google Scholar]
- 44.Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. R-Spondin2 is a secreted activator of Wnt/β-catenin signaling and is required for Xenopus myogenesis. Dev Cell. 2004;7:525–534. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Wei Q, Yokota C, Semenov MV, Doble B, Woodgett J, He X. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and β-catenin signaling. J Biol Chem. 2007;282:15903–15911. doi: 10.1074/jbc.M701927200. [DOI] [PubMed] [Google Scholar]
- 46.Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 47.Bell SM, Schreiner CM, Wert SE, Mucenski ML, Scott WJ, Whitsett JA. R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development. 2008;135:1049–1058. doi: 10.1242/dev.013359. [DOI] [PubMed] [Google Scholar]
- 48.Tomaselli S, Megiorni F, De Bernardo C, Felici A, Marrocco G, Maggiulli G, Grammatico B, et al. Syndromic true hermaphroditism due to an R-spondin1 (RSPO1) homozygous mutation. Hum Mutat. 2008;29:220–226. doi: 10.1002/humu.20665. [DOI] [PubMed] [Google Scholar]
- 49.Blaydon DC, Ishii Y, O’Toole EA, Unsworth HC, Teh MT, Ruschendorf F, Sinclair C, et al. The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet. 2006;38:1245–1247. doi: 10.1038/ng1883. [DOI] [PubMed] [Google Scholar]
- 50.Hoang B, Moos M, Jr, Vukicevic S, Luyten FP. Primary structure and tissue distribution of FRZB, a novel protein related to Drosophila frizzled, suggest a role in skeletal morphogenesis. J Biol Chem. 1996;271:26131–26137. doi: 10.1074/jbc.271.42.26131. [DOI] [PubMed] [Google Scholar]
- 51.Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, et al. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- 53.Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R. Wise, a context-dependent activator and inhibitor of Wnt signaling. Development. 2003;130:4295–4305. doi: 10.1242/dev.00674. [DOI] [PubMed] [Google Scholar]
- 54.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 55.Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 56.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 57.Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302:179–183. doi: 10.1016/s0378-1119(02)01106-x. [DOI] [PubMed] [Google Scholar]
- 58.Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 2000;19:1010–1022. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sokol SY, Klingensmith J, Perrimon N, Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development. 1995;121:1637–1647. doi: 10.1242/dev.121.6.1637. [DOI] [PubMed] [Google Scholar]
- 61.Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 1994;8:118–130. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- 62.Sussman DJ, Klingensmith J, Salinas P, Adams PS, Nusse R, Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Dev Biol. 1994;166:73–86. doi: 10.1006/dbio.1994.1297. [DOI] [PubMed] [Google Scholar]
- 63.Noordermeer J, Klingensmith J, Perrimon N, Nusse R. Dishevelled and armadillo act in the wingless signaling pathway in Drosophila. Nature. 1994;367:80–83. doi: 10.1038/367080a0. [DOI] [PubMed] [Google Scholar]
- 64.Yanagawa S, van Leeuwen F, Wodarz A, Klingensmith J, Nusse R. The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev. 1995;9:1087–1097. doi: 10.1101/gad.9.9.1087. [DOI] [PubMed] [Google Scholar]
- 65.Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- 66.Willert K, Brink M, Wodarz A, Varmus H, Nusse R. Casein kinase 2 associates with and phosphorylates dishevelled. EMBO J. 1997;16:3089–3096. doi: 10.1093/emboj/16.11.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun TQ, Lu B, Feng JJ, Reinhard C, Jan YN, Fantl WJ, Williams LT. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signaling. Nat Cell Biol. 2001;3:628–636. doi: 10.1038/35083016. [DOI] [PubMed] [Google Scholar]
- 68.Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 69.Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, et al. Casein kinase 1γ couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- 71.Baig-Lewis S, Peterson-Nedry W, Wehrli M. Wingless/Wnt signal transduction requires distinct initiation and amplification steps that both depend on Arrow/LRP. Dev Biol. 2007;306:94–111. doi: 10.1016/j.ydbio.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Macdonald BT, Yokota C, Tamai K, Zeng X, He X. Wnt signal amplification: activity, cooperativity and regulation of multiple intracellular PPPSP motifs in the Wnt coreceptor LRP6. J Biol Chem. 2008;283:16115–16123. doi: 10.1074/jbc.M800327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 75.Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- 77.Peifer M, Wieschaus E. The segment polarity gene armadillo encodes a functionally modular protein that is the Drosophila homolog of human plakoglobin. Cell. 1990;63:1167–1176. doi: 10.1016/0092-8674(90)90413-9. [DOI] [PubMed] [Google Scholar]
- 78.Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siegfried E, Wilder EL, Perrimon N. Components of wingless signaling in Drosophila. Nature. 1994;367:76–80. doi: 10.1038/367076a0. [DOI] [PubMed] [Google Scholar]
- 80.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, Lee JJ, et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 81.Forde JE, Dale TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci. 2007;64:1930–1944. doi: 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Siegfried E, Chou TB, Perrimon N. wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell. 1992;71:1167–1179. doi: 10.1016/s0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- 83.He X, Saint-Jeannet JP, Woodgett JR, Varmus HE, Dawid IB. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature. 1995;374:617–622. doi: 10.1038/374617a0. [DOI] [PubMed] [Google Scholar]
- 84.Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim Biophys Acta. 1997;1332:F127–F147. doi: 10.1016/s0304-419x(97)00008-5. [DOI] [PubMed] [Google Scholar]
- 85.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 86.Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- 87.Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, et al. Association of the APC gene product with β-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 88.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 90.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, et al. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 91.Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 92.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, et al. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 94.Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of β-catenin. J Biol Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 95.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, et al. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 96.Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, et al. Axin-mediated CKI phosphorylation of β-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 98.Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, et al. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 99.Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signaling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- 100.Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X. β-Trcp couples β-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci USA. 1999;96:6273–6278. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spencer E, Jiang J, Chen ZJ. Signal-induced ubiquitination of IκBα by the F-box protein Slimb/β-TrCP. Genes Dev. 1999;13:284–294. doi: 10.1101/gad.13.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBαand β-catenin and stimulates IκBαubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eklof Spink K, Fridman SG, Weis WI. Molecular mechanisms of β-catenin recognition by adenomatous polyposis coli revealed by the structure of an APC-β-catenin complex. EMBO J. 2001;20:6203–6212. doi: 10.1093/emboj/20.22.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dajani R, Fraser E, Roe SM, Yeo M, Good VM, Thompson V, Dale TC, et al. Structural basis for recruitment of glycogen synthase kinase 3beta to the axin-APC scaffold complex. EMBO J. 2003;22:494–501. doi: 10.1093/emboj/cdg068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ha NC, Tonozuka T, Stamos JL, Choi HJ, Weis WI. Mechanism of phosphorylation-dependent binding of APC to β-catenin and its role in β-catenin degradation. Mol Cell. 2004;15:511–521. doi: 10.1016/j.molcel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 106.Spink KE, Polakis P, Weis WI. Structural basis of the Axin-adenomatous polyposis coli interaction. EMBO J. 2000;19:2270–2279. doi: 10.1093/emboj/19.10.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xing Y, Clements WK, Kimelman D, Xu W. Crystal structure of a β-catenin/axin complex suggests a mechanism for the β-catenin destruction complex. Genes Dev. 2003;17:2753–2764. doi: 10.1101/gad.1142603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xing Y, Clements WK, Le Trong I, Hinds TR, Stenkamp R, Kimelman D, Xu W. Crystal structure of a β-catenin/APC complex reveals a critical role for APC phosphorylation in APC function. Mol Cell. 2004;15:523–533. doi: 10.1016/j.molcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 109.Xing Y, Takemaru K, Liu J, Berndt JD, Zheng JJ, Moon RT, Xu W. Crystal structure of a full-length β-catenin. Structure. 2008;16:478–487. doi: 10.1016/j.str.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakamura Y, Umehara T, Hamana H, Hayashizaki Y, Inoue M, Kigawa T, Shirouzu M, et al. Crystal structure analysis of the PHD domain of the transcription co-activator Pygopus. J Mol Biol. 2007;370:80–92. doi: 10.1016/j.jmb.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 111.Sampietro J, Dahlberg CL, Cho US, Hinds TR, Kimelman D, Xu W. Crystal structure of a β-catenin/BCL9/Tcf4 complex. Mol Cell. 2006;24:293–300. doi: 10.1016/j.molcel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 112.Wu G, Xu G, Schulman BA, Jeffrey PD, Harper JW, Pavletich NP. Structure of a β-TrCP1-Skp1-β-catenin complex: destruction motif binding and lysine specificity of the SCF (β-TrCP1) ubiquitin ligase. Mol Cell. 2003;11:1445–1456. doi: 10.1016/s1097-2765(03)00234-x. [DOI] [PubMed] [Google Scholar]
- 113.Huber AH, Nelson WJ, Weis WI. Three-dimensional structure of the armadillo repeat region of β-catenin. Cell. 1997;90:871–882. doi: 10.1016/s0092-8674(00)80352-9. [DOI] [PubMed] [Google Scholar]
- 114.Liu J, Xing Y, Hinds TR, Zheng J, Xu W. The third 20 amino acid repeat is the tightest binding site of APC for β-catenin. J Mol Biol. 2006;360:133–144. doi: 10.1016/j.jmb.2006.04.064. [DOI] [PubMed] [Google Scholar]
- 115.Yang J, Zhang W, Evans PM, Chen X, He X, Liu C. Adenomatous polyposis coli (APC) differentially regulates β-catenin phosphorylation and ubiquitination in colon cancer cells. J Biol Chem. 2006;281:17751–17757. doi: 10.1074/jbc.M600831200. [DOI] [PubMed] [Google Scholar]
- 116.Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases β-catenin from the axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3β regulates its stability. J Biol Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 119.Hocevar BA, Mou F, Rennolds JL, Morris SM, Cooper JA, Howe PH. Regulation of the Wnt signaling pathway by disabled-2 (Dab2) EMBO J. 2003;22:3084–3094. doi: 10.1093/emboj/cdg286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hsu W, Zeng L, Costantini F. Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem. 1999;274:3439–3445. doi: 10.1074/jbc.274.6.3439. [DOI] [PubMed] [Google Scholar]
- 121.Yang J, Wu J, Tan C, Klein PS. PP2A:B56epsilon is required for Wnt/β-catenin signaling during embryonic development. Development. 2003;130:5569–5578. doi: 10.1242/dev.00762. [DOI] [PubMed] [Google Scholar]
- 122.Seeling JM, Miller JR, Gil R, Moon RT, White R, Virshup DM. Regulation of β-catenin signaling by the B56 subunit of protein phosphatase 2A. Science. 1999;283:2089–2091. doi: 10.1126/science.283.5410.2089. [DOI] [PubMed] [Google Scholar]
- 123.Gao ZH, Seeling JM, Hill V, Yochum A, Virshup DM. Casein kinase I phosphorylates and destabilizes the β-catenin degradation complex. Proc Natl Acad Sci USA. 2002;99:1182–1187. doi: 10.1073/pnas.032468199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bos CL, Diks SH, Hardwick JC, Walburg KV, Peppelenbosch MP, Richel DJ. Protein phosphatase 2A is required for mesalazine-dependent inhibition of Wnt/β-catenin pathway activity. Carcinogenesis. 2006;27:2371–2382. doi: 10.1093/carcin/bgl071. [DOI] [PubMed] [Google Scholar]
- 125.Ratcliffe MJ, Itoh K, Sokol SY. A positive role for the PP2A catalytic subunit in Wnt signal transduction. J Biol Chem. 2000;275:35680–35683. doi: 10.1074/jbc.C000639200. [DOI] [PubMed] [Google Scholar]
- 126.Bos CL, Kodach LL, van den Brink GR, Diks SH, van Santen MM, Richel DJ, Peppelenbosch MP, et al. Effect of aspirin on the Wnt/β-catenin pathway is mediated via protein phosphatase 2A. Oncogene. 2006;25:6447–6456. doi: 10.1038/sj.onc.1209658. [DOI] [PubMed] [Google Scholar]
- 127.Li X, Yost HJ, Virshup DM, Seeling JM. Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus. EMBO J. 2001;20:4122–4131. doi: 10.1093/emboj/20.15.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bajpai R, Makhijani K, Rao PR, Shashidhara LS. Drosophila Twins regulates Armadillo levels in response to Wg/Wnt signal. Development. 2004;131:1007–1016. doi: 10.1242/dev.00980. [DOI] [PubMed] [Google Scholar]
- 129.Luo W, Peterson A, Garcia BA, Coombs G, Kofahl B, Heinrich R, Shabanowitz J, et al. Protein phosphatase 1 regulates assembly and function of the β-catenin degradation complex. EMBO J. 2007;26:1511–1521. doi: 10.1038/sj.emboj.7601607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, et al. Wilms tumor suppressor WTX negatively regulates WNT/β-catenin signaling. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- 131.Rivera MN, Kim WJ, Wells J, Driscoll DR, Brannigan BW, Han M, Kim JC, et al. An X chromosome gene, WTX, is commonly inactivated in Wilms tumor. Science. 2007;315:642–645. doi: 10.1126/science.1137509. [DOI] [PubMed] [Google Scholar]
- 132.Fagotto F, Gluck U, Gumbiner BM. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of β-catenin. Curr Biol. 1998;8:181–190. doi: 10.1016/s0960-9822(98)70082-x. [DOI] [PubMed] [Google Scholar]
- 133.Yokoya F, Imamoto N, Tachibana T, Yoneda Y. β-catenin can be transported into the nucleus in a Ran-unassisted manner. Mol Biol Cell. 1999;10:1119–1131. doi: 10.1091/mbc.10.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long X. Rac1 activation controls nuclear localization of β-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cong F, Varmus H. Nuclear-cytoplasmic shuttling of Axin regulates subcellular localization of β-catenin. Proc Natl Acad Sci USA. 2004;101:2882–2887. doi: 10.1073/pnas.0307344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rosin-Arbesfeld R, Townsley F, Bienz M. The APC tumor suppressor has a nuclear export function. Nature. 2000;406:1009–1012. doi: 10.1038/35023016. [DOI] [PubMed] [Google Scholar]
- 137.Henderson BR. Nuclear-cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover. Nat Cell Biol. 2000;2:653–660. doi: 10.1038/35023605. [DOI] [PubMed] [Google Scholar]
- 138.Neufeld KL, Zhang F, Cullen BR, White RL. APC-mediated downregulation of β-catenin activity involves nuclear sequestration and nuclear export. EMBO Rep. 2000;1:519–523. doi: 10.1093/embo-reports/kvd117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Krieghoff E, Behrens J, Mayr B. Nucleo-cytoplasmic distribution of β-catenin is regulated by retention. J Cell Sci. 2006;119:1453–1463. doi: 10.1242/jcs.02864. [DOI] [PubMed] [Google Scholar]
- 140.Townsley FM, Cliffe A, Bienz M. Pygopus and Legless target Armadillo/β-catenin to the nucleus to enable its transcriptional co-activator function. Nat Cell Biol. 2004;6:626–633. doi: 10.1038/ncb1141. [DOI] [PubMed] [Google Scholar]
- 141.Tolwinski NS, Wieschaus E. Armadillo nuclear import is regulated by cytoplasmic anchor Axin and nuclear anchor dTCF/Pan. Development. 2001;128:2107–2117. doi: 10.1242/dev.128.11.2107. [DOI] [PubMed] [Google Scholar]
- 142.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 143.Brunner E, Peter O, Schweizer L, Basler K. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- 144.Brannon M, Brown JD, Bates R, Kimelman D, Moon RT. XCtBP is a XTcf-3 co-repressor with roles throughout Xenopus development. Development. 1999;126:3159–3170. doi: 10.1242/dev.126.14.3159. [DOI] [PubMed] [Google Scholar]
- 145.Billin AN, Thirlwell H, Ayer DE. β-catenin-histone deacetylase interactions regulate the transition of LEF1 from a transcriptional repressor to an activator. Mol Cell Biol. 2000;20:6882–6890. doi: 10.1128/mcb.20.18.6882-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, et al. Identification of a Wnt/Dvl/β-Catenin→Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–685. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- 147.Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, et al. Drosophila Tcf and Groucho interact to repress Wingless signaling activity. Nature. 1998;395:604–608. doi: 10.1038/26982. [DOI] [PubMed] [Google Scholar]
- 148.Roose J, Molenaar M, Peterson J, Hurenkamp J, Brantjes H, Moerer P, van de Wetering M, et al. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature. 1998;395:608–612. doi: 10.1038/26989. [DOI] [PubMed] [Google Scholar]
- 149.Levanon D, Goldstein RE, Bernstein Y, Tang H, Goldenberg D, Stifani S, Paroush Z, et al. Transcriptional repression by AML1 and LEF-1 is mediated by the TLE/Groucho corepressors. Proc Natl Acad Sci USA. 1998;95:11590–11595. doi: 10.1073/pnas.95.20.11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Palaparti A, Baratz A, Stifani S. The Groucho/transducin-like enhancer of split transcriptional repressors interact with the genetically defined amino-terminal silencing domain of histone H3. J Biol Chem. 1997;272:26604–26610. doi: 10.1074/jbc.272.42.26604. [DOI] [PubMed] [Google Scholar]
- 151.Daniels DL, Weis WI. β-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol. 2005;12:364–371. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- 152.Hsu SC, Galceran J, Grosschedl R. Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with β-catenin. Mol Cell Biol. 1998;18:4807–4818. doi: 10.1128/mcb.18.8.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Belenkaya TY, Han C, Standley HJ, Lin X, Houston DW, Heasman J, Lin X. Pygopus encodes a nuclear protein essential for wingless/Wnt signaling. Development. 2002;129:4089–4101. doi: 10.1242/dev.129.17.4089. [DOI] [PubMed] [Google Scholar]
- 154.Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, et al. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear β-catenin-TCF complex. Cell. 2002;109:47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- 155.Parker DS, Jemison J, Cadigan KM. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development. 2002;129:2565–2576. doi: 10.1242/dev.129.11.2565. [DOI] [PubMed] [Google Scholar]
- 156.Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signaling pathway. Nat Cell Biol. 2002;4:367–373. doi: 10.1038/ncb786. [DOI] [PubMed] [Google Scholar]
- 157.Soliman MA, Riabowol K. After a decade of study-ING, a PHD for a versatile family of proteins. Trends Biochem Sci. 2007;32:509–519. doi: 10.1016/j.tibs.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 158.Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of β-catenin in vertebrates. EMBO J. 2000;19:1839–1850. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cox RT, Pai LM, Kirkpatrick C, Stein J, Peifer M. Roles of the C terminus of Armadillo in Wingless signaling in Drosophila. Genetics. 1999;153:319–332. doi: 10.1093/genetics/153.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with β-catenin to activate gene expression. J Cell Biol. 2000;149:249–254. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 162.Goldman PS, Tran VK, Goodman RH. The multifunctional role of the co-activator CBP in transcriptional regulation. Recent Prog Horm Res. 1997;52:103–119. [PubMed] [Google Scholar]
- 163.Mosimann C, Hausmann G, Basler K. Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with β-catenin/Armadillo. Cell. 2006;125:327–341. doi: 10.1016/j.cell.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 164.Wei Y, Renard CA, Labalette C, Wu Y, Levy L, Neuveut C, Prieur X, et al. Identification of the LIM protein FHL2 as a coactivator of β-catenin. J Biol Chem. 2003;278:5188–5194. doi: 10.1074/jbc.M207216200. [DOI] [PubMed] [Google Scholar]
- 165.Hecht A, Litterst CM, Huber O, Kemler R. Functional characterization of multiple transactivating elements in β-catenin, some of which interact with the TATA-binding protein in vitro. J Biol Chem. 1999;274:18017–18025. doi: 10.1074/jbc.274.25.18017. [DOI] [PubMed] [Google Scholar]
- 166.Barker N, Hurlstone A, Musisi H, Miles A, Bienz M, Clevers H. The chromatin remodelling factor Brg-1 interacts with β-catenin to promote target gene activation. EMBO J. 2001;20:4935–4943. doi: 10.1093/emboj/20.17.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bauer A, Huber O, Kemler R. Pontin52, an interaction partner of β-catenin, binds to the TATA box binding protein. Proc Natl Acad Sci USA. 1998;95:14787–14792. doi: 10.1073/pnas.95.25.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bauer A, Chauvet S, Huber O, Usseglio F, Rothbacher U, Aragnol D, Kemler R, et al. Pontin52 and reptin52 function as antagonistic regulators of β-catenin signaling activity. EMBO J. 2000;19:6121–6130. doi: 10.1093/emboj/19.22.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 170.Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. Bioessays. 2002;24:91–98. doi: 10.1002/bies.10028. [DOI] [PubMed] [Google Scholar]
- 171.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 172.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, et al. Loss of APC in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, et al. β-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 176.Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 177.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, et al. Identification of c-Myc as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 178.Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, Vass JK, et al. Myc deletion rescues APC deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 179.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. III Evidence from columnar, enteroendocrine, and mucous cells in the adult mouse. Am J Anat. 1981;160:77–91. doi: 10.1002/aja.1001600107. [DOI] [PubMed] [Google Scholar]
- 180.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, et al. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 181.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 182.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumor growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 183.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 184.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 186.van de Wetering M, Oosterwegel M, Dooijes D, Clevers H. Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. EMBO J. 1991;10:123–132. doi: 10.1002/j.1460-2075.1991.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Travis A, Amsterdam A, Belanger C, Grosschedl R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor alpha enhancer function. Genes Dev. 1991;5:880–894. doi: 10.1101/gad.5.5.880. [DOI] [PubMed] [Google Scholar]
- 188.Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R. Redundant regulation of T cell differentiation and TCRα gene expression by the transcription factors LEF-1 and TCF-1. Immunity. 1998;8:11–20. doi: 10.1016/s1074-7613(00)80454-9. [DOI] [PubMed] [Google Scholar]
- 189.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, et al. A role for Wnt signaling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 190.Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, Radtke F. β-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of β- and γ-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 192.Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, Kuttler F, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of β-catenin and γ-catenin. Blood. 2008;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- 193.Trowbridge JJ, Xenocostas A, Moon RT, Bhatia M. Glycogen synthase kinase-3 is an in vivo regulator of hematopoietic stem cell repopulation. Nat Med. 2006;12:89–98. doi: 10.1038/nm1339. [DOI] [PubMed] [Google Scholar]
- 194.Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 195.Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive β-catenin activation. Nat Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 196.Murdoch B, Chadwick K, Martin M, Shojaei F, Shah KV, Gallacher L, Moon RT, et al. Wnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivo. Proc Natl Acad Sci USA. 2003;100:3422–3427. doi: 10.1073/pnas.0130233100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci USA. 2007;104:15436–15441. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fuchs E. cratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 201.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 202.Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- 203.Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 204.Lo Celso C, Prowse DM, Watt FM. Transient activation of β-catenin signaling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumors. Development. 2004;131:1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- 205.Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of β-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.van Mater D, Kolligs FT, Dlugosz AA, Fearon ER. Transient activation of β-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003;17:1219–1224. doi: 10.1101/gad.1076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 208.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 209.Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–183. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 211.Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 212.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 213.Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, et al. Wnt signaling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 214.Niwa H. How is pluripotency determined and maintained? Development. 2007;134:635–646. doi: 10.1242/dev.02787. [DOI] [PubMed] [Google Scholar]
- 215.Kielman MF, Rindapaa M, Gaspar C, van Poppel N, Breukel C, van Leeuwen S, Taketo MM, et al. APC modulates embryonic stem-cell differentiation by controlling the dosage of β-catenin signaling. Nat Genet. 2002;32:594–605. doi: 10.1038/ng1045. [DOI] [PubMed] [Google Scholar]
- 216.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 217.Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. Wnt/β-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci USA. 2007;104:5668–5673. doi: 10.1073/pnas.0701331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22:746–755. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 220.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 221.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 222.Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 223.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 224.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 225.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 226.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 227.Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M, Laurent-Puig P, et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following APC loss in the mouse intestine. Development. 2005;132:1443–1451. doi: 10.1242/dev.01700. [DOI] [PubMed] [Google Scholar]
- 228.Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S, Matsumoto H, et al. Rapid colorectal adenoma formation initiated by conditional targeting of the APC gene. Science. 1997;278:120–123. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- 229.Munemitsu S, Albert I, Rubinfeld B, Polakis P. Deletion of an amino-terminal sequence β-catenin in vivo and promotes hyperphosporylation of the adenomatous polyposis coli tumor suppressor protein. Mol Cell Biol. 1996;16:4088–4094. doi: 10.1128/mcb.16.8.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Liu W, Dong X, Mai M, Seelan RS, Taniguchi K, Krishnadath KK, Halling KC, et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating β-catenin/TCF signaling. Nat Genet. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- 231.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang JG, Wang DF, Lv BJ, Si JM. A novel mouse model for colitis-associated colon carcinogenesis induced by 1,2-dimethylhydrazine and dextran sulfate sodium. World J Gastroenterol. 2004;10:2958–2962. doi: 10.3748/wjg.v10.i20.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang W, Chen X, Kato Y, Evans PM, Yuan S, Yang J, Rychahou PG, et al. Novel cross talk of Kruppel-like factor 4 and β-catenin regulates normal intestinal homeostasis and tumor repression. Mol Cell Biol. 2006;26:2055–2064. doi: 10.1128/MCB.26.6.2055-2064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ghaleb AM, McConnell BB, Nandan MO, Katz JP, Kaestner KH, Yang VW. Haploinsufficiency of Kruppel-like factor 4 promotes adenomatous polyposis coli ependent intestinal tumorigenesis. Cancer Res. 2007;67:7147–7154. doi: 10.1158/0008-5472.CAN-07-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Isaacs W, De Marzo A, Nelson WG. Focus on prostate cancer. Cancer cell. 2002;2:113–116. doi: 10.1016/s1535-6108(02)00103-4. [DOI] [PubMed] [Google Scholar]
- 236.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nature Rev. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 237.Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, Sun Z. Linking β-catenin to androgen-signaling pathway. J Biol Chem. 2002;277:11336–11344. doi: 10.1074/jbc.M111962200. [DOI] [PubMed] [Google Scholar]
- 238.Mulholland DJ, Cheng H, Reid K, Rennie PS, Nelson CC. The androgen receptor can promote β-catenin nuclear translocation independently of adenomatous polyposis coli. J Biol Chem. 2002;277:17933–17943. doi: 10.1074/jbc.M200135200. [DOI] [PubMed] [Google Scholar]
- 239.Chesire DR, Ewing CM, Gage WR, Isaacs WB. In vitro evidence for complex modes of nuclear β-catenin signaling during prostate growth and tumorigenesis. Oncogene. 2002;21:2679–2694. doi: 10.1038/sj.onc.1205352. [DOI] [PubMed] [Google Scholar]
- 240.Sharma M, Chuang WW, Sun Z. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3β inhibition and nuclear β-catenin accumulation. J Biol Chem. 2002;277:30935–30941. doi: 10.1074/jbc.M201919200. [DOI] [PubMed] [Google Scholar]
- 241.Mulholland DJ, Read JT, Rennie PS, Cox ME, Nelson CC. Functional localization and competition between the androgen receptor and T-cell factor for nuclear β-catenin: a means for inhibition of the Tcf signaling axis. Oncogene. 2003;22:5602–5613. doi: 10.1038/sj.onc.1206802. [DOI] [PubMed] [Google Scholar]
- 242.Song LN, Herrell R, Byers S, Shah S, Wilson EM, Gelmann EP. β-catenin binds to the activation function 2 region of the androgen receptor and modulates the effects of the N-terminal domain and TIF2 on ligand-dependent transcription. Mol Cell Biol. 2003;23:1674–1687. doi: 10.1128/MCB.23.5.1674-1687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Masiello D, Chen SY, Xu Y, Verhoeven MC, Choi E, Hollenberg AN, Balk SP. Recruitment of β-catenin by wild-type or mutant androgen receptors correlates with ligand-stimulated growth of prostate cancer cells. Mol Endocrinol. 2004;18:2388–2401. doi: 10.1210/me.2003-0436. [DOI] [PubMed] [Google Scholar]
- 244.Verras M, Brown J, Li X, Nusse R, Sun Z. Wnt3a growth factor induces androgen receptor-mediated transcription and enhances cell growth in human prostate cancer cells. Cancer Res. 2004;64:8860–8866. doi: 10.1158/0008-5472.CAN-04-2370. [DOI] [PubMed] [Google Scholar]
- 245.Gerstein AV, Almeida TA, Zhao G, Chess E, Shih IeM, Buhler K, Pienta K, et al. APC/CTNNB1 (β-catenin) pathway alterations in human prostate cancers. Genes Chromosomes Cancer. 2002;34:9–16. doi: 10.1002/gcc.10037. [DOI] [PubMed] [Google Scholar]
- 246.Watanabe M, Kakiuchi H, Kato H, Shiraishi T, Yatani R, Sugimura T, Nagao M. APC gene mutations in human prostate cancer. J Clin Oncol. 1996;26:77–81. doi: 10.1093/oxfordjournals.jjco.a023188. [DOI] [PubMed] [Google Scholar]
- 247.Voeller HJ, Truica CI, Gelmann EP. β-catenin mutations in human prostate cancer. Cancer Res. 1998;58:2520–2523. [PubMed] [Google Scholar]
- 248.Gounari F, Signoretti S, Bronson R, Klein L, Sellers WR, Kum J, Siermann A, et al. Stabilization of β-catenin induces lesions reminiscent of prostatic intraepithelial neoplasia, but terminal squamous transdifferentiation of other secretory epithelia. Oncogene. 2002;21:4099–4107. doi: 10.1038/sj.onc.1205562. [DOI] [PubMed] [Google Scholar]
- 249.Bierie B, Nozawa M, Renou JP, Shillingford JM, Morgan F, Oka T, Taketo MM, et al. Activation of β-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene. 2003;22:3875–3887. doi: 10.1038/sj.onc.1206426. [DOI] [PubMed] [Google Scholar]
- 250.Persad S, Troussard AA, McPhee TR, Mulholland DJ, Dedhar S. Tumor suppressor PTEN inhibits nuclear accumulation of β-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation. J Cell Biol. 2001;153:1161–1174. doi: 10.1083/jcb.153.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Verras M, Sun Z. β-catenin is involved in insulin-like growth factor 1-mediated transactivation of the androgen receptor. Mol Endocrinol. 2005;19:391–398. doi: 10.1210/me.2004-0208. [DOI] [PubMed] [Google Scholar]
- 252.Suksaweang S, Lin CM, Jiang TX, Hughes MW, Widelitz RB, Chuong CM. Morphogenesis of chicken liver: identification of localized growth zones and the role of β-catenin/Wnt in size regulation. Dev Biol. 2004;266:109–122. doi: 10.1016/j.ydbio.2003.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Monga SP, Monga HK, Tan X, Mule K, Pediaditakis P, Michalopoulos GK. β-catenin antisense studies in embryonic liver cultures: role in proliferation, apoptosis, and lineage specification. Gastroenterology. 2003;124:202–216. doi: 10.1053/gast.2003.50000. [DOI] [PubMed] [Google Scholar]
- 254.Micsenyi A, Tan X, Sneddon T, Luo JH, Michalopoulos GK, Monga SP. β-catenin is temporally regulated during normal liver development. Gastroenterology. 2004;126:1134–1146. doi: 10.1053/j.gastro.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 255.Cadoret A, Ovejero C, Terris B, Souil E, Levy L, Lamers WH, Kitajewski J, et al. New targets of β-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene. 2002;21:8293–8301. doi: 10.1038/sj.onc.1206118. [DOI] [PubMed] [Google Scholar]
- 256.Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of β-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology. 2006;43:817–825. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- 257.Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, Vasseur-Cognet M, et al. APC tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell. 2006;10:759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 258.Colnot S, Decaens T, Niwa-Kawakita M, Godard C, Hamard G, Kahn A, Giovannini M, et al. Liver-targeted disruption of APC in mice activates β-catenin signaling and leads to hepatocellular carcinomas. Proc Natl Acad Sci USA. 2004;101:17216–17221. doi: 10.1073/pnas.0404761101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Buendia MA. Genetics of hepatocellular carcinoma. Semin Cancer Biol. 2000;10:185–200. doi: 10.1006/scbi.2000.0319. [DOI] [PubMed] [Google Scholar]
- 260.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 261.Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 262.Harada N, Oshima H, Katoh M, Tamai Y, Oshima M, Taketo MM. Hepatocarcinogenesis in mice with β-catenin and Ha-ras gene mutations. Cancer Res. 2004;64:48–54. doi: 10.1158/0008-5472.can-03-2123. [DOI] [PubMed] [Google Scholar]
- 263.Chan E, Gat U, McNiff JM, Fuchs E. A common human skin tumor is caused by activating mutations in β-catenin. Nature Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- 264.Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, et al. Cutaneous cancer stem cell maintenance is dependent on β-catenin signaling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]