Abstract
Post-traumatic stress disorder (PTSD) is a stress-related mental disorder caused by traumatic experience. Single-prolonged stress (SPS) is one of the animal models proposed for PTSD. Rats exposed to SPS showed enhanced inhibition of the hypothalamo-pituitary-adrenal (HPA) axis, which has been reliably reproduced in patients with PTSD. Mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) in the hippocampus regulate HPA axis by glucocorticoid negative feedback. Abnormalities in negative feedback are found in PTSD, suggesting that GR and MR might be involved in the pathophysiology of these disorders.
In the present study, we performed immunohistochemistry and western blotting to examine the changes in hippocampal MR- and GR-expression after SPS. Immunohistochemistry revealed decreased MR- and GR-immunoreactivity (ir) in the CA1 of hippocampus in SPS animals. Change in GR sub-distribution was also observed, where GR-ir was shifted from nucleus to cytoplasm in SPS rats. Western blotting showed that SPS induced significantly decreased MR- and GR-protein in the whole hippocampus, although the degree of decreased expression of both receptors was different. Meanwhile, we also found the MR/GR ratio decreased in SPS rats. In general, SPS induced down-regulation of MR- and GR-expression. These findings suggest that MR and GR play critical roles in affecting hippocampal function. Changes in MR/GR ratio may be relevant for behavioral syndrome in PTSD.
Keywords: post-traumatic stress disorder, mineralocorticoid receptor, glucocorticoid receptor, hippocampus
I. Introduction
The hippocampus has two types of adrenal steroid receptors, Type I (mineralocorticoid receptor, MR) and Type II (glucocorticoid receptor, GR). They play important roles in the HPA axis through their effect on glucocorticoid negative feedback [7]. Both receptors differ in their affinity for glucocorticoids, with MR demonstrating a higher affinity for cortisol and GR demonstrating a lower one. In addition, their distribution in rodent brain differs, with MR found predominantly in limbic areas, particularly the hippocampus, and GR more widely distributed across all brain regions [7]. Both receptors may exert different effects on abnormal negative feedback in post-traumatic stress disorder (PTSD).
PTSD is an anxiety disorder for certain severe psychological consequences of exposure to, or confrontation with, stressful events that a person experiences as highly traumatic. It has been reported that stress can lead to changes in the neuroendocrine system, thereby affecting the immune system and metabolic system [17]. In the neuroendocrine system, the hypothalamic-pituitary-adrenal axis (HPA-axis) is the most important, and glucocorticoid is the next most important factor. Decreased negative feedback is found in major depression and enhanced negative feedback has been found in PTSD [31]. PTSD patients exhibit hippocampal volume loss [8, 28], lower corticosteroid levels [30] and decreased responsiveness to stress [6]. These data suggest that PTSD is characterized by an abnormal HPA-axis. Chronic stress and prolonged glucocorticoid exposure may damage the hippocampus, thus raising the possibility that PTSD may induce hippocampal degeneration.
The importance of the activation of MR and GR-mediated effect by glucocorticoid are observed in psychiatric disorders. Activation of these receptors can modulate the system associated with memory, behavioral responsibility, anxiety and fear [1]. Hippocampal MR is important in terms of controlling the inhibitory tone along the HPA axis. This effect of corticosterone via MR is modulated by GR that becomes progressively occupied after stress. Thus, animals with increased MR capacity have decreased neuroendocrine responsibility to stress [19]. Antidepressants increase expression of hippocampal MR and decrease basal and stress-induced HPA activity. Blockade of MR and GR produced anxiety and startle [13] and MR antagonism induced enhanced fear [14]. These studies examined whether changes in hippocampal MR and GR could mediate both behavioral/emotional changes in psychiatric disorders. On the other hand, changes of hippocampal neurons were observed with both chronic absence and overexposure to corticosteroids, indicating that corticosterone-receptors are of crucial importance for hippocampal damage. Degeneration and associated loss of synaptic function could be prevented by treatment with MR ligands [29]. MR and GR in maternal separation condition induce glucocorticoid resistance and apoptosis in the hippocampus [32]. Effects of MR and GR on hippocampal neurogenesis suggest both receptors are key factors affecting the changes in hippocampal structure and function in PTSD.
In the present study, we demonstrated that a specific stress paradigm, SPS (single prolonged stress), induces hypersensitive glucocorticoid fast feedback, and proposed that it is a specific animal model of PTSD. To examine the link between PTSD and corticosteroid receptors in the hippocampus, we studied expression of MR and GR after SPS stress using immunohistochemistry and western blotting.
II. Materials and Methods
Animals
Male Wistar rats (150 g–200 g) were used for all experiments. Rats were housed under temperature-controlled (22±1°C) conditions, and maintained at 12:12 light/dark cycle (lights on at 07:00 and off at 19:00) with free access to food and water. All procedures were approved by the Institutional Animal Care. Experimental procedures were undertaken following an adaptation period of 5–6 days.
Experimental procedure
Fourteen animals were divided into two groups: 1) control group; 2) SPS group. Control animals remained in their home cages with no handling for 7 days and were killed at the same time as SPS groups. SPS-rats had SPS procedure on the first day. The single session of prolonged stress consisted of: restraint for 2 hr, followed by forced swim for 20 min (24°C), followed by ether anesthesia [15, 25]. They were then allowed to remain in their home cages without interference and were killed 7 days later.
Immunohistochemistry
Rats of control group and SPS group were anaesthetized with 50 mg/kg body weight sodium pentobarbital, and the brains were removed from the skull after perfusion fixation with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer saline (PBS). The brains were then quickly frozen using powdered dry ice and cut into 12 µm thick frontal sections on a cryostat. After being dried, the sections were stored at −20°C before immunohistochemistry.
The sections were treated with 5% bovine serum albumin (BSA), 0.3% Triton X-100 in PBS for 30 min at room temperature (RT) to block non-specific staining. The sections were then incubated with rabbit polyclonal antibody against MR (Santa Cruz, CA, USA; 1:300) or rabbit polyclonal antibody against GR (Santa Cruz; 1:1,000) in 2%BSA-PBS for 24 hr at 4°C. After being washed with PBS, the sections were incubated with biotinylated-goat anti anti-rabbit IgG (Boster, China; 1:100) for 2 hr and then with streptoavidin-biotin peroxidase complex (SABC) for 1 hr. The sections were washed four times with PBS after each of incubation and subsequently incubated with 3,3'-diaminobenzidine (DAB) and H2O2. To assess nonspecific staining, a few sections in every experiment were incubated in buffer without primary antibody.
Western blotting
Freshly frozen hippocampus of control rats and SPS rats were respectively homogenized with sample buffer containing 200 mM Tris-buffered saline, pH 7.5 (TBS), 4% sodium dodecyl sulfate (SDS), 20% glycerol, 10% 2-mercaptoethanol and were denatured by boiling for 3 min. Sample (50 µg/lane) were loaded on a 7.5% SDS-polyacrylamide gel (PAGE), and electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Millipore Corp., Bedford, MA, USA) from the gel by a semi-dry blotting apparatus (Bio-Rad Laboratories, Inc, Hercules, CA, USA). The blotted membrane was then blocked with 1.5% skim milk, 0.05% Tween-20 in TBS (TBST) at 4°C overnight and then incubated with rabbit polyclonal antibody against MR (Santa Cruz, USA; 1:300) or rabbit polyclonal antibody against GR (Santa Cruz, USA; 1:500) at 4°C for 24 hr. Blots were washed three times with TBST, and then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG second antibody (Bio-Rad Laboratories, Inc, USA; 1:2,000) for 2 hr at room temperature. After the incubation, blots were washed three times with TBST before visualization by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Buckinghamshire, UK). To confirm equal protein loading the same blots were re-incubated with antibodies specific for β-actin (Abcam, British; 1:1,000). Immunoreaction for β-actin was detected with the ECL.
Data Analysis
For evaluation of the immunohistochemical signals, sections of the same level of the hippocampus were chosen according to the autoradiograms, the brain atlas of Paxinos and Watson [22] and histological criteria. Ten neurons of hippocampal CA1, which had been enlarged 100 times under the microscope, were randomly chosen from each section. We used Scion Image Analysis System to detect the Optical density (OD) of neurons from 3 tissue sections per brain of seven rats. After correcting intensity, we obtained all OD values and then calculated the average OD of each group.
For the western blotting, each of band intensity was calculated as the average of measurements from seven brains per group. The method was the same as above. Both of the results were evaluated by SPSS13.0 software. The MR or GR data in the control and SPS groups were expressed as mean±standard deviation, and by unpaired samples t-test between the groups. P value less than 0.05 was considered statistically significant.
III. Results
Immunohistochemical observation of MR and GR
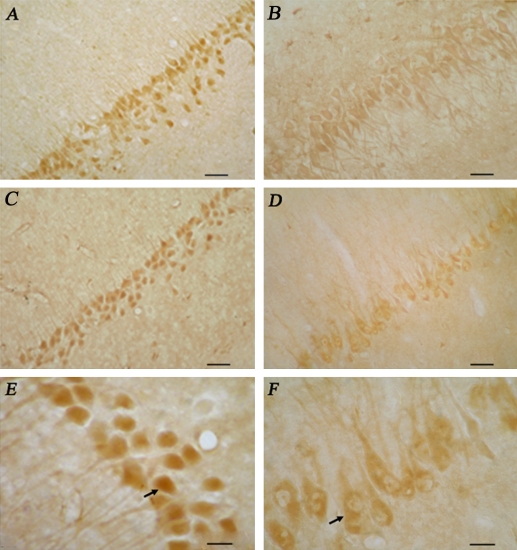
We observed the MR- and GR-ir in the neurons in CA1 of hippocampus in the controls and SPS rats (Fig. 1). In the control rats, MR-ir was distributed in the cytoplasm and nucleus (Fig. 1A), while GR-ir was distributed mainly in the nucleus (Fig. 1C). In the SPS rat, decreased MR-ir (Fig. 1B) or GR-ir (Fig. 1D) was observed in the CA1 region. The change in MR-ir was more conspicuous than that in GR-ir. We found a change in GR-distribution in SPS rats, some neurons (black arrows) expressed GR-ir not only in the nucleus but also in the cytoplasm (Fig. 1F). However, almost all of the neurons in the control rats expressed GR-ir in nucleus (Fig. 1E). We did not find any change in MR-distribution between control and SPS (data not shown). Quantitative analysis also revealed decreased MR- and GR-ir in the SPS in comparison with control rat (Fig. 2).
Fig. 1.
Expression of MR-ir (A, B) and GR-ir (C, D, E and F) in the CA1 of the hippocampus in control groups (left panels) and SPS groups (right panels). Arrows showed that some neurons expressed GR-ir in nucleus and cytoplasm of E and F. Bars=200 µm (A, B, C and D), 75 µm (E and F).
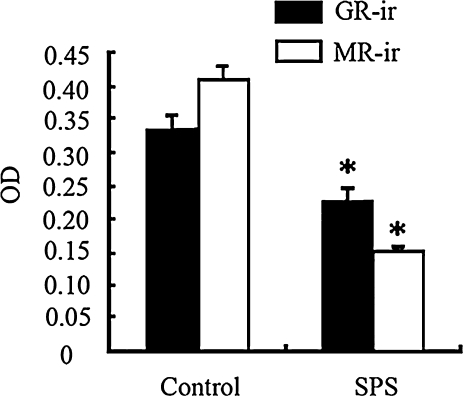
Fig. 2.
Quantitative analysis of MR- and GR-ir in the CA1 in control and SPS rats MR and GR-ir were significantly difference from control group (* p<0.05).
Western blotting result of MR and GR
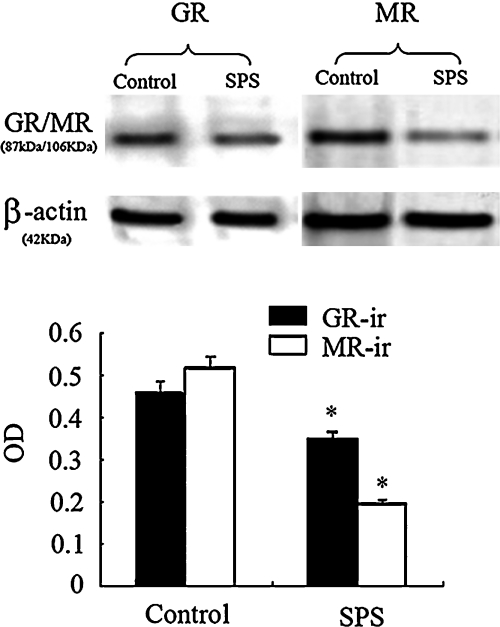
Immunoreactive signals for MR, GR and β-actin appeared on Western blotting at 106 kDa, 87 kDa and 42 kDa respectively (data not shown). Changes of MR and GR expression in the hippocampal region between control and SPS rats are presented in Figure 3. Using western blotting we disclosed the presence of MR and GR in the hippocampal area. Figure 3 showed a significant down-regulation of hippocampal MR and GR protein in SPS rats in comparison with control rats (p<0.05).
Fig. 3.
Image (upper panel) and quantitative analysis result (lower panel) of MR and GR expression in the hippocampus of control and SPS rats using western blotting. Decreased MR and GR expression were observed in the SPS rats in comparison with control rats (* p<0.05). To confirm equal protein loading the same blots were re-hybridized with antibodies specific for β-actin.
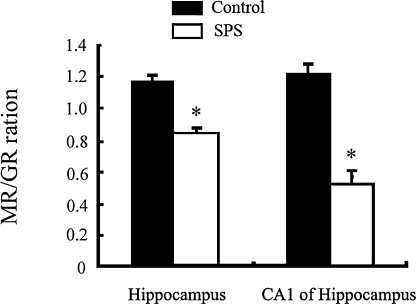
The MR/GR ratios
We have examined the MR/GR ratios in CA1 and whole hippocampus (Fig. 4). There was a decrease in MR/GR ratio in CA1 and hippocampus in the SPS rats, compared to respective control (p<0.05).
Fig. 4.
MR/GR ratio in CA1 and hippocampus in SPS rats and control rats. SPS rats group are significantly lower than the controls not only in the CA1, but also in the whole hippocampus (* p<0.05, in comparison with control groups).
IV. Discussion
In present study, we examined the SPS induced down regulation of MR and GR protein expression and the change in MR/GR ratio in the hippocampus. Moreover, we found that SPS induced different degrees of regulation in GR-ir distribution in the hippocampus by immunochemistry.
SPS produced decrease expression of MR and GR in the hippocampus. Hippocampal MR is involved in the maintenance of basal HPA activity throughout the circadian cycle by corticosterone. A number of studies examined the importance of hippocampal MR in controlling HPA axis. Liberzon et al. examined decreased hippocampal MR mRNA in the same animal model [15]. The decrease in MR suggests that lower MR does not alter the increasing sensitivity to negative feedback, or alternatively, that MR plays a less prominent role in the feedback regulation [15]. On the other hand, MR carries a neuron-protective function in the hippocampus [2]. Abnormally low or down-regulated hippocampal MR in PTSD patients may induce large tissue loss. On the behavioral level, increased MR function is implicated in decreased anxiety/fear related behaviors in animals. Lower MR function is associated with increased anxiety/fear response [19]. A decrease of hippocampal MR in PTSD could be responsible for the related clinical phenomena including increased startle-response, enhanced autonomic reactivity, hypervigilance, and increased anxiety.
On the other hand, hippocampal glucocorticoid receptors play central roles in glucorticoid negative feedback regulation. Various research groups have suggested that the overactivity of the HPA axis in depression may be due to an abnormality of the GR at the limbic-hippocampal level [10, 21]. PTSD patients exhibit hypersensitive negative feedback and fortified dexamethasone negative feedback. The increase of GR mRNA in the hippocampus was also observed in the SPS-7 days animal [15]. These data suggest that an increase in GR function occurs in PTSD patients. The increase in GR mRNA in hippocampus is involved in enhanced fast feedback in PTSD even in the presence of lower MR mRNA. Decreased GR protein level in the present study was not suspected. Although the degree of decreased GR protein is less than that of MR protein, and decreased GR is still maintained at a high level in comparison with MR, it is well known that the changes in mRNA levels do not imply a similar regulation at the level of the receptors’ protein. There are two possibilities: 1) some of the activated corticosteroid receptors were not detected by the antibodies we used. 2) Different animal species used in experiments caused different expression between mRNA and protein.
Low level of glucocorticoids might also induce the changes in the structures of MR and GR-protein. The activation of MR and GR or maintenance of MR- and GR-ir is an important factor affecting hippocampal neuronal survival [9]. A previous study showed that the activation of MR or GR blocked adrenalectomy-induced cell death. Moreover, strong immunoreactivity for MR and GR was found in postnatal 1 week rats [27]. The first week of life is characterized by a high degree of neuronal morphogenesis and cell migration, especially in the hippocampus [4]. We also found decreased neuronal numbers in the hippocampus after SPS (data not shown), which suggested that decreased MR- and GR-ir may have induced hippocampal neuronal death in the SPS animals. In general, MR and GR play important roles in negative feedback and hippocampus change in PTSD patients. It is possible that there is a selection for roles of MR and GR in PTSD [15], and that they may induce the proliferative activity in hippocampal neurons to maintain the structure of hippocampus [12]. This could explain both normal hippocampus size and intact negative feedback in some PTSD patients.
We found altered MR/GR ratio of both CA1-subregion and all hippocampus in the SPS animals. The hippocampus receives multimodal representations of stimulus information by CA1 pyramidal cells. CA1 cells are especially affected following corticosterone administration [11]. In addition, the GR receptor is located mostly in the CA1-subregion of the hippocampus. Thus both of these receptors in CA1 are related with PTSD. We also found that the MR/GR ratio in the SPS group was lower than that of the control group.
The balance in actions mediated by the two types of corticosteroid receptors in these neurons appears critical for neuronal excitability, stress responsibility, and behavioral adaptability [3]. Dysregulation of MR/GR balance brings CA1 neurons into a vulnerable state with consequences for regulation of the stress response and enhanced vulnerability to disease in genetically predisposed individuals. Behavioral reactivity and extinction of avoidance conditioning are impaired in adrenalectomized animals, restored with low dose corticosterone replacement in MR activation and impaired with high dose replacement in both MR and GR activation [18]. MR closes to saturation with low basal concentrations of corticosterone, while high corticosterone concentrations during stress occupy both MRs and GRs. De Kloet and Joels reported similar differential effects of predominantly MR versus GR and MR occupation on ion permeability [1]. Antagonist effects of MR and GR could explain the importance of MR/GR ratio in these systems, since the change in MR/GR ratio might alter physiological output [15]. Our data showed decreased ratio, with down-regulation MR and GR. Interestingly, previous studies also suggest different regulation patterns in MR and GR expression with decreased MR/GR ratio in PTSD. The first is up-regulation of both receptors, with GR up to 129% of control levels and MR up to 114% of control levels. The authors speculated that the changes might be associated with the changes in negative feedback [26]. The second is down-regulation of MR and unaltered GR [23]. The third is down-regulation of MR and up-regulation of GR [15]. The decreased MR suggests that the lower MR in the presence of the higher GR does not alter the increased sensitivity to negative feedback. In general, GR is kept at a higher level in comparison with MR in SPS rats, whenever GR is decreased, increased or unchanged. Too low or too high levels of corticosteroid hormones during stress result in MR/GR ratio change, impaired information processing, and enhanced vulnerability of specific hippocampal neurons [3]. Well documented animal studies show apoptotic cell death and altered neurogenesis after adrenalectomy. It is proposed that the maintenance of corticosteroid homeostasis and MR/GR ratio mediated effects limit vulnerability to stress-related disease.
Meanwhile, we also found a shift in GR distribution from nucleus to cytoplasm by immunohistochemistry in hippocampal CA1. It is conjectured that low corticosterone under PTSD conditions induced GR cytoplasmic distribution. This would suggest that the affinity of glucocorticoid with GR declined, which could induce default of the neuronal growth and compromise the function of the hippocampus. Although we did not measure the corticosterone concentration in the blood, we supposed that a change in GR distribution was relative to the corticosterone level. Much evidence has accumulated to suggest that stress-related adrenal steroid hormones modulate brain and cognitive function, and may play a pivotal role in the enhancement or impairment of memory processes. It is possible that corticosterone reduces the overall risks and symptoms of PTSD by inhibiting excessive retrieval of traumatic memories [24].
Furthermore, we have examined the colocalization of MR and GR in the hippocampus [5]. MR and GR form not only homodimers, but also heterodimers [16, 20]. It is significant that MR-GR heterodimers plays an essential role in mediating response to corticosteroids in some tissues. In particular, receptor heterodimers may contribute to the biphasic excitatory response of hippocampal neurons to corticosterone [3]. In the present study, we only studied the changes in expression of both receptors after SPS. It is not known whether homodimers, heterodimers, or both caused the decreased expression of MR and GR. These findings may be relevant for behavioral syndrome seen in PTSD and we hope to investigate these questions in further experiments.
V. Acknowledgments
We wish to thank all of the staff members in the China Medical University Experiment Center for their technical support. This research was supported by a grant from the National Natural Science Foundation of China (30600341).
VI. References
- 1.De Kloet E. R., Joels M. Functional implications of brain corticosteroid receptor diversity. Cell. Mol. Neurobiol. 1993;13:433–455. doi: 10.1007/BF00711582. [DOI] [PubMed] [Google Scholar]
- 2.de Kloet E. R., Sutanto W., van den Berg D. T., Carey M. P., van Haarst A. D., Hornsby C. D., Meijer O. C., Rots N. Y., Oitzl M. S. Brain mineralocorticoid receptor diversity: functional implications. J. Steroid Biochem. Mol. Biol. 1993;47:183–190. doi: 10.1016/0960-0760(93)90073-6. [DOI] [PubMed] [Google Scholar]
- 3.De Kloet E. R., Vreugdenhil E., Oitzl M. S., Joels M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 4.Galeeva A., Ordyan N., Pivina S., Pelto-Huikko M. Expression of glucocorticoid receptors in the hippocampal region of the rat brain during postnatal development. J. Chem. Neuroanat. 2006;31:216–225. doi: 10.1016/j.jchemneu.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Han F., Ozawa H., Matsuda K., Nishi M., Kawata M. Colocalization of mineralocorticoid receptor and glucocorticoid receptor in the hippocampus and hypothalamus. Neurosci. Res. 2005;51:371–381. doi: 10.1016/j.neures.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Heim C. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 7.Herman J. P., Patel P. D., Akil H., Watson S. J. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol. Endocrinol. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- 8.Heuser I., Lammer C. H. Stress and the brain. Neurobiol. Aging. 2003;24:69–76. doi: 10.1016/s0197-4580(03)00048-4. [DOI] [PubMed] [Google Scholar]
- 9.Hu Z., Yuri K., Ozawa H., Lu H., Yang Y., Ito T., Kawata M. Adrenalectomy-induced granule cell death is predicated on the disappearance of glucocorticoid receptor immunoreactivity in the rat hippocampal granule cell layer. Brain Res. 1997;778:293–301. doi: 10.1016/s0006-8993(97)01047-0. [DOI] [PubMed] [Google Scholar]
- 10.Juruena M. F., Cleare A. J., Bauer M. E., Pariante C. M. Molecular mechanism of GR sensitivity and relevance for affective disorders for special issue. Acta Neuropsychiatrica. 2003;15:354–367. doi: 10.1046/j.1601-5215.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 11.Kadar T., Dachir S., Shukitt H. B., Levy A. Sub-regional hippocampal vnlnerability in various animal models leading to cognitive dysfunction. J. Neural Transm. 1998;105:987–1004. doi: 10.1007/s007020050107. [DOI] [PubMed] [Google Scholar]
- 12.Kataoka Y., Tamura Y., Cui Y., Yamada H. Neural activity-dependent cellular proliferation in the rat cerebral cortex. Acta Histochem. Cytochem. 2005;38:93–98. [Google Scholar]
- 13.Korte S. M., de Boer S. F., de Kloet E. R., Bohus B. Anxiolytic-like effects of selective mineralocorticoid and glucocorticoid antagonists on fear-enhanced behavior in the elevated plus-maze. Psychoneuroendocrinology. 1995;20:385–394. doi: 10.1016/0306-4530(94)00069-7. [DOI] [PubMed] [Google Scholar]
- 14.Korte S. M., Korte-Bouws G. A., Koob G. F., de Kloet E. R., Bohus B. Mineralcocorticoid and glucocorticoid receptors antagonists in animal model of anxiety. Pharmacol. Biochem. Behav. 1996;54:261–267. doi: 10.1016/0091-3057(95)02172-8. [DOI] [PubMed] [Google Scholar]
- 15.Liberzon I., Flagel S. B., Vazquez D. M., Young E. A. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. J. Neuroendocrinol. 1999;11:11–17. doi: 10.1046/j.1365-2826.1999.00288.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu W., Wang J., Sauter N. K., Pearce D. Steroid receptor heterodimerization demonstrated in vitro and in vivo. Proc. Natl. Acad. Sci. U S A. 1995;92:12480–12484. doi: 10.1073/pnas.92.26.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagura H., Nagura Y., Fukudo S., Sasano H. Neuroendocrine-immune interactions and starvation in mucosal immunity and mucosal inflammation. Acta Histochem. Cytochem. 2003;36:287–292. [Google Scholar]
- 18.Oital M. S., Fluttert M., de Kloet E. R. The effects of corticosterone on reacitivity to spatial novelty in mediated by central mineralcocorticoid receptors. Eur. J. Neurosci. 1994;6:1072–1079. doi: 10.1111/j.1460-9568.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 19.Oitzl M. S., Van Haarst A. D., Sutanto W., de Kloet E. R. Corticosterone, brain mineralocorticoid receptors (MRs) and the activity of the hypothalamic-pituitary-adrenal (HPA) axis: the Lewis rat as an example of increased central MR capacity and a hyporesponsive HPA axis. Psychoneuroendocrinology. 1995;20:655–675. doi: 10.1016/0306-4530(95)00003-7. [DOI] [PubMed] [Google Scholar]
- 20.Ou X. M., Storring J. M., Kushwaha N., Albert P. R. Heterodimerization of mineralocorticoid and glucocorticoid receptors at a novel negative response element of the 5-HT1A receptor gene. J. Biol. Chem. 2001;276:14299–14307. doi: 10.1074/jbc.M005363200. [DOI] [PubMed] [Google Scholar]
- 21.Pariante C. M., Miller A. H. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G., Watson C. (CD-Rom) Academic Press; San Diego: 1998. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 23.Reul J. M., Probst J. C., Skutella T., Hirschmann M., Stec I. S., Montkowski A., Landgraf R., Holsboer F. Increased stress-induced adrenocorticotropin response after long-term intracerebroventricular treatment of rats with antisense mineralocorticoid receptor oligodeoxynucleotides. Neuroendocrinology. 1997;65:189–199. doi: 10.1159/000127272. [DOI] [PubMed] [Google Scholar]
- 24.Schelling G., Richter M., Roozendaal B., Rothenhausler H. B., Krauseneck T., Stoll C. Exposure to high stress in the intensive care unit may have negative effects on health-related quality-of-life outcomes after cardiac surgery. Crit. Care Med. 2003;31:1971–1980. doi: 10.1097/01.CCM.0000069512.10544.40. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi T., Morinobu S., Iwamoto Y., Yamawali S. Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacology. 2006;189:165–173. doi: 10.1007/s00213-006-0545-6. [DOI] [PubMed] [Google Scholar]
- 26.Van Dijken H. H., Mos J., van der Heyden J. A., Tilders F. J. Characterization of stress-induced long-term behavioural changes in rats: evidence in favor of anxiety. Physiol. Behav. 1992;52:945–951. doi: 10.1016/0031-9384(92)90375-c. [DOI] [PubMed] [Google Scholar]
- 27.Van Eekelen J. A., Bohn M. C., de kloet E. R. Postnatal ontogeny of mineralcocoricoid and glucocorticoid receptor gene expression in the regions of rat tel and diencephalon. Brain Res. Dev. Brain Res. 1991;61:33–43. doi: 10.1016/0165-3806(91)90111-u. [DOI] [PubMed] [Google Scholar]
- 28.Winter H., Irle E. Hippocampal volume in adult burn patients with and without posttraumatic stress disorder. Am. J. Psychiatry. 2004;161:2194–2200. doi: 10.1176/appi.ajp.161.12.2194. [DOI] [PubMed] [Google Scholar]
- 29.Woolley C. S., Gould E., Sakai R. R., Spencer R. L., McEwen B. S. Effects of aldosterone or RU28362 treatment on adrenalectomy-induced cell death in the dentate gyrus of the adult rat. Brain Res. 1991;554:312–315. doi: 10.1016/0006-8993(91)90207-c. [DOI] [PubMed] [Google Scholar]
- 30.Yehuda R., Giller E. L., Southwick S. M., Lowy M. T., Mason J. W. Hypothalamic-pituitary-adrenal dysfunction in post-traumatic stress disorder. Biol. Psychiatry. 1991;30:1031–1048. doi: 10.1016/0006-3223(91)90123-4. [DOI] [PubMed] [Google Scholar]
- 31.Young E. A., Haskett R. F., Grunhaus L., Pande A., Weinberg V. M., Watson S. J., Akil H. Increased circadian activation of the hypothalamic pituitary adrenal axis in depressed patients in the evening. Arch. Gen Psychiatry. 1994;51:701–707. doi: 10.1001/archpsyc.1994.03950090033005. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L. X., Smith M. A., Li X. L., Weiss S. R. B., Post R. M. Apoptosis of hippocampal neurons after amygdala kindled seizures. Brain Res. Mol. Brain Res. 1998;55:198–208. doi: 10.1016/s0169-328x(97)00316-1. [DOI] [PubMed] [Google Scholar]