Abstract
Age-related atresia of ovarian follicles is characterized by apoptosis of the constituent cells. Recent studies have indicated that dysfunction of the proteasome and endoplasmic reticulum and subsequent apoptosis in the presence of oxidative stress have relevance to aging. The aim of this study was to assess the involvement of these processes in age-related follicular atresia. Formalin-fixed, paraffin-embedded sections of ovaries obtained at surgery from 74 women (age: 21–54 y) were examined with the terminal deoxynucleotidyl transferase-mediated, dUTP-biotin nick-end labeling (TUNEL) method and an immunohistochemical technique. Primary antibodies used in immunohistochemistry were against pentosidine, ubiquitin and caspase 12. Histological localization of these substances in oocytes was observed by light microscopy, and labeling indices of these cells were evaluated by regression analysis. Positive signals for pentosidine, ubiquitin, caspase 12, and TUNEL were detectable in oocytes of the primordial, primary and their atretic follicles. Regression analysis revealed an age-related increase in the labeling indices for pentosidine, ubiquitin, caspase 12, and TUNEL. These results suggest that pentosidine accumulation in human oocytes is related to apoptosis and increases with age. Further studies will be necessary to clarify the involvement of pentosidine accumulation, proteasome inhibition, and endoplasmic reticulum stress in age-related apoptosis of oocytes in human ovaries.
Keywords: aging, apoptosis, oocyte, oxidative stress, proteasome inhibition
I. Introduction
The age-related decrease in the number of ovarian follicles critically impairs the female reproductive capability in humans. During the fetal period, the number of ovarian follicles in an ovary begins to increase at 4–5 gestational weeks, reaches a maximum (approximately seven million) at 20 gestational weeks, begins to decrease at 24 gestational weeks, and declines to about forty thousand at birth. The number continues to decline after birth and disappears almost completely before menopause [39]. The loss of ovarian follicles is termed follicular atresia, which is mediated by apoptosis of the constituent cells [7, 16]. In addition, the hypothesis that the age-related apoptosis of oocytes primarily occurs in the fetal and premenopausal periods is evidenced by the observations that DNA fragmentation is prominently seen in oocytes in the primordial and primary follicles, although the primordial and primary follicles are not influenced by sex hormones [7, 42]. Thus, it is conjectured that there is an unknown, sex hormone-independent apoptotic mechanism involved in aging.
It is well known that oxidative stress in the body increases with aging [14, 26]. Recent investigations in obstetrics and gynecology have suggested a close link of oxidative stress to issues associated with female reproductive functions including ovulation and luteinization [1, 10]. It is known that the reactive intermediates, which include aldehydes (carbonyls) formed during glycoxidation, alter and impair cell signaling, resulting in inflammation, apoptosis or both [20, 40]. These unstable aldehydes simultaneously bind to nucleic acids and proteins to become stable carbonyl-modified end adducts such as pentosidine, which are readily detectable by immunochemical and immunohistochemical approaches [34]. Thus, the detection of these adducts in human tissues is a useful way to obtain evidence for oxidative stress there.
Endoplasmic reticulum (ER) participates in protein quality control to prevent misfolding of the molecules by the ubiquitin-mediated, ATP-dependent, proteasome (ubiquitin-proteasome) system (UPS) [4, 22]. Both the ER system and the UPS have been shown to be disrupted in aging and in several pathological conditions [27, 41]. Moreover, several in vitro studies have suggested that increased oxidative stress and subsequent glycoxidation damage both the ER and the proteasome [2, 5, 17, 25, 35]. Damage to the ER stress gives rise to accumulation of misfolded proteins in the ER, triggering caspase 12-mediated apoptosis [28].
A recent report suggested that oxidative stress has an important role for oocyte apoptosis, aging of oocyte and female fertility [8]. However, there are few studies that report the relation between glycoxidation or carbonyl-modified end adducts and oocyte apoptosis. Given these observations, it is hypothesized that the age-related increased oxidative stress amplifies carbonyl stress, alters cell signaling, and deprives oocytes of normal functions of the ER and proteasome, leading to apoptosis induction of the cells. To test this hypothesis, we determined the involvement of carbonyl stress and proteasome inhibition in the age-related apoptosis of human oocytes, using the immunoperoxidase method [29], and the TUNEL method [12, 30]. We also performed a semiquantitative analysis of our morphological data.
II. Materials and Methods
Cases
The investigation was carried out on 74 premenopausal women (ages: 21–54 y) who underwent oophorectomy for benign gynecological diseases and carcinomas of the uterine cervix. According to reports of relationship between oxidative stress and chronic diseases [26], we determined the exclusion criteria were as follows: age ≥55 years, BMI >30 kg/m2, frequent weight-reducing diets, smoking, elevated triglycerides or total cholesterol, abnormal kidney or liver function, untreated hypertension (>160/90 mmHg), personal history of chronic disease (diabetes, stroke, cardiovascular disease, rheumatoid arthritis), and ovarian tumors. The study adhered to the principles of the Helsinki Declaration, and the Institutional Review Boards of the Tokyo Women’s Medical University approved and supervised the study protocol. Written informed consent for experimental use of ovaries was obtained from all subjects.
Tissue preparation
Ovaries obtained at surgery were fixed in 10% formalin, dehydrated, and embedded in paraffin. Serial 3-µm-thick sections of each ovary were used for histological, immunohistochemical and TUNEL analyses. Histological examination was done on sections stained with hematoxylin-eosin (H&E). The immunohistochemical and TUNEL procedures are described below.
Primary antibodies
For immunohistochemical staining, we employed the following primary antibodies: a mouse monoclonal IgG1 against pentosidine (Clone: 4D7) at a concentration of 1.0 µg/mL [34], a rabbit polyclonal IgG against ubiquitin (Cat No. Z-458) at a dilution of 1:500 [32]. The antibody to pentosidine was a kind gift from Dr. R. Nagai (Department of Biochemistry, Kumamoto University Graduate School of Medicine). The anti-ubiquitin antibody and the anti-caspase 12 antibody were purchased from Dako Cytomation (Kyoto, Japan), ProSci (Poway, CA, USA), and Molecular Probes (Eugene, OR, USA), respectively.
Immunohistochemical technique
Immunohistochemistry was performed according to the following steps. Prior to staining for only caspase 12 among these antigens, antigen retrieval pretreatment was required. Sections for caspase 12 staining were processed for 10 min at 121°C with autoclaving in citrate buffer, pH 6.0. Sections were deparaffinized, rehydrated, quenched for 10 min at 4°C with 3% H2O2 to block endogenous peroxidase activity, rinsed in 100 mM phosphate-buffered saline (PBS), pH 7.6, treated for 30 min at room temperature with 3% nonimmune animal serum in PBS to block nonspecific antibody binding, and subsequently incubated overnight at 4°C with the primary antibodies. Antibody binding was visualized by the avidin-biotin-immunoperoxidase complex (ABC) method using the appropriate Vectastain ABC kits (Vector Laboratories, Burlingame, CA, USA) in accordance with the manufacturer’s instructions. The chromogen was 3,3'-diaminobenzidine tetrahydrochloride (DAB), and the counterstain was hematoxylin. Negative reaction control sections were prepared by omission of the primary antibodies or by incubation with nonimmune animal IgG, instead of the antibodies, derived from the same species as those producing the antibodies. Epithelial cells of proximal tubuli in the kidney of diabetic nephropathy served as positive reaction controls for pentosidine [40]. Neurofibrillary tangles in hippocampal pyramidal neurons in the brain of Alzheimer’s disease served as a positive reaction control for ubiquitin [32]. Cardiocytes in the heart served as a positive reaction control for caspase 12 [28]. Immunohistochemical identification of substances on the sections was performed by light microscopy and roughly verified by comparison with their consecutive sections stained with H&E and immunostained for others.
TUNEL method
Sections were deparaffinized, rehydrated, pretreated for 30 min at room temperature with 20 µg/mL proteinase K (Sigma Chemical, St. Louis, MO, USA) in 50 mM Tris-buffered saline, at pH 7.5 (TBS) for digestion of nuclear protein, quenched for 10 min at 4°C with 3% H2O2, rinsed in TBS, and subsequently incubated for 60 min in a humidified atmosphere at 37°C with the TUNEL reaction solution. This solution consisted of TdT buffer (Invitrogen) containing 0.3 U/µL TdT (Invitrogen, Tokyo, Japan) and 0.01 M digoxigenin-11-dUTP (Roche Diagnostics, Tokyo, Japan). The TUNEL reaction was stopped by immersion in TBS for 15 min at 37°C. Sections were then incubated for 30 min at room temperature with a peroxidase-conjugated anti-digoxigenin antibody (Roche) at a dilution of 1:50, using DAB as the chromogen and hematoxylin as the counterstain. Lymphocytes in the germinal center of mucosal lymph follicles in the appendix were used as a positive reaction control [12]. Sections processed with omission of TdT gave a negative reaction control.
Analysis of the labeling indices
The density of follicles in an ovary was defined as the total number of oocytes in the primordial, primary and their atretic follicles in a constant field of the cortex. The three largest step-wised H&E-stained sections of each ovary were digitally scanned and imported into a personal computer using Photoshop image software, and the entire cortical area was measured using an NIH image software. The number of oocytes was visually counted with a light microscope. Subsequently, the follicular density was expressed as the average value of the number of oocytes per mm2 field in the three sections from each case.
The labeling index of follicles in an ovary was defined as the percentage of oocytes with positive signals for the examined substances in the primordial, primary and their atretic follicles. Positive reaction controls for these substances were used according to the previous studies [4, 11, 12, 34, 35]. The numbers of total and signal-positive oocytes were visually counted on each section, and the labeling index was expressed as the average value of percentage of signal-positive oocytes in three randomly-selected sections from each case. The relationship between aging and data of the labeling index in the examined cases was evaluated by regression analysis, and their respective linear equation (y = ax + b) and correlation coefficient (r) were obtained.
Statistics
Referring to a textbook of statistics [6], we defined the grading of statistical significance in regression analysis as follows: (i) r2 values less than 0.0625 is considered faintly correlated; (ii) r2 values from 0.0625 to 0.25 is weakly correlated; (iii) r2 values from 0.25 to 0.5625 is moderately correlated; and (iv) r2 values more than 0.5625 is strongly correlated.
III. Results
Histological observations and relationship between follicular density and aging
The cortical areas of the examined ovaries ranged from a minimum of 16 to a maximum of 261 mm2. Primordial, primary, secondary, Graafian and atretic follicles were found in the ovarian cortex. The total number of primordial, primary and atretic follicles per ovary varied from case to case, and ranged from 1 to 184. Atretic follicles were characterized by nuclear condensation and somatic shrinkage of oocytes surrounded by a single layer of involuted granulosa cells [13]. The major axis of oocytes in the atretic follicles ranged from 30 to 70 µm. As described previously [39], the follicular density decreased age-dependently (data not shown).
Localization of immunohistochemical and TUNEL signals
Signals for pentosidine, ubiquitin, caspase 12 and TUNEL were detected in a subset of oocytes (Fig. 1). These signals exhibited a homogenous pattern, but differed in subcellular localization: pentosidine immunoreactivity was confined to the cytoplasm; ubiquitin immunoreactivity was found mainly in the cytoplasm but also in the nucleus; caspase 12 immunoreactivity was found mainly in the nucleus but also in the cytoplasm; and TUNEL positivity was restricted to the nucleus. None of these signals was detected in the negative control sections, whereas all of these signals were detected at reasonable locations in the positive control sections, confirming the propriety of our immunohistochemical and TUNEL procedures.
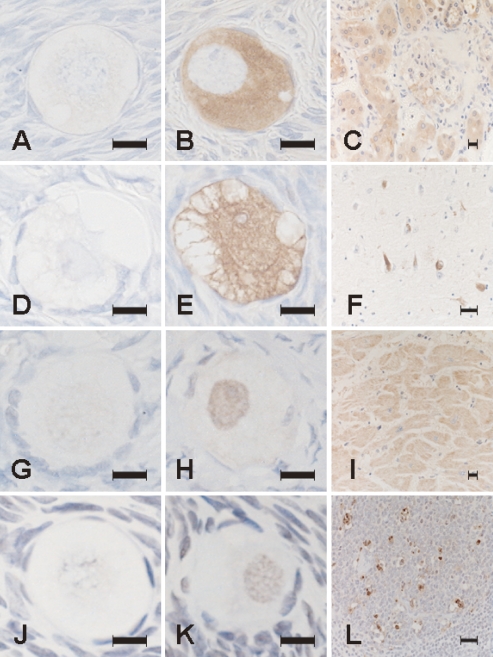
Fig. 1.
Photomicrographs of sections processed with immunohistochemical staining for pentosidine (A–C), ubiquitin (D–F), caspase 12 (G–I) and TUNEL stainings (J–L). Positive signals for these substances were undetectable on negative reaction control sections for pentosidine (A), ubiquitin (D), caspase 12 (G), and TUNEL (J). Pentosidine immunoreactivity is localized in the cytoplasm of oocytes (B) and in the cytoplasm of epithelial cells of proximal tubuli in the kidney of diabetic nephropathy as a positive reaction control (C). Ubiquitin immunoreactivity is localized in the cytoplasm and nucleus of oocytes (E) and in neurofibrillary tangles in the cerebral cortex of Alzheimer’s disease as a positive reaction control (F). Caspase 12 immunoreactivity is localized in the nucleus of oocytes indicative of nuclear translocation of the activated form (H) and in the cytoplasm of cardiocytes of the heart as a positive reaction control with a dotlike pattern indicative of microsomal localization of the inactive form (I). TUNEL positivity is localized in the nucleus of oocytes (K) and in large lymphocytes in the germinal center of a mucosal lymph follicle in catarrhal appendicitis as a positive reaction control (L). Bar=50 µm.
In order to determine whether these signals colocalize in the same oocyte or not, we examined serial sections from ten cases (three for twenties, thirties, forties and one for fifties of age). As shown in Figure 2, colocalization of these signals was demonstrated in a majority of oocytes in all cases. We used two sets of 3 serial sections, because the size of oocytes was too small for 4 serial sections.
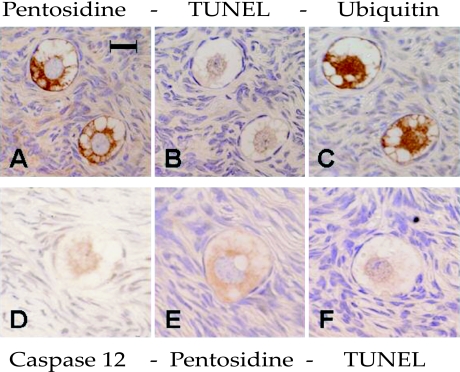
Fig. 2.
Comparison of localization between positive signals for immunohistochemical and TUNEL staining on two groups of consecutive sections (A–C) and (D–F). Pentosidine immunoreactivity is localized in the cytoplasm of oocytes (A, E). TUNEL positivity is in the nucleus of oocytes (B, F). Ubiquitin immunoreactivity is localized in the cytoplasm and nucleus of oocytes (C). Caspase 12 is mainly localized in the nucleus of an oocyte (D). Bar=50 µm.
Relationships between labeling indices and aging
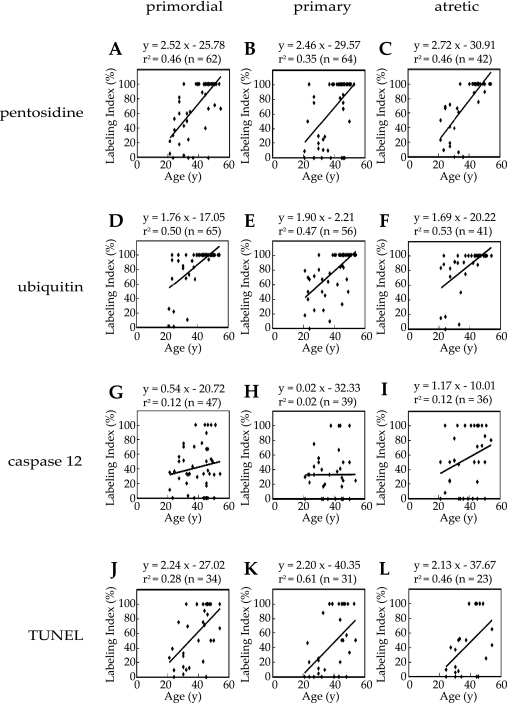
To evaluate the relationship between pentosidine, ubiquitin and caspase 12 immunoreactivities or TUNEL positivity and aging, regression analysis was performed using labeling index. The pentosidine labeling index was moderately positive-correlated with aging in oocytes in the primordial (Fig. 3A), and primary (Fig. 3B) follicles, while it was strongly positive-correlated with aging in oocytes in the atretic (Fig. 3C) follicles. The ubiquitin labeling index was also moderately positive-correlated with aging in oocytes in the premordial (Fig. 3D), primary (Fig. 3E), atretic (Fig. 3F) follicles. The caspase 12 labeling index was weakly positive-correlated with aging in oocytes in the premordial (Fig. 3G) and atretic (Fig. 3I) follicles, while was faintly correlated with aging in oocytes in the primary (Fig. 3H) follicles. The TUNEL labeling index was moderately positive-correlated with aging in oocytes in the primordial (Fig. 3J) and atretic (Fig. 3L) follicles, while it was strongly positive-correlated with aging in oocytes in the primary (Fig. 3K) follicles.
Fig. 3.
Relationship between aging and the pentosidine, ubiquitin, caspase 12 and TUNEL labeling index in oocytes. The graphs show correlation between age and the labeling index of pentosidine in the primordial (A), primary (B) and atretic (C), the labeling index of ubiquitin in the primordial (D), primary (E) and atretic (F), the labeling index of caspase 12 in the primordial (G), primary (H) and atretic (I), and the labeling index of TUNEL in the primordial (J), primary (K) and atretic (L) follicles.
IV. Discussion
The formation processes of glycoxidation-induced carbonyl-modified end products, synonymous with advanced glycation end products (AGEs), are diverse [33, 34]. Pentosidine is produced by oxidative crosslinking of the protein arginine and lysine residues with pentose. Previous investigations have suggested that glycolytic activity is lower in oocytes compared to granulosa cells, and energy metabolism in oocytes depends on the products of glycolysis, such as pyruvate, which is supplied by the surrounding granulosa cells [19, 38]. These observations could indicate that oocytes uptake and metabolize glucose, mainly to pentose, and that intracellular levels of pentose in oocytes are much higher than those of glucose in oocytes. Recent studies have demonstrated increased intracellular accumulation of pentosidine in aging [18], and in age-related disorders such as neurodegenerative disorders and intervertebral discopathies [27, 37], that is to say, pentosidine may gradually occur in response to mild oxidative stress and accumulate in the body for lengthy periods. Thus, our result that pentose-derived pentosidine accumulation in oocytes increased with age was consistent with previous studies.
Our findings of the colocalization of positive signals for pentosidine, ubiquitin, caspase 12 and TUNEL in oocytes in a subset of the primordial, primary and atretic follicles could be seen as collateral evidence that intracellular pentosidine accumulation and subsequent proteasome inhibition and ER stress induce apoptosis of these cells. Several studies have shown that proteasome inhibition is caused by increased oxidative stress and subsequent carbonyl stress by exposure of the cell to AGEs [2, 27]. Ubiquitinated (misfolded) proteins accumulate in the cells when the UPS is disrupted [23], and misfolded proteins accumulate in the ER in several pathological conditions including diabetes mellitus, ischemic heart and brain diseases, and neurodegenerative diseases [15, 25]. In addition, some studies have suggested that a close relationship exists between oxidative stress, aging, ER stress and proteasome inhibition [9, 21, 25, 41]. ER stress-induced apoptosis is mediated by the emancipation of caspase 12 from a complex consisting of its inactive precursors [28, 36]. The activated form of caspase 12 induces apoptosis directly in the nucleus or via caspase 3 activation [11, 28]. Another study reported that it arises from the binding of Fas ligand, expressed on the cell surface of oocytes, to its receptor Fas expressed on the cell surface of granulosa cells, resulting in the activation of caspases 8, 9 and 3 to induce apoptosis of granulosa cells [3]. However, we could not find apoptosis of oocytes via caspase 8, 9 and 3 activation because of the lack of antibodies for the activated form of caspase 8, 9 and 3.
A recent experimental study on mice has indicated that caspase 3 activity significantly increases in aged oocytes compared with young oocytes, and that both caspase 8 and caspase 9 activities significantly decrease in aged oocytes compared with young oocytes [31]. This reminds us of the implications for caspase 12 activation in apoptosis of aged oocytes. However, in light of the lack of correlation between the caspase 12 labeling index and aging in oocytes in the primary follicles, it should be considered with the caveat that oocyte apoptosis in the primary follicles is not necessarily mediated only by caspase 12 but rather may be mediated collaboratively by caspase 8 and caspase 9. Because proteasome inhibition-induced apoptosis is mediated not only by triggering ER stress but also by additional mechanisms, proteasome inhibition allows intranuclear accumulation of phosphorylated p53 that induces the caspase 9-mediated apoptosis [24].
However, there are some limitations in this study. In this study, it is unclear whether the examined ovaries were in the luteal or follicular phase. However, since pentosidine gradually accumulates in the body over several years, it is evident that there is no significant change in the accumulating levels during the relatively short, 28-day menstrual cycle. This study focuses on the primordial, primary and atretic follicles as markers of aging because they are not significantly influenced by sex hormones. Furthermore, we have attempted to employ the immunohistochemical technique of caspase 8 and caspase 9. But the caspase 8 antibody which is commercially available has a positive reaction to both active and inactive caspase 8, making it impossible to distinguish between the active and the inactive forms morphologically. Further, the positive signals for caspase 9 were very unclear, and accordingly we have not showed the results for caspase 8 and caspase 9.
In conclusion, these results suggested that pentosidine accumulation in human oocytes was related to apoptosis and increased with age. Further studies are necessary to clarify the involvement of pentosidine accumulation, proteasome inhibition and endoplasmic reticulum stress in age-related apoptosis of oocytes in human ovaries. Determining the cellular and molecular mechanisms of toxic cell signaling in age-related oocyte apoptosis should improve understanding of the aging and infertility processes and to develop appropriate therapeutic strategies.
V. Acknowledgements
The authors wish to thank Drs. Koichiro Takagi, Motohiko Aiba, Satoru Shimizu, and Naoto Yamaguchi for their valuable suggestions, and Drs. Ryoji Nagai and Koji Uchida (Department of Biochemistry, Kumamoto University Graduate School of Medicine) for their kind gift of antibodies.
VI. References
- 1.Agarwal A., Gupta S., Sharma R. K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulteau A. L., Verbeke P., Petropoulos I., Chaffotte A. F., Friguet B. Proteasome inhibition in glyoxal-treated fibroblasts and resistance of glycated glucose-6-phosphate dehydrogenase to 20 S proteasome degradation in vitro. J. Biol. Chem. 2001;276:45662–45668. doi: 10.1074/jbc.M105374200. [DOI] [PubMed] [Google Scholar]
- 3.Chen Q., Yano T., Matsumi H., Osuga Y., Yano N., Xu J., Wada O., Koga K., Fujiwara T., Kugu K., Taketani Y. Cross-talk between Fas/Fas ligand system and nitric oxide in the pathway subserving granulosa cell apoptosis: a possible regulatory mechanism for ovarian follicle atresia. Endocrinology. 2005;146:808–815. doi: 10.1210/en.2004-0579. [DOI] [PubMed] [Google Scholar]
- 4.Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Exp. Biol. Med. 2006;231:1197–1211. doi: 10.1177/153537020623100705. [DOI] [PubMed] [Google Scholar]
- 5.Ciechanover A., Schwartz A. L. The ubiquitin system: pathogenesis of human diseases and drug targeting. Biochim. Biophys. Acta. 2004;1695:3–17. doi: 10.1016/j.bbamcr.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Dawson B., Trapp R. G. Lange Medical Books-McGraw-Hill. Medical Pub. Division; New York: 2004. Basic & Clinical Biostatistics. [Google Scholar]
- 7.Depalo R., Nappi L., Loverro G., Bettocchi S., Caruso M. L., Valentini A. M., Selvaggi L. Evidence of apoptosis in human primordial and primary follicles. Hum. Reprod. 2003;18:2678–2682. doi: 10.1093/humrep/deg507. [DOI] [PubMed] [Google Scholar]
- 8.Ebisch I. M., Thomas C. M., Peters W. H., Braat D. D., Steegers-Theunissen R. P. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum. Reprod. Update. 2007;13:163–174. doi: 10.1093/humupd/dml054. [DOI] [PubMed] [Google Scholar]
- 9.Fribley A., Zeng Q., Wang C. Y. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol. Cell. Biol. 2004;24:9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii J., Iuchi Y., Okada F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod. Biol. Endocrinol. 2005:3–43. doi: 10.1186/1477-7827-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita E., Kouroku Y., Jimbo A., Isoai A., Maruyama K., Momoi T. Caspase-12 processing and fragment translocation into nuclei of tunicamycin-treated cells. Cell Death Differ. 2002;9:1108–1114. doi: 10.1038/sj.cdd.4401080. [DOI] [PubMed] [Google Scholar]
- 12.Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr. Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 14.Harman D. The free radical theory of aging. Antioxid. Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T., Saito A., Okuno S., Ferrand-Drake M., Dodd R. L., Chan P. H. Damage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neurons. J. Cereb. Blood Flow Metab. 2005;25:41–53. doi: 10.1038/sj.jcbfm.9600005. [DOI] [PubMed] [Google Scholar]
- 16.Hussein M. R. Apoptosis in the ovary: molecular mechanisms. Hum. Reprod. Update. 2005;11:162–177. doi: 10.1093/humupd/dmi001. [DOI] [PubMed] [Google Scholar]
- 17.Hyun D. H., Lee M. H., Halliwell B., Jenner P. Proteasomal dysfunction induced by 4-hydroxy-2,3-trans-nonenal, an end-product of lipid peroxidation: a mechanism contributing to neurodegeneration? J. Neurochem. 2002;83:360–370. doi: 10.1046/j.1471-4159.2002.01125.x. [DOI] [PubMed] [Google Scholar]
- 18.Jono T., Kimura T., Takamatsu J., Nagai R., Miyazaki K., Yuzuriha T., Kitamura T., Horiuchi S. Accumulation of imidazolone, pentosidine and N(epsilon)-(carboxymethyl)lysine in hippocampal CA4 pyramidal neurons of aged human brain. Pathol. Int. 2002;52:563–571. doi: 10.1046/j.1320-5463.2002.01390.x. [DOI] [PubMed] [Google Scholar]
- 19.Leese H. J., Barton A. M. Production of pyruvate by isolated mouse cumulus cells. J. Exp. Zool. 1985;234:231–236. doi: 10.1002/jez.1402340208. [DOI] [PubMed] [Google Scholar]
- 20.Leonarduzzi G., Arkan M. C., Basaga H., Chiarpotto E., Sevanian A., Poli G. Lipid oxidation products in cell signaling. Free Radic. Biol. Med. 2000;28:1370–1378. doi: 10.1016/s0891-5849(00)00216-1. [DOI] [PubMed] [Google Scholar]
- 21.Lewis M. D., Roberts B. J. Role of CYP2E1 activity in endoplasmic reticulum ubiquitination, proteasome association, and the unfolded protein response. Arch. Biochem. Biophys. 2005;436:237–245. doi: 10.1016/j.abb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y., Hendershot L. M. The unfolding tale of the unfolded protein response. Cell. 2001;107:827–830. doi: 10.1016/s0092-8674(01)00623-7. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Vicente M., Sovak G., Cuervo A. M. Protein degradation and aging. Exp. Gerontol. 2005;40:622–633. doi: 10.1016/j.exger.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Melino G. Discovery of the ubiquitin proteasome system and its involvement in apoptosis. Cell Death Differ. 2005;12:1155–1157. doi: 10.1038/sj.cdd.4401740. [DOI] [PubMed] [Google Scholar]
- 25.Menendez-Benito V., Verhoef L. G., Masucci M. G., Dantuma N. P. Endoplasmic reticulum stress compromises the ubiquitin-proteasome system. Hum. Mol. Genet. 2005;14:2787–2799. doi: 10.1093/hmg/ddi312. [DOI] [PubMed] [Google Scholar]
- 26.Miyata T., Kurokawa K., Van Ypersele De Strihou C. Advanced glycation and lipoxidation end products: role of reactive carbonyl compounds generated during carbohydrate and lipid metabolism. J. Am. Soc. Nephrol. 2000;11:1744–1752. doi: 10.1681/ASN.V1191744. [DOI] [PubMed] [Google Scholar]
- 27.Munch G., Shepherd C. E., McCann H., Brooks W. S., Kwok J. B., Arendt T., Hallupp M., Schofield P. R., Martins R. N., Halliday G. M. Intraneuronal advanced glycation endproducts in presenilin-1 Alzheimer’s disease. Neuroreport. 2002;13:601–604. doi: 10.1097/00001756-200204160-00013. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B. A., Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 29.Nakane P. K. Simultaneous localization of multiple tissue antigens using the peroxidase-labeled antibody method: a study on pituitary glands of the rat. J. Histochem. Cytochem. 1968;16:557–560. doi: 10.1177/16.9.557. [DOI] [PubMed] [Google Scholar]
- 30.Otsuki Y. Various methods of apoptosis detection. Acta Histochem. Cytochem. 2000;33:235–241. [Google Scholar]
- 31.Papandile A., Tyas D., O’Malley D. M., Warner C. M. Analysis of caspase-3, caspase-8 and caspase-9 enzymatic activities in mouse oocytes and zygotes. Zygote. 2004;12:57–64. doi: 10.1017/s0967199404002588. [DOI] [PubMed] [Google Scholar]
- 32.Perry G., Friedman R., Shaw G., Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc. Natl. Acad. Sci. U S A. 1987;84:3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibata N., Nagai R., Miyata S., Jono T., Horiuchi S., Hirano A., Kato S., Sasaki S., Asayama K., Kobayashi M. Nonoxidative protein glycation is implicated in familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation. Acta Neuropathol. 2000;100:275–284. doi: 10.1007/s004019900173. [DOI] [PubMed] [Google Scholar]
- 34.Shibata N., Nagai R., Uchida K., Horiuchi S., Yamada S., Hirano A., Kawaguchi M., Yamamoto T., Sasaki S., Kobayashi M. Morphological evidence for lipid peroxidation and protein glycoxidation in spinal cords from sporadic amyotrophic lateral sclerosis patients. Brain Res. 2001;917:97–104. doi: 10.1016/s0006-8993(01)02926-2. [DOI] [PubMed] [Google Scholar]
- 35.Shibata T., Iio K., Kawai Y., Shibata N., Kawaguchi M., Toi S., Kobayashi M., Kobayashi M., Yamamoto K., Uchida K. Identification of a lipid peroxidation product as a potential trigger of the p53 pathway. J. Biol. Chem. 2006;281:1196–1204. doi: 10.1074/jbc.M509065200. [DOI] [PubMed] [Google Scholar]
- 36.Siman R., Flood D. G., Thinakaran G., Neumar R. W. Endoplasmic reticulum stress-induced cysteine protease activation in cortical neurons: effect of an Alzheimer’s disease-linked presenilin-1 knock-in mutation. J. Biol. Chem. 2001;276:44736–44743. doi: 10.1074/jbc.M104092200. [DOI] [PubMed] [Google Scholar]
- 37.Sivan S. S., Tsitron E., Wachtel E., Roughley P., Sakkee N., van der Ham F., Degroot J., Maroudas A. Age-related accumulation of pentosidine in aggrecan and collagen from normal and degenerate human intervertebral discs. Biochem. J. 2006;399:29–35. doi: 10.1042/BJ20060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugiura K., Pendola F. L., Eppig J. J. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev. Biol. 2005;279:20–30. doi: 10.1016/j.ydbio.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 39.te Velde E. R., Scheffer G. J., Dorland M., Broekmans F. J., Fauser B. C. Developmental and endocrine aspects of normal ovarian aging. Mol. Cell. Endocrinol. 1998;145:67–73. doi: 10.1016/s0303-7207(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 40.Toyokuni S. Reactive oxygen species-induced molecular damage and its application in pathology. Pathol. Int. 1999;49:91–102. doi: 10.1046/j.1440-1827.1999.00829.x. [DOI] [PubMed] [Google Scholar]
- 41.van der Vlies D., Woudenberg J., Post J. A. Protein oxidation in aging: endoplasmic reticulum as a target. Amino Acids. 2003;25:397–407. doi: 10.1007/s00726-003-0025-9. [DOI] [PubMed] [Google Scholar]
- 42.Wu J., Zhang L., Wang X. Maturation and apoptosis of human oocytes in vitro are age-related. Fertil. Steril. 2000;74:1137–1141. doi: 10.1016/s0015-0282(00)01597-1. [DOI] [PubMed] [Google Scholar]