Abstract
Artemisinin-based combination therapy (ACT) is currently promoted as a strategy for treating both uncomplicated and severe falciparum malaria, targeting asexual blood-stage Plasmodium falciparum parasites. However, the effect of ACT on sexual-stage parasites remains controversial. To determine the clearance of sexual-stage P. falciparum parasites from 342 uncomplicated, and 217 severe, adult malaria cases, we reviewed and followed peripheral blood sexual-stage parasites for 4 wk after starting ACT. All patients presented with both asexual and sexual stage parasites on admission, and were treated with artesunate-mefloquine as the standard regimen. The results showed that all patients were asymptomatic and negative for asexual forms before discharge from hospital. The percentages of uncomplicated malaria patients positive for gametocytes on days 3, 7, 14, 21, and 28 were 41.5, 13.1, 3.8, 2.0, and 2.0%, while the percentages of gametocyte positive severe malaria patients on days 3, 7, 14, 21, and 28 were 33.6, 8.2, 2.7, 0.9, and 0.9%, respectively. Although all patients were negative for asexual parasites by day 7 after completion of the artesunate-mefloquine course, gametocytemia persisted in some patients. Thus, a gametocytocidal drug, e.g., primaquine, may be useful in combination with an artesunate-mefloquine regimen to clear gametocytes, so blocking transmission more effectively than artesunate alone, in malaria transmission areas.
Keywords: Plasmodium falciparum, gametocyte clearance time, uncomplicated malaria, severe malaria, artemisinin-based combination therapy, Thailand
INTRODUCTION
Antimalarial drug treatments against Plasmodium falciparum malaria target asexual blood-stage parasites, which are responsible for clinical diseases and death. Gametocytes, the sexual stages of the malaria parasite, do not cause clinical disease; they are produced in the human host but remain in a state of arrested cell development until ingested by a feeding mosquito. The subsequent development of mosquito-specific stages results in infection of the mosquito salivary glands with sporozoites and malaria transmission to humans. With each subsequent blood meal, parasites are transmitted to a new person, resulting in the spread of malaria among the human population. Gametocytes are thus vital to the maintenance of the malaria transmission cycle.
The effects of antimalarial drugs on gametocytes and their infectiousness for vector mosquitoes have been studied and illustrated some factors that might be associated with gametocyte carriage, such as treatment regimens, disease severity, anemia, a recrudescence infection fever, and duration of symptoms [1-3]. Those studies also raised questions about the policy for use of antimalarial drugs for malaria treatment. However, some studies have shown that treatment with artemisinin derivatives (in combination with other drugs) results in lower gametocyte carriage rates and density, and less infection of the treated individuals than non-artemisinin regimens [4-6]. At present, artemisinin-based combination therapies (ACTs) are widely recommended for the treatment of both uncomplicated and severe falciparum malaria. However, few data exist about the dynamic changes of gametocytemia after ACT, in both uncomplicated and severe falciparum malaria. To investigate this, we attempted to evaluate the effects of artesunate, in artesunate-mefloquine combination therapy, on sexual-stage parasites in both uncomplicated and severe falciparum malaria patients.
MATERIALS AND METHODS
Study site and recruitment procedures
The medical records of 559 adult falciparum malaria patients were reviewed. Each of these patients had (1) been admitted to the Hospital for Tropical Diseases, Bangkok, Thailand, for treatment of acute P. falciparum malaria, January 1998-December 2006, either male or female, body weight ≥ 40 kg, age ≥ 15 yr old; (2) been positive for both asexual- and sexual-stage P. falciparum parasites on admission before treatment, with microscopic confirmation; (3) been enrolled in the artesunate-mefloquine drug protocols; (4) no history of pre-treatment with an antimalarial drug; (5) remained in hospital for 4 wk following the initiation of antimalarial therapy; (6) provided blood samples for blood examinations, e.g., sexual and asexual parasite counts every 6 hr until clear, then daily. Patients fulfilled the following selection criteria, (1) clearance of asexual forms of P. falciparum from the peripheral blood with therapy; (2) no evidence of concomitant non-falciparum malaria infection on admission or during follow-up; (3) no reappearance of asexual-form parasitemia during the 4-wk study period. In addition, since the administration of blood might affect immune response, we studied only patients who did not receive blood transfusions. Among the patients, 342 had uncomplicated malaria, and 217 had severe malaria, as classified by World Health Organization criteria (2000) [7]. The present study was reviewed and approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Baseline studies
Age, gender, body mass index (BMI), initial fever and duration of fever before admission, history of malaria, initial parasite counts (both sexual and asexual forms), and liver and spleen size, were recorded. Baseline laboratory data, e.g., red-blood-cell count, hemoglobin, hematocrit, white-blood-cell count, and platelet count, were determined by automated cell counter (Advia 120 Hematology System, Siemens Medical Solutions Diagnostics; commercial reagents by Roche Diagnostics, Berlin, Germany). The following biochemical parameters were measured using a Cobas Integra 400 (Roche Diagnostics), with commercial reagents from the same supplier: bilirubin, aminotransferases, alkaline phosphatase, proteins, blood urea, creatinine, glucose, cholesterol, sodium, and potassium. Thick and thin blood films were prepared from fingerprick blood samples and stained with Giemsa. Peripheral blood concentrations of either asexual or sexual forms of P. falciparum were estimated by counting the number per 200 white blood cells on thick smears, multiplied by white-blood-cell count, or by counting the number of either asexual or sexual forms per 1,000 erythrocytes on thin smears, multiplied by the red-blood-cell count.
Antimalarial chemotherapies
Antimalarial chemotherapy for uncomplicated malaria: a single dose of oral artesunate (200 mg/day) with mefloquine (8 mg/kg/day) was given daily for 3 days.
Antimalarial chemotherapy for severe malaria: artesunate 2.4 mg/kg i.v. as a loading dose, then 1.2 mg/kg i.v. 12-hourly until oral artesunate (50 mg/tablet) could be taken, at the dose 200 mg/day to complete 5 days of treatment, with the addition of 2 doses of oral mefloquine (25 mg/kg given in divided doses 8 hr apart) given 12 hr after the last dose of artesunate.
No patient received any other antimalarial drugs, e.g., primaquine, as pre- or post-treatment course.
Follow up studies over 4 wk
Body temperature was recorded every 6 hr until it decreased to 37.5℃ and remained stable for the next 48 hr, then daily. Blood parasite (both asexual and sexual forms) concentrations were estimated every 6 hr until cleared, then daily. Clinical laboratory tests were performed on admission and weekly until day 28 post-dosing, as appropriate. Radical cure was defined as the absence of asexual parasites during the 28-day follow-up. Parasite clearance time (PCT) was the time from the initiation of treatment until the first time a slide was negative for the asexual parasite forms, and remained negative for the next 24 hr. Gametocyte clearance time (GCT) was the time from the initiation of treatment until the first time a slide was gametocyte negative, and remained so for the next 24 hr. Fever clearance time (FCT) was defined as the time from the start of treatment until the body temperature subsided to 37.5℃ and remained at or below this temperature for the next 48 hr. Agreement to remain in hospital was signed pre-enrollment by the patients or their legal guardians, and financial compensation was provided to all patients for the 28-day follow-up period.
Statistical analysis
Quantitative and qualitative data were expressed as mean with SD and number of observations with percentages (%), respectively. All P-values reported were from 2-tailed testing, and statistical significance was set at 0.05. Descriptive statistics were used to summarize baseline values and demographic data. The chi-square or Fisher's exact tests were used to compare proportions as appropriate, and the t-test was used to analyze continuous data.
RESULTS
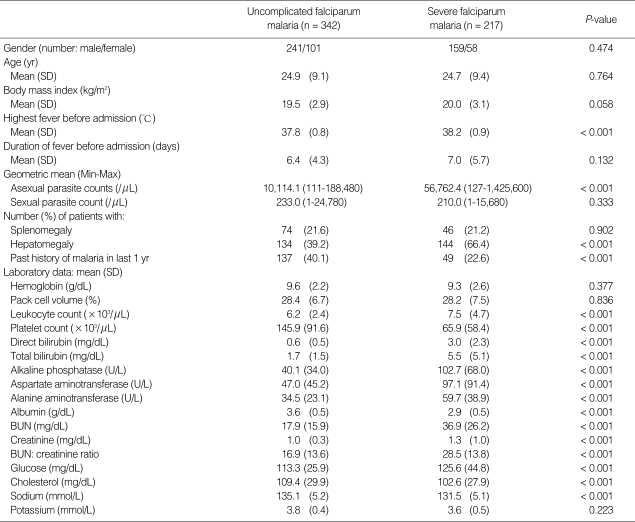
Of the 559 malaria patients enrolled in the present study, 342 had uncomplicated, and 217 had severe falciparum malaria. Demographic data and pretreatment characteristics are shown in Table 1. The majority had contracted malaria infection along the Thailand-Myanmar border.
Table 1.
Pre-treatment clinical and laboratory data of the patients (n = 559)
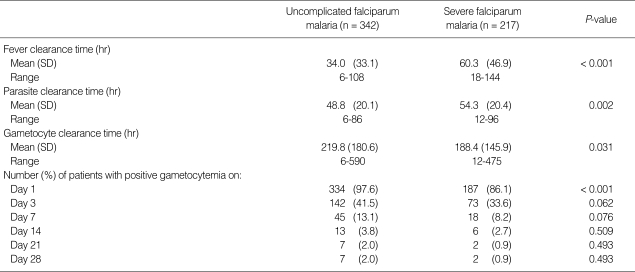
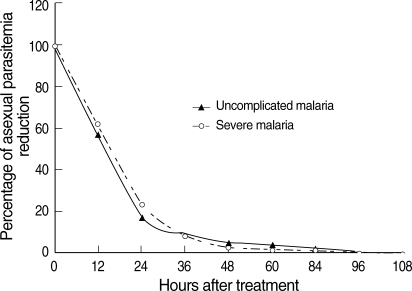
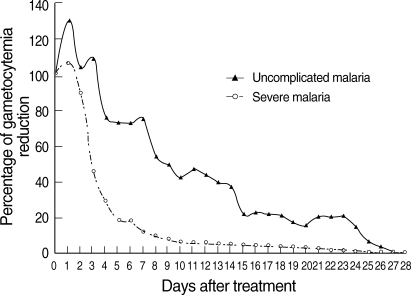
All patients completed courses of artesunate-mefloquine therapy and were asymptomatic and negative for asexual forms before discharge from hospital. However, 7 patients (2.0%) in the uncomplicated malaria group and 2 in the severe malaria group (0.9%) were still positive for gametocytemia before discharge from hospital. Those 9 patients were therefore excluded from GCT calculations. The therapeutic results for the 2 study groups are shown in Table 2. The percentages of uncomplicated malaria patients positive for gametocytes on days 3, 7, 14, 21, and 28 were 41.5, 13.1, 3.8, 2.0, and 2.0%, respectively. The percentages of severe malaria patients positive for gametocytes on days 3, 7, 14, 21, and 28 were 33.6, 8.2, 2.7, 0.9, and 0.9%, respectively. FCT and asexual-PCT in the uncomplicated malaria patients were significantly shorter than the severe malaria patients (P < 0.001 and P < 0.002, respectively). However, GCT in the uncomplicated malaria patients was significantly longer than the severe malaria patients (P = 0.031). When the percentage reduction in asexual parasitemia was plotted against hours post-treatment, the comparative asexual parasite clearance times for the uncomplicated malaria and severe malaria patient groups were similar (Fig. 1). Regarding GCT, gametocytes cleared significantly faster in severe than uncomplicated malaria (Fig. 2). In severe malaria, gametocytemia density peaked during the first 3 days post-admission and decreased sharply to < 10% after completion of the 7-day artesunate-mefloquine course. In uncomplicated malaria, gametocytemia density also peaked during the first 3 days post-admission, but slowly decreased to < 10% between post-treatment wk 3 and 4.
Table 2.
Therapeutic responses (n = 559)
Fig. 1.
Post-treatment percentage reduction in asexual parasitemia.
Fig. 2.
Post-treatment percentage reduction in sexual parasitemia.
DISCUSSION
For malaria control programs, the ideal antimalarial or antimalarial combination should clear asexual parasitemia and its associated clinical symptoms and signs within the shortest possible time. ACTs are widely promoted as a strategy to counteract the increasing resistance of P. falciparum to antimalarials. In addition, ACTs are used in many countries to prevent disease transmission and reduce the risk of drug resistance [8]. In the present study, we showed the asexual and sexual parasites clearances of using artesunate-mefloquine to treat uncomplicated and severe falciparum malaria in an area of multidrug resistance. The study was based on a follow-up period of 28 days. The results showed that artesunate given together with mefloquine remains effective and rapidly clears asexual parasitemia without undue side-effects. Regarding the therapeutic results, our results showed fever and asexual parasites cleared within 7 days post-treatment. These results were consistent with previous studies [9-11].
The previous studies suggested that the prevalence of gametocytemia during treatment varies according to the antimalarial drug regimen used [12,13]. In the present study, the activity of artesunate in an artesunate-mefloquine regimen against sexual-stage parasites in adult falciparum malaria was well established, particularly in severe malaria. Interestingly, PCT was significantly longer in severe malaria than uncomplicated malaria, whereas GCT was significantly shorter in severe malaria than uncomplicated malaria. Parenteral administration of artesunate might clear gametocytes faster than oral artesunate. The slower asexual parasite clearance in the severe malaria group is likely to reflect higher baseline parasitemia. Based on our result, it also gives an idea of how rapidly gametocytes disappear. The half-life of gametocytes in uncomplicated malaria group in Fig. 2 seem much longer about 8 days to get from 100% to 50% and from 50% to 25% implying a half-life of around 8 days. After complete antimalarial treatment, 13.1% of uncomplicated malaria and 8.2% of severe malaria patients had gametocytemia on day 7. If the patients were discharged from hospital and returned to a malaria endemic area early, e.g., on day 7, a gametocytocidal drug e.g., primaquine, would be useful to block gametocyte transmission.
Previous studies have shown that ACTs can reduce malaria transmission in the community [14,15]. However, the 90% gametocytemia clearance times cited in these studies were > 20 days post-treatment. In our study, both groups of patients had < 5% gametocytemia on day 21 post-treatment. Artemisinin derivatives may kill young gametocytes, not mature gametocytes [8]. This may explain the persistence of gametocytes after 3- and 7-day courses of treatment in uncomplicated and severe malaria, respectively. Therefore, artesunate in artesunate-mefloquine regimen is not totally kill gametocyte observed on day 7 and day 28. Adding primaquine in ACT may be more effective in eradication of gametocyte than ACT alone. In conclusion, the activity of ACTs against sexual-stage P. falciparum parasites should be further monitored in areas of malaria transmission, including the study to investigate other factors affecting gametocyte clearance in both patients who already developed gametocytemia on admission and patients who develop patent gametocytemia after the onset of treatment. This will provide information to inform drug policymaking in those areas, and can be used in relevant areas.
ACKNOWLEDGEMENTS
We thank the nurses and laboratory technicians of the Hospital for Tropical Diseases for their excellent care of the patients and help with the present study. The English of this manuscript was improved by Mr. Paul R Adams, Specialist, Research and Academic Affairs Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. We thank Faculty of Tropical Medicine, Mahidol University for page charge support. The present study was supported partly by a Mahidol University Research Grant and a Health and Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare of Japan (H18-SHINKOU-IPPAN-009).
References
- 1.Targett G, Drakeley C, Jawara M, Von Seidlein L, Colman R, Deen J, Pinder M, Doherty T, Sutherland C, Walraven G, Milligan P. Artesunate reduces but does not prevent post treatment transmission of Plasmodium falciparum to Anopheles gambiae. J Infect Dis. 2001;183:1254–1259. doi: 10.1086/319689. [DOI] [PubMed] [Google Scholar]
- 2.Hallett RL, Sutherland CJ, Alexander N, Ord R, Jawara M. Combination therapy counteracts the enhanced transmission of drug-resistant malaria parasites to mosquitoes. Antimicrob Agents Chemother. 2004;48:3940–3943. doi: 10.1128/AAC.48.10.3940-3943.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nacher M, Silachamroon U, Singhasivanon P, Wilairatana P, Phumratanaprapin W, Fontanet A, Looareesuwan S. Comparison of artesunate and chloroquine activities against Plasmodium vivax gametocytes. Antimicrob Agents Chemother. 2004;48:2751–2752. doi: 10.1128/AAC.48.7.2751-2752.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doherty JF, Sadiq AD, Bayo L, Alloueche A, Milligan P. A randomized safety and tolerability trial of artesunate plus sulfadoxine-pyrimethamine versus sulfadoxine-pyrimethamine alone for treatment of uncomplicated malaria in Gambian children. Trans R Soc Trop Med Hyg. 1999;93:543–546. doi: 10.1016/s0035-9203(99)90376-0. [DOI] [PubMed] [Google Scholar]
- 5.Drakeley CJ, Jawara M, Targett GA, Walravan G, Obiske U. Addition of artesunate to chloroquine for treatment of Plasmodium falciparum malaria in Gambian children causes a significant but short-lived reduction in infectiousness for mosquitoes. Trop Med Int Health. 2004;9:53–61. doi: 10.1046/j.1365-3156.2003.01169.x. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland CJ, Ord R, Dunyo S, Jawara M, Drakeley CJ. Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med. 2005;2:e92. doi: 10.1371/journal.pmed.0020092. (doi: 10.1371/journal.pmed.0020092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Severe falciparum malaria. Tran R Soc Trop Med Hyg. 2000;94(S1):1–90. [PubMed] [Google Scholar]
- 8.World Health Organization. Guidelines for the treatment of malaria. 1st ed. Geneva, Switzerland: WHO; 2006. pp. 133–143. [Google Scholar]
- 9.Silachamroon U, Krudsood S, Thanachartwet W, Tangpukdee N, Leowattana W, Chalermrut K, Srivilairit S, Wilairatana P, Thimasarn K, Looareesuwan S. An open, randomized trial of three-day treatment with artesunate combined with a standard dose of mefloquine divided over either two or three days, for acute, uncomplicated falciparum malaria. Southeast Asian J Trop Med Public Health. 2005;36:591–596. [PubMed] [Google Scholar]
- 10.Piyaphanee W, Krudsood S, Tangpukdee N, Thanachartwet W, Silachamroon U, Phophak N, Duangdee C, Haoharn O, Faithong S, Wilairatana P, Leowattana W, Looareesuwan S. Emergence and clearance of gametocytes in uncomplicated Plasmodium falciparum malaria. Am J Trop Med Hyg. 2006;74:432–435. [PubMed] [Google Scholar]
- 11.Tangpukdee N, Krudsood S, Thanachatwet V, Pengruksa C, Phophak N, Kano S, Li G, Brittenham GM, Looareesuwan S, Wilairatana P. Efficacy of artequick versus artesunate-mefloquine in the treatment of acute uncomplicated falciparum malaria in Thailand. Southeast Asian J Trop Med Public Health. 2008;39:1–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Bousema JT, Schneider P, Gouana LC, Drakeley CJ, Tostmann A, Houben R, Githure JI, Ord R, Sutherland CJ, Omar SA, Sauerwein RW. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J Infect Dis. 2006;193:1151–1159. doi: 10.1086/503051. [DOI] [PubMed] [Google Scholar]
- 13.Sowunmi A, Gbotosho GO, Happi CT, Adedeji AA, Fehintola FA, Folaria OA, Tambo E, Fateye BA. Therapeutic efficacy and effects of artemether-lumefantrine and amodiaquine-sulfalene-pyrimethamine on gametocyte carriage in children with uncomplicated Plasmodium falciparum malaria in Southwestern Nigeria. Am J Trop Med Hyg. 2007;77:235–241. [PubMed] [Google Scholar]
- 14.van den Broek I, Kitz C, Attas SA, Libama F, Balasegaram M, Guthmann JP. Efficacy of three artemisinin combination therapies for the treatment of uncomplicated Plasmodium falciparum malaria in the Republic of Congo. Malar J. 2006;5:113. doi: 10.1186/1475-2875-5-113. (doi:10.2286/1475-2875-5-113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali E, Mackinnon MJ, Abdel-Muhsin AA, Ahmed S, Walliker D, Babiker HA. Increased density but not prevalence of gametocytes following drug treatment of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 2006;100:176–183. doi: 10.1016/j.trstmh.2005.04.021. [DOI] [PubMed] [Google Scholar]