Abstract
Theileria annulata, a protozoan parasite of cattle and domestic buffaloes, is transmitted by ticks of the genus Hyalomma, and causes a disease named Mediterranean or tropical theileriosis. In this research 50 cattle naturally infected with Theileria annulata were treated with the extract of the plant Peganum harmala. The treatment was continued for 5 days, the dose of the extract being 5 mg/kg per day. After the treatment, 39 cattle responded to the treatment and recovered, but 11 did not respond to the treatment and died. The recovery rate of animals treated with the extract of the plant Peganum harmala was 78%.
Keywords: Theileria annulata, cattle, tropical theileriosis, Peganum harmala
INTRODUCTION
Theileria annulata is a protozoan parasite of cattle and domestic buffaloes, and is transmitted by ticks of the genus Hyalomma. This protozoan causes a disease named Mediterranean or tropical theileriosis. The disease affects cattle in a wide strip that covers southern Europe, northern Africa, and the Middle East (Iran), and reaches the south of the former USSR, India, and China (Purnell, 1978). Tropical theileriosis represents a major threat to crossbred and purebred cattle in Iran. During the last 4 decades scientists in the Razi and other institutes throughout the world have worked to find a potent compound to cure theileriosis (Hashemi-Fesharki, 1991). Parvaquone and buparvaquone are 2 effective drugs against tropical theileriosis. The recovery rate of animals treated with parvaquone was 60.7% and with buparvaquone it was 88.7% (Hashemi-Fesharki, 1991). Parvaquone and buparvaquone are chemical drugs which infiltrate in muscles of cattle. They are not easily and quickly eliminated from the body of animals (McHardy et al., 1985), which can constitute a public health hazard if milk and meat of treated animals are consumed by humans, but the extract of Peganum harmala is a natural drug which does not infiltrate in muscles of cattle (Puzii and Serov, 1983).
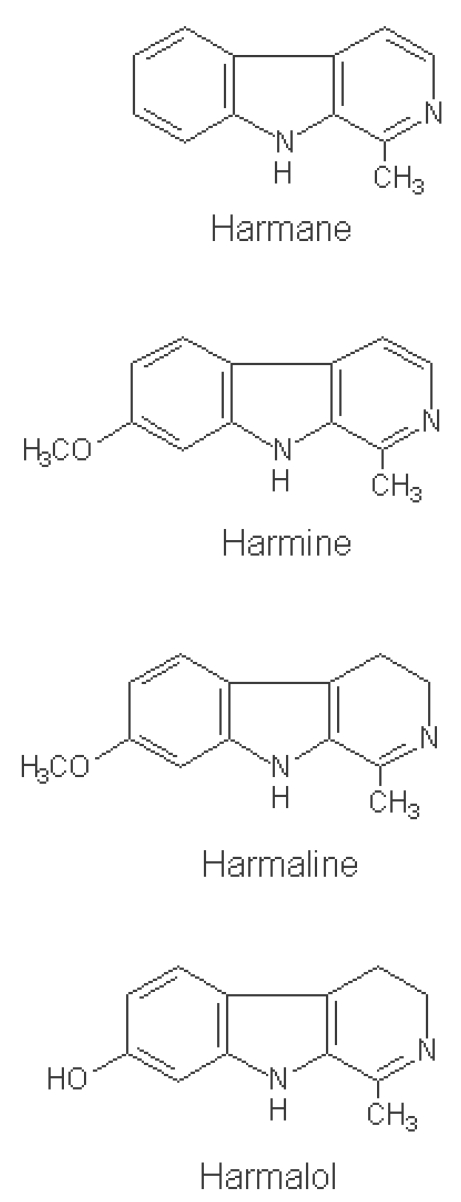
Peganum harmala is the plant from which harmine was first isolated, as well as a source of harmaline and tetrahydroharmine. This plant grows in semi-arid conditions like Iran. It originated from Central Asia, and is held in high esteem throughout Asia Minor as a medicinal plant. The pharmacologically active compounds of P. harmala are several alkaloids. These include β-carbolines, such as harmine, harmaline (identical with harmidine), harmalol, and harman (Fig. 1), and quinazoline derivatives, such as vasicine and vasicinone (Kamel et al., 1970). Alkaloid compounds well illustrate the diversity of antiprotozoal compounds found in P. harmala plants (Wright and Phillipson, 1990), and among the several alkaloids, harmaline (harmidine, C13H14N2O) has been found to be the major active alkaloid (Budavari and O'neil, 1996). In the present study, it has been tried to evaluate therapeutic effects of the extract of P. harmala on natural tropical theileriosis in cattle.
Fig. 1.
Chemical structure of β-carboline alkaloids.
MATERIALS AND METHODS
Fifty naturally infected cattle with tropical theileriosis were selected. These cattle were from different ages and breeds (crossbreed and native breed). Rectal temperatures and schizont parasitosis values were scored on a 3-point scale (Table 1) as described by Mbwambo et al. (2002). Only animals which were at the primary phase of their disease were used. Animals that attended to at an early stage of the infection were those whose lymph node biopsy smears showed fairly numerous to numerous schizonts, and only rare piroplasm parasitemia was observed. In addition, only one or both prescapular lymph nodes were slightly to moderately enlarged with temperature reaction between 39.5 and 40.9℃.
Table 1.
Scores of rectal temperature and Theileria annulata schizonts in cattle
Animals were totally managed by the farmers, and our responsibility was to screen the disease-suspected animals and to conduct treatments accordingly. Rectal temperatures of cattle were measured daily in the morning until dropped to normal values. Vigor, appetite, visible mucous membranes and other signs were observed clinically every day. Indicative signs of a possible T. annulata infection were enlargement of the prescapular glands, accompanied by rectal temperatures above 39.5℃. The peripheral ear vein was punctured for preparation of thin blood smears. Lymph node biopsy smears from enlarged prescapular glands were collected using a 16-gauge needle, 1 inch long. Thin blood and lymph node biopsy smears were air-dried, fixed in absolute methanol, stained with Giemsa's stain for 45 min and examined under microscope for presence or absence of piroplasms and schizonts of T. annulata.
The aerial parts of P. harmala were collected around Isfahan province, Iran. The plant was taxonomically identified by botanists in the Department of Biology (Shiraz University, Shiraz, Iran). An extract of P. harmala was prepared from the seeds of the plant according to the method described by Manske and Holmes (1952). The finally resulting concentrated extract was sterilized by ultraviolet then dried below 70℃ in an oven.
Treatment with the extract containing the alkaloids of P. harmala was performed intramuscularly at a dose of 5 mg/kg body weight once daily for 5 days (Mirzaiedehaghi, 2006). All cattle with rectal temperatures ≥ 39.5℃ and diagnosed positive for T. annulata schizonts in lymph node biopsy smears were treated with the extract of P. harmala.
RESULTS
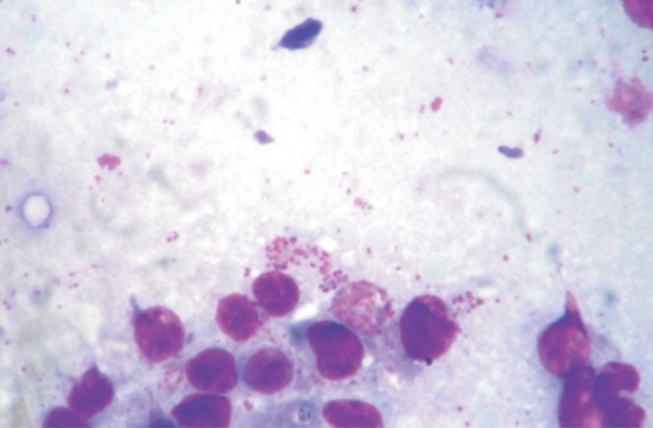
Lymph node biopsy smears from enlarged prescapular glands showed schizonts of T. annulata (Fig. 2) before treatment but 15 ± 3 days after beginning of the treatment most of cattle (39 cases) did not show schizonts of T. annulata.
Fig. 2.
A lymph node smear showing Theileria annulata schizonts in lymphoblastoid cells. Giemsa stain. x 1,000.
The disease symptoms and parasite existence in most of cattle (39 cases) disappeared, and all animals recovered 15 ± 3 days after beginning of the treatment. The parasitemia and the body temperature in most of cattle (39 cases) were reduced after the treatment, and finally they didn't show any parasitemia and had normal body temperature 15 ± 3 days after beginning of the treatment. Clinical symptoms in 11 cattle became severe and resulted in their death. Also parasitemia, the body temperature, and schizonts of T. annulata in these cattle were raised and finally caused their death.
Thirty-nine (78%) out of 50 infected cattle responded to the treatment with the extract of P. harmala, and 11 (22%) of 50 infected cattle did not respond to the treatment with the extract of P. harmala and died (Table 2).
Table 2.
Number of cattle treated with Peganum harmala extract at an early stage of Theileria annulata infection, doses used and outcome of treatment
DISCUSSION
Therapeutic evaluations for medicinal plants are essential because of the growing interests in alternative therapies and the therapeutic use of natural products. Natural products have potential in the search for new and selective agents for the treatment of important tropical diseases caused by protozoans (Wright and Phillipson, 1990). Natural products can be lead to compounds, allowing the design and rational planning of new drugs, biomimetic synthesis development, and discovery of new therapeutic properties that are not yet attributed to known compounds (Hamburger and Hostettmann, 1991).
In the present study, we report the antitheilerial activity of P. harmala extract on T. annulata. This activity represents an exciting advance in the search for antitheilerial agents from natural sources, since significant effects against the protozoan were demonstrated. Cattle treated with the extract of P. harmala were considered as "cured" when their body temperature returned to normal and schizonts were not detectable for 3 consecutive days. The results indicate therapeutic effects of the extract of P. harmala for the treatment of tropical theileriosis. Though researches have been rarely performed on the treatment of tropical theileriosis with the extract of P. harmala in cattle, the results of the present study were approximately in agreement with the results of other researches which were done on the effects of the total alkaloids of P. harmala for the treatment of tropical theileriosis (Vecherkin et al., 1977; Puzii et al., 1982; Hu et al., 1997; Fan et al., 1997).
The recovery rate of cattle from theileriosis was 78%, but in our previous study on natural malignant theileriosis in sheep, it was 65% (Mirzaiedehaghi, 2006). The reason for this discrepancy in the success rate of these 2 studies could be attributed to the different extracts of P. harmala. In the present study, the extract of seeds was used, whereas, in the previous research, a chloroform extract of the stem and leaves were used. The extract of seeds contains more alkaloids. Also other researches expressed different recovery rates. For example, in a research by Puzii et al. (1982), the recovery rate of total alkaloids of P. harmala on tropical theileriosis was 93%. This difference may have been caused by different application methods, because in the present study the extract of P. harmala was injected to cattle intramuscularly, but other researchers including Puzii et al. (1982) administered it intravenously. Also in the treatment of cattle in the present study, additional treatments were not applied and this can explain the lower cure rate in comparison with the other researchers. Additional treatments were necessary for more cattle to recover and of great importance for the protection of animals from hepatic and renal damage induced by poisonous substances. The poisonous substances were mainly produced by metabolism of the parasites.
Though the antiprotozoan mechanisms of P. harmala extract on T. annulata have been unknown yet, alkaloid compounds well illustrate the diversity of antiprotozoal compounds found in P. harmala plants (Wright and Phillipson, 1990), and among the several alkaloids (harmine, harmaline, harmalol, harman, vasicine and vasicinon) derived from P. harmala extracts, harmaline (harmidine, C13H14N2O) has been found to be the major active alkaloid and quite soluble in dilute acids (Budavari and O'neil, 1996). In the present study, diluted acetic acid was used to extract the alkaloids (Manske and Holmes, 1952), and although not specifically tested by us, it is probable that our extract contained this specific alkaloid, and must have been effective against T. annulata, also we demonstrated that P. harmala extracts showed excellent antitheilerial activities. A cell cycle analysis using flow cytometry suggested that, although harmine interferes with the cell division, it does not induce apoptosis in Leishmania donovani promastigotes. The results using a confocal microscopy supported that the cell death could be attributed to necrosis due to non-specific membrane damage (Lala et al., 2004).
ACKNOWLEDGMENTS
The author wishes to thank Mr. Mansour Aminzadeh for his technical assistance and Mrs. Diane Simpson for editing this article.
References
- 1.Budavari S, O'neil MJ. The Merk Index. 12nd ed. New Jersey, USA: CRC Press; 1996. pp. 4644–4645. [Google Scholar]
- 2.Fan B, Liang J, Men J, Gao F, Li G, Zhao S, Hu T, Dang P, Zhang L. Effect of total alkaloid of Peganum harmala L. in the treatment of experimental haemosporidian infections in cattle. Trop Anim Health Prod. 1997;29(4, suppl):77S–83S. doi: 10.1007/BF02632937. [DOI] [PubMed] [Google Scholar]
- 3.Hamburger M, Hostettmann K. Bioactivity in plants: the link between phytochemistry and medicine. Phytochemistry. 1991;30:3864–3874. [Google Scholar]
- 4.Hashemi-Fesharki R. Chemotherapeutic value of parvaquone and buparvaquone against Theileria annulata infection of cattle. Res Vet Sci. 1991;50:204–207. doi: 10.1016/0034-5288(91)90107-y. [DOI] [PubMed] [Google Scholar]
- 5.Hu T, Fan B, Liang J, Zhao S, Dang P, Gao F, Dong M. Observations on the treatment of natural haemosporidian infections by total alkaloid of Peganum harmala L. in cattle. Trop Anim Health Prod. 1997;29(4, suppl):72S–76S. doi: 10.1007/BF02632936. [DOI] [PubMed] [Google Scholar]
- 6.Kamel S, Ibrahim L, Afifi A, Hamza S. Major alkaloidal constituents of the Egyptian plant, Peganum harmala. UAR J Vet Sci. 1970;7:71–86. [Google Scholar]
- 7.Lala S, Pramanick S, Mukhopadhyay S, Bandyopadhyay S, Basu MK. Harmine: evaluation of its antileishmanial properties in various vesicular delivery systems. J Drug Target. 2004;12:165–175. doi: 10.1080/10611860410001712696. [DOI] [PubMed] [Google Scholar]
- 8.Manske RHF, Holmes HL. The Alkaloids: Chemistry and Pharmacology. New York, USA: Academic Press; 1952. p. 393. [Google Scholar]
- 9.Mbwambo HA, Sudi FF, Mkonyi PA, Mfinanga JM, Mella ES, Ngovi CJ. Comparative studies of the efficacy of parvaquone and parvaquone-plus-frusemide in the treatment of Theileria parva infection (East Coast fever) in cattle. Vet Parasitol. 2002;108:195–205. doi: 10.1016/s0304-4017(02)00195-4. [DOI] [PubMed] [Google Scholar]
- 10.0McHardy N, Wekesa LS, Hudson AT, Randall AW. Antitheilerial activity of BW720C (buparvaquone): a comparison with parvaquone. Res Vet Sci. 1985;39:29–33. [PubMed] [Google Scholar]
- 11.Mirzaiedehaghi M. Treatment of natural ovine malignant theileriosis with a chloroform extract of the plant Peganum harmala. Onderstepoort J Vet Res. 2006;73:153–155. [PubMed] [Google Scholar]
- 12.Purnell RE. Theileria annulata as a hazard to cattle in countries on the northern Mediterranean littoral. Vet Sci Commun. 1978;2:3–10. [Google Scholar]
- 13.Puzii AD, Serov VM. Persistence of pegarmin (a preparation of the alkaloids of Peganum harmala) in the body (of cattle) Veterinariia. 1983;(No. 5):62–64. [Google Scholar]
- 14.Puzii AD, Vecherkin SS, Toptaev VI, Tsyganova GA, Duisheev NA. Tests for teratogenic and embryotoxic properties of pegarmin (alkaloid of the plant Peganum) and its therapeutic use in cattle infected with Theileria annulata. Trudy Vsesoyuznogo Instituta Eksperimental' noi Veterinarii. 1982;56:31–38. [Google Scholar]
- 15.Vecherkin SS, Puzii AD, Romakhov VG, Tribunskii MP. Harmal alkaloids in theileriasis. Veterinariia. 1977;(No. 10):77–78. [PubMed] [Google Scholar]
- 16.Wright CW, Phillipson JD. Natural products and the development of selective antiprotozoal drugs. Phytother Res. 1990;4:127–139. [Google Scholar]