Abstract
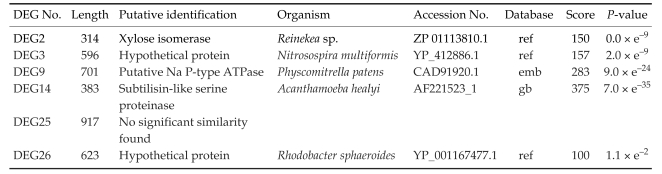
To examine the expressed gene profile during encystation of Acanthamoeba castellanii Castellani, we used differentially expressed gene (DGE) screening by RT-PCR with 20 sets of random primers. From this analysis, we found that approximately 16 genes showed upregulation during encystation. We chose 6 genes, which had relatively higher expression levels, for further investigation. Based on homology search in database, DEG2 showed 55% of similarity with xylose isomerase, DEG9 showed 37% of similarity with Na P-type ATPase, and DEG14 showed 77% of similarity with subtilisin-like serine proteinase. DEG3 and DEG26 were identified as hypothetical proteins and DEG25 exhibited no significant similarity to any known protein. Encystation of Acanthamoeba has been suggested to be a process to resist adverse environmental or nutritional conditions. Further characterization studies of these genes may provide us with more information on the encystation mechanism of Acanthamoeba.
Keywords: Acanthamoeba, differentially expressed gene, encystation
Acanthamoeba is an opportunistic pathogen responsible for several diseases in humans, such as granulomatous amebic encephalitis (GAE), amebic keratitis and dermatitis (Marciano-Cabral and Carbral, 2003). Acanthamoeba comprises 2 distinct stages, i.e., trophozoite and cyst. The trophozoite is the vegetative form, which conducts movement, phagocytosis, metabolism, and cell division (Baumann and Murphy, 1995; Wang and Ahearn, 1997). The cyst is formed from a trophozoite, when desiccation, starvation, or other adverse condition prevails, and is conversed to the trophozoite, when conditions are favorable (Weisman, 1976; Cordingley et al., 1996). Because trophozoites can transform to cysts under adverse conditions, such as host immune responses or chemical treatments in human infections (McClellan et al., 2002), it is difficult to treat the ameba infection with almost all kinds of antibiotics. If it is possible to understand the molecular mechanisms of encystation and to disrupt its process, it will be easier to treat the infection. It will also be applicable to drug development against other cyst forming pathogenic protozoa. To compare the gene expression between trophozoites and cysts, we conducted differentially expressed gene screening with Acanthamoeba castellanii Castellani (American Type Culture Collections; ATCC #30011, Beltsville, Maryland, USA).
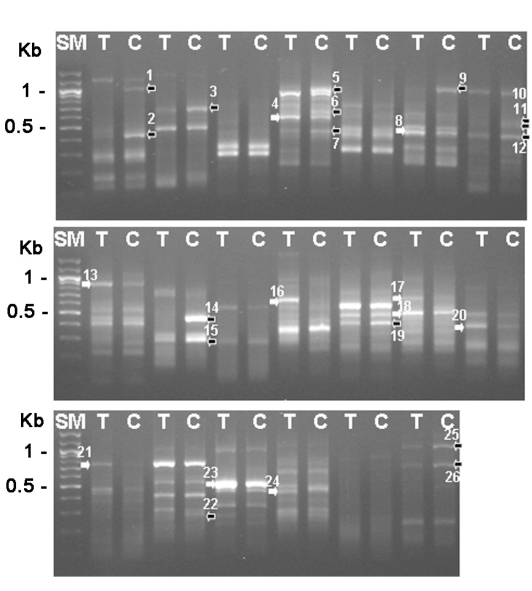
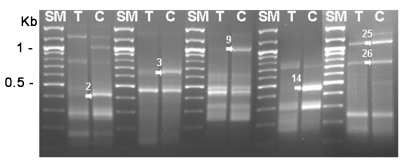
Acanthamoeba was obtained from ATCC and was cultured axenically in PYG medium and induced encystation by the procedure of Bowers and Korn (1969). The total RNAs were purified from trophozoites and 3-day cysts, and their cDNAs were made, which was followed by the differentially expressed gene (DEG) screening with 20 sets of random primers (Seegen, Seoul, Korea). From this analysis between trophozoites and cysts, we found that approximately 16 genes were up-regulated and 10 genes were down-regulated during encystation (Fig. 1). Then, 6 genes (DEG2, 3, 9, 14, 25 and 26) showing significant up-regulation were confirmed the differential expression between cysts and trophozoites by DEG analysis again (Fig. 2). The expression pattern of these genes may suggest roles of the gene products in the encystation of Acanthamoeba. The genes were sequenced followed by submission for blast search (Table 1). Based on blast search results, the DEG2 protein showed 55% sequence similarity with xylose isomerase, DEG9 showed 37% similarity with Na P-type ATPase, DEG14 showed 77% similarity with subtilisin-like serine proteinase. DEG3 and DEG26 were identified as hypothetical proteins and DEG25 exhibited no significant similarity to any known protein at database.
Fig. 1.
Gel electrophoresis analysis with differentially expressed gene screening results (26 genes). SM; 100 bp ladder, T; trophozoite, C; 3-day cyst, black arrows; reduced genes during encystation, white arrows; increased genes during encystation.
Fig. 2.
Re-amplification of DEG2, 3, 9, 14, 25 and 26 between cysts and trophozoites. SM; 100 bp ladder, T; trophozoite, C; 3-day cyst, arrows; increased genes during encystation.
Table 1.
Significant matches of DEGs with database sequences of other organisms
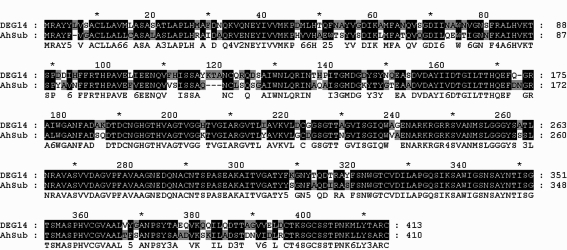
Interestingly, DEG14 showed significant sequence similarity with subtilisin-like serine proteinase (AhSub), which was reported to be a secretory enzyme and a potential virulence factor (Hong et al.,2000). Until now, many proteinases of Acanthamoeba, such as serine, cysteine, and metallo-proteinases, have been suggested to be involved in the pathogenesis or phagocytosis (Mitro et al., 1994; Hong et al., 2000; Kong et al., 2000; Hong et al., 2002; Kim et al., 2006; Serrano-Luna et al., 2006). We identified full length ORF of DEG14 of A. castellanii by RACE-PCR. Based on the sequence analysis, DEG14 belonged to the serine protease family subtilisin group (data not shown). The deduced amino acid sequence of DEG14 showed 76% similarity with that of AhSub (Fig. 3). Here, we identified 6 genes induced by encystations, while its expression in the trophozoite is very low or absent. Further studies on these genes may provide more understanding on the encystation mechanisms of Acanthamoeba.
Fig. 3.
Comparision of the deduced amino acid sequences of DEG14 with AhSub (subtilisin-like serine proteinase).
Footnotes
This work was supported by the Korea Research Foundation Grant funded by Korea Government (MOEHRD, Basic Research Promotion Fund) (KRF-2005-204-E00027) and the Brain Korea 21 Project in 2007.
References
- 1.Baumann O, Murphy DB. Microtubule-associated movement of mitochondria and small particles in Acanthamoeba castellanii. Cell Motil Cytoskeleton. 1995;32:305–317. doi: 10.1002/cm.970320407. [DOI] [PubMed] [Google Scholar]
- 2.Bowers B, Korn ED. The fine structure of Acanthamoeba castellanii (Neff strain). II. Encystment. J Cell Biol. 1969;41:786–805. doi: 10.1083/jcb.41.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordingley JS, Wills RA, Villemez CL. Osmolarity is an independent trigger of Acanthamoeba castellanii differentiation. J Cell Biochem. 1996;61:167–171. doi: 10.1002/(SICI)1097-4644(19960501)61:2%3C167::AID-JCB1%3E3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Hong YC, Hwang MY, Yun HC, Yu HS, Kong HH, Yong TS, Chung DI. Isolation and characterization of a cDNA encoding a mammalian cathepsin L-like cysteine proteinase from Acanthamoeba healyi. Korean J Parasitol. 2002;40:17–24. doi: 10.3347/kjp.2002.40.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong YC, Kong HH, Ock MS, Kim IS, Chung DI. Isolation and characterization of a cDNA encoding a subtilisin-like serine proteinase (ahSUB) from Acanthamoeba healyi. Mol Biochem Parasitol. 2000;111:441–446. doi: 10.1016/s0166-6851(00)00326-1. [DOI] [PubMed] [Google Scholar]
- 6.Kim WT, Kong HH, Ha YR, Hong YC, Jeong HJ, Yu HS, Chung DI. Comparison of specific activity and cytopathic effects of purified 33 kDa serine proteinase from Acanthamoeba strains with different degree of virulence. Korean J Parasitol. 2006;44:321–330. doi: 10.3347/kjp.2006.44.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong HH, Kim TH, Chung DI. Purification and characterization of a secretory serine proteinase of Acanthamoeba healyi isolated from GAE. J Parasitol. 2000;86:12–17. doi: 10.1645/0022-3395(2000)086[0012:PACOAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClellan K, Howard K, Mayhew E, Niederkorn J, Alizadeh H. Adaptive immune responses to Acanthamoeba cysts. Exp Eye Res. 2002;75:285–293. [PubMed] [Google Scholar]
- 10.Mitro K, Bhagavathiammai A, Zhou OM, Bobbett G, McKerrow JH, Chokshi R, Chokshi B, James ER. Partial characterization of the proteolytic secretions of Acanthamoeba polyphaga. Exp Parasitol. 1994;78:377–385. doi: 10.1006/expr.1994.1041. [DOI] [PubMed] [Google Scholar]
- 11.Serrano-Luna Jde J, Cervantes-Sandoval I, Calderon J, Navarro-Garcia F, Tsutsumi V, Shibayama M. Protease activities of Acanthamoeba polyphaga and Acanthamoeba castellanii. Can J Microbiol. 2006;52:16–23. doi: 10.1139/w05-114. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Ahearn DG. Effect of bacteria on survival and growth of Acanthamoeba castellanii. Curr Microbiol. 1997;34:212–215. doi: 10.1007/s002849900170. [DOI] [PubMed] [Google Scholar]
- 13.Weisman RA. Differentiation in Acanthamoeba castellanii. Annu Rev Microbiol. 1976;30:189–219. doi: 10.1146/annurev.mi.30.100176.001201. [DOI] [PubMed] [Google Scholar]