Abstract
Protozoan parasites of the genus Leishmania cause a number of important human diseases. One of the key determinants of parasite infectivity and survival is the surface glycoconjugate lipophosphoglycan (LPG). In addition, LPG is shown to be useful as a transmission blocking vaccine. Since culture supernatant of parasite promastigotes is a good source of LPG, we made attempts to characterize functions of the culture supernatant, and membrane LPG isolated from metacyclic promastigotes of Leishmania major. The purification scheme included anion-exchange chromatography, hydrophobic interaction chromatography and cold methanol precipitation. The purity of supernatant LPG (sLPG) and membrane LPG (mLPG) was determined by SDS-PAGE and thin layer chromatography. The effect of mLPG and sLPG on nitric oxide (NO) production by murine macrophages cell line (J774.1A) was studied. Both sLPG and mLPG induced NO production in a dose dependent manner but sLPG induced significantly higher amount of NO than mLPG. Our results show that sLPG is able to promote NO production by murine macrophages.
Keywords: Leishmania major, soluble LPG, membrane LPG, nitric oxide, macrophages
INTRODUCTION
Protozoan parasites of genus Leishmania cause a number of important human diseases including cutaneous, mucocutaneous and visceral leishmaniasis. During their digenetic life cycle, these parasites alternate between an extracellular promastigote stage and an intracellular amastigote stage that reside within the phagolysosome compartment of macrophages in mammalian hosts (Descoteaux and Turco, 2002; Handman and Bullen, 2002).
One of the key determinants of parasite infectivity and survival in these environments is the glycoconjugate lipophosphoglycan (LPG). LPG from all Leishmania species has same structure and composed of a repeating phosphorylated disaccharide unit attached via a phosphosaccharide core to the phosphatidylinositol, 1-O-alkyl-2-lysophosphatidylinositol (McConville et al., 1992; Turco and Descoteaux, 1992).
New strategies for control of leishmaniasis are needed since chemotherapy such as antimonial drugs is prolonged, expensive, associated with side effects and relapses. Vector control has limitations and a vaccine which may be the best approach is not available. LPG has several functions and has been implicated in binding of parasites to epithelial cells of the sandfly mid-gut (Davies et al., 1990), receptor mediated phagocytosis of macrophages (Sacks, 1989), toll-like receptor 2 (TLR2) signaling (de Veer et al., 2003) and stimulation of NK cells (Becker et al., 2003). Therefore, its efficacy as a transmission blocking vaccine has been proposed (Toui et al., 2001).
It has long been shown that promastigotes of 3 species Leishmania mexicana, L. donovani and L. major shed LPG into the culture medium by release of micelles from the cell surface. Like the cell-associated LPG, culture supernatant LPG is amphiphilic and composed of a lysoalkylphosphatidylinositol-phosphosaccharide core connected to species-specific phosphosaccharide repeats and oligosaccharide caps. All these 3 species release hydrophilic phosphoglycan (Ilg et al., 1994). Since purification of LPG from culture medium is easier than from the parasite membrane it is reasonable to use the culture supernatant for LPG preparation as the source of this antigen for vaccine studies. However, due to some structural differences between cell-associated and culture supernatant LPG it is necessary to assess immunological properties of these 2 forms of LPGs.
Therefore, we assessed functional properties of parasite membrane isolated LPG (mLPG) and culture supernatant LPG (sLPG). The effect of LPGs on nitric oxide (NO) production was studied on a murine macrophage cell line, J774.1A. The functional difference between these 2 forms of LPG is discussed.
MATERIALS AND METHODS
Parasite culture
The strain of L. major used in this study was the vaccine strain (MRHO/IR/75/ER). The infectivity of the parasites was maintained by regular passage in susceptible BALB/c mice. The parasites were cultured in the RPMI-1640 medium (Sigma, Chemical Co., St. Louis, U.S.A.) supplemented with 10% FBS (Sigma), 292 µg/ml L-glutamine (Sigma) and 4.5 mg/ml glucose. The starting parasite inoculum was 1 × 106/ml. Under these culture conditions, the stationary phase of parasite growth was obtained in 6 days as determined by peanut agglutination assay.
Membrane LPG purification
Membrane LPG (mLPG) from 1011 L. major promastigotes was extracted and purified as previously described (McConville and Bacic, 1988). Briefly, the cell membrane was extracted sequentially with 10 ml of each solvent at 4℃ as follows: chloroform/methanol/water (3:2:1), 4 mM MgCl2, and chloroform/methanol/water (10:10:3). LPG was extracted from the resulting de-lipidated residue fraction by 4 extractions at 4℃ with 10 ml of solvent E (water: ethanol: ethyl ether: pyridine: ammonium hydroxide, 420:420:140:28:0.5). The solvent E extract was dried under reduced pressure, re-suspended in 5% 1-propanol/200 mM ammonium acetate, and applied to a column of octyl-Sepharose FF (12 × 3 cm, Pharmacia) and eluted with 50% 1-propanol. After purification, the purity was analyzed by SDS-PAGE/periodate silver nitrate staining (for glycolipid detection), Coomassie blue staining (for protein detection) and thin layer chromatography (TLC). Lipopolysaccharide (LPS) was used as standard. The contents of phosphate, protein and glycan were also determined.
Soluble LPG purification
Soluble LPG was purified by ion-exchange chromatography, hydrophobic interaction chromatography and cold methanol precipitation from culture medium (Ilg et al., 1996). Briefly, 5 liters of L. major promastigote culture medium were loaded onto a DEAE-sepharose FF column (12 × 3 cm, Pharmacia) with flow rate of 1 ml/min. The column was washed with 200 ml of 100 mM NaCl, 20 mM Tris-HCl, pH 7.5. The elution was performed by a linear gradient of 100-500 mM NaCl in 20 mM Tris-HCl, pH = 7.5, at a flow rate of 0.5 ml/min. Phosphoglycan was detected in the eluate by phosphate and protein determination and TLC.
Fractions containing phosphoglycan were pooled, adjusted with 1 M ammonium sulfate and loaded onto an octyl-sepharose FF column (12 × 3 cm, Pharmacia). After washing with 60 ml ammonium sulfate, the bounded LPG was eluted with 50% 1-propanol. Again, the fractions were analyzed for the presence of LPG as described above.
Since the octyl-sepharose bound material was eluted with 50% 1-propanol, further ultra-filtration (Amicon, 10000) was done. The fraction was treated with proteinase K (10 µg/ml, 18 hr, 37℃) and precipitated with cold methanol (-20℃, 18 hr). The pellet contained LPG. After purification, the purity was analyzed by SDS-PAGE/periodate silver nitrate staining (for glycolipid detection), Coomassie blue staining (for protein detection) and TLC. LPS was used as standard. The contents of phosphate, protein and glycan were also determined.
Biochemical analysis
Protein was determined by Bradford method. Phosphate was determined by the Ames method (Ames, 1966). Glycan contents were determined by phenol-sulfuric acid method (Rao and Pattabiraman, 1989). Discontinuous SDS-PAGE was performed on 4% stacking and 14% separating gel according to Leammli method. After electrophoresis, the gels were stained with periodate/silver nitrate (Tsai and Frasch, 1982) and Coomassie blue.
Thin layer chromatography (TLC)
Samples were spotted on silica gel 60 TLC plate (aluminum backed) and developed with solvent E (water: ethanol: ethyl ether: pyridine: ammonium hydroxide, 420:420:140:28:0.5) and then detected with α- naphthol/sulfuric acid at 100℃ for 15 min.
LAL test
In order to indicate the presence of gram negative bacterial endotoxin (LPS), Limulus amoebocyte lysate (LAL) assay was performed based on manufacturer's instruction (LAL Kit, Charles Riever Endosafe, T2092 CTK7, U.S.A.).
Macrophage culture and NO measurement
The murine macrophage cell line (J774.1A) was obtained from cell bank of Institute Pasteur of Iran. The cells were cultured in DMEM medium containing 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 10% heat-inactivated fetal calf serum.
Adherent macrophages were scraped from flask and washed with warm medium (25℃). Cells were counted and their viability was determined by trypan blue dye exclusion. J774.1A cells were cultured in 24 well plates (106 cells/ml, 1 ml/well) and incubated in 37℃, 5% CO2 for 18 hr to adhere and non-adherent cells were removed. mLPG and sLPG at different concentrations in absence and presence of 0.2 mM L-NMMA (iNOS inhibitor) were added to some wells (Leiw et al., 1997). The supernatants were collected after 48 hr and NO production was determined by Griess reagent (Miranda et al., 2001). Briefly, to 100 µl of culture medium, 100 µl of vanadium chloride (III) and 50 µl of Griess reagents [1:1 (v/v) of 0.1% naphthylethylenediaminedihydrochloride (NED) in H2O + 2% sulphanilamide in 5% H3PO4] was added and incubated at 37℃ for 40 min and the absorbance was read at 540 nm.
Statistical analysis
Statistical significance (P < 0.05) was analyzed by Student's t-test using SPSS version 10.
RESULTS
mLPG purification
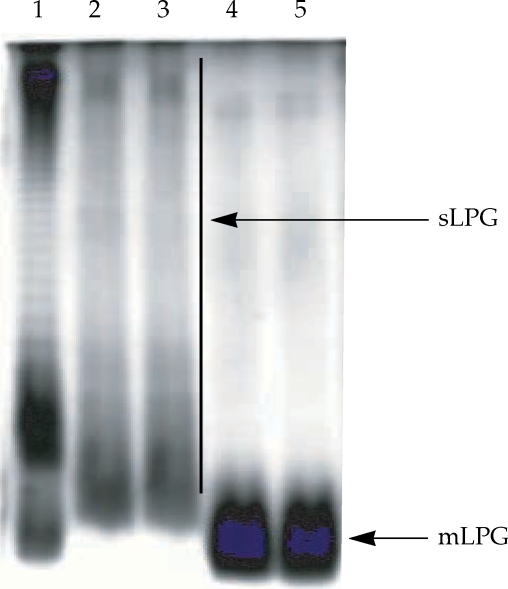
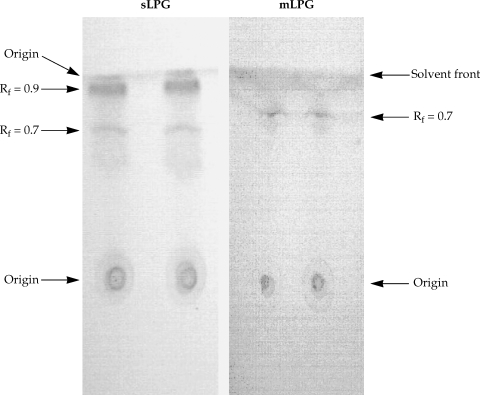
The L. major mLPG was extracted and purified by solvent extraction, hydrophobic interaction chromatography and cold methanol precipitation. The efficiency of purification was analyzed by SDS-PAGE and TLC. One short smear at the end of gel was observed in SDS-PAGE/silver nitrate stained gel (Fig. 1) but no band was observed when the gel stained with Coomassie blue. In TLC plate, one band at Rf = 0.7 was observed (Fig. 2). The total amount of glycan and phosphate was calculated and showed that from 1011 promastigotes 10 mg of mLPG was obtained. The total amount of phosphate was 3.8 mg. Therefore, the ratio of glycan/phosphate was approximately 2.62.
Fig. 1.
Gel electrophoresis profile of purified mLPG and sLPG from Leishmania major promastigotes. Purified mLPG and sLPG (10 µg) were subjected to SDS-PAGE in 14% separating gel and stained with periodate/silver nitrate. The wells are loaded as follows: Lane 1 by LPS (from Salmonella, Sigma) as standard. Lanes 2-3 by sLPG. Lanes 4-5 by mLPG. Since LPG is not a globular molecule, ordinary molecular weight markers containing proteins are not a good reference. Therefore, LPS was used as control.
Fig. 2.
Thin layer chromatography of Leishmania major sLPG and mLPG. After extraction of mLPG and sLPG with octylsepharose and methanol precipitation, 10 µg of sample were chromatographed on silica gel 60 in solvent E then detected by α- naphthol /H2SO4 at 100℃ for 15 min.
sLPG purification
Soluble LPG from culture medium was purified with ion-exchange chromatography, hydrophobic interaction chromatography and cold methanol precipitation. The efficiency of purification was analyzed by SDS-PAGE and TLC. In the SDS-PAGE/silver nitrate staining, one broad smear from top of gel to the end was observed (Fig. 1). By TLC, one band at Rf = 0.9 and one band at Rf = 0.7 were detected (Fig. 2).
In addition the amount of glycan and phosphate of sLPG was determined. The amount of glycan in sLPG preparation was 50 mg and the amount of phosphate was 5.4 mg. Thus the ratio of glycan/phosphate was 9.26.
On the basis of silver staining, there is significant molecular weight difference between mLPG and sLPG that is due to glycan portion since the ratio of glycan/phosphate in mLPG and sLPG were 2.62 and 9.26, respectively.
Endotoxin determination of sLPG and mLPG
The results of LAL test showed that these samples contain less than 300 pg endotoxin per milligram of phosphoglycan (permissive level of LPS for biological sample; 1 ng/ml).
Effects of sLPG and mLPG on nitric oxide production
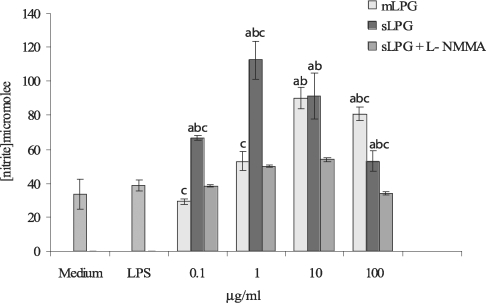
The macrophage cell line J774.1A was cultured in DMEM medium and stimulated with various concentrations of LPG (0.1, 1, 10, 100 µg/ml). After 48 hr incubation, the supernatants were collected and analyzed for NO production. We observed that mLPG induced NO production in a dose dependent manner (Fig. 3). The optimal concentration of mLPG for NO production was 10 µg/ml. NO production was increased up to the optimum dose and then decreased with high concentration of mLPG. The same pattern of NO production was demonstrated with sLPG but the optimal concentration of sLPG for NO production was 1 µg/ml (Fig. 3). In order to show the specificity of NO production, iNOS inhibitor was added to some wells containing sLPG or mLPG. In the presence of the inhibitor, NO production was completely blocked.
Fig. 3.
Effect of Leishmania major mLPG and sLPG on NO production in J774.1A macrophage cell line. Macrophages were cultured (106 cell/ml) in DMEM medium in the presence of LPG (0.1, 1, 10 and 100 µg/ml) alone and with L-NMMA (2 mM) as nitric oxide synthase inhibitor. After 48 hr supernatant were analyzed for NO production by Griess reagent. The bars are mean +/- SD for three times study. Letters indicate significant differences at P < 0.05. a: compared with untreated cells (medium). b: compared with LPS treated cells. c: sLPG treated cells compared with mLPG treated cells.
It is noteworthy that sLPG showed more stimulatory effect on NO production and the differences between sLPG and mLPG was found to be statistically significant (P < 0.05).
DISCUSSION
Lipophosphoglycan is the major macromolecule on the promastigote surface and is thought to play a key role in immunity against Leishmania. Unraveling the ways, in which the structures of the LPG are modified in different developmental stages, has provided insights into the structure-function relationship of these molecules and has also suggested important roles for Leishmania LPG. The most striking differences in LPG structure between Leishmania species lie in the phosphorylated oligosaccharide repeats and their side chains (Descoteaux and Turco, 2002; Handman and Bullen, 2002). As judged by SDS-PAGE, LPG is a poly-disperse family of molecules, with molecular weight ranging from 5 to 40 kDa (McConville et al., 1992; Turco and Descoteaux, 1992). In this study, SDS-PAGE data indicated that mLPG was observed as a short smear at the end of gel, however, sLPG was observed as a broad smear from the top to the end of gel which could be due to longer glycan portion of sLPG compared to mLPG. Our compositional analysis of mLPG and sLPG indicated that they are very similar. The only clear difference was the smaller repeat unit number for mLPG compared with sLPG, which is consistent with the previous report (McConville et al., 1992).
Based on the glycan component, 1011 parasite promastigotes yielded 10 mg mLPG, however, the yield of the culture medium spent for the above number of parasite promastigotes was 50 mg sLPG. Therefore, culture medium is a good source for LPG preparation.
There is now a good clinical and experimental body of evidence that control of cutaneous leishmaniasis is obtained through the following circuit: activated macrophages produce IL-12 which drives Th1 cell differentiation and proliferation. Th1 cells produce IFN-γ which activates macrophages to kill Leishmania parasites through NO production. It is well documented that NO production is the key step in leishmaniacidal activity of murine macrophages (Leiw et al., 1997; Ropert and Gazzinelli, 2000; Balaraman et al., 2004). Therefore, in the present study, we measured NO production by J774.1A cell line in response to LPG. However, it has been previously indicated that Leishmania directly inhibits the synthesis of NO and indirectly block the development of Th1 cells via the inhibition of IL-12 synthesis by macrophages stimulated with IFN-γ and LPS in a dose-dependent manner. All the inhibitory effect lies on the alkylglycerol fraction (Proudfoot et al., 1996; Cunningham, 2002; Olivier et al., 2005). In contrast, LPG, especially phosphoglycan (PG) induces a large amount of NO when added together with or soon after IFN-γ priming, however, the individual repeats and lipid core are not effective (Proudfoot et al., 1996).
Apparently, the intact structure of LPG is important for synergy with IFN-γ and also for binding of L. major to macrophages. By using synthetic fragments of PG it has been shown that the longer PG fragment is more able to synergize with IFN-γ to induce NO synthesis (Proudfoot et al., 1996). Our study demonstrates that both of the membrane and culture supernatant LPG promote NO production by murine macrophages. It is noteworthy that sLPG has higher stimulatory effect on NO synthesis than mLPG which is probably due to the higher number of glycan repeat units. Therefore, this finding is consistent with results of previous studies which showed that the larger LPG has higher stimulatory effect on the NO synthesis than the smaller LPG (Proudfoot et al., 1996).
Thus, LPG of Leishmania can strongly regulate the expression of iNOS and the leishmanicidal activity of murine macrophages. Glycosilphosphatidylinositol (GIPL) inhibits the induction of NO synthesis by macrophages, thereby contributes to the survival of the parasite. However, the host masks LPG to enhance NO synthesis. Finally, the parasite sheds mLPG as soon as it enters the macrophages to inhibit subsequent NO synthesis by the host (Proudfoot et al., 1995).
Nonetheless, LPG would be a promising transmission blocking vaccine candidate against leishmaniasis since LPG purified from Leishmania promastigotes was studied for vaccination in animal models. Sandflies which had previously fed on LPG-immunized mice showed the lowest infection rates compared to control sandflies fed on saline immunized mice (P < 0.05). A significant number of procyclic promastigotes, the first developmental form of the parasite in culture as well as in the sandfly was observed in sandflies which fed on LPG-immunized mice (P < 0.05) (Toui et al., 2001). Therefore, it is reasonable to postulate that sLPG would be a vaccine candidate.
In conclusion, mLPG and sLPG of L. major have the same biochemical content but with different molecular weights which may be due to the difference of the number of repeat units. sLPG is a stronger stimulator for NO synthesis in murine J774.1A cell lines than mLPG.
References
- 1.Ames BN. Assay of inorganic phosphate, total phosphate and phosphatases. Methods in Enzymol. 1966;8:115–118. [Google Scholar]
- 2.Balaraman S, Tewary P, Singh VK, Madhubala R. Leishmania donovani induces interferon regulatory factor in murine macrophages: a host defense response. Biochem Biophys Res Commun. 2004;317:639–647. doi: 10.1016/j.bbrc.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 3.Becker I, Salaiza N, Aguirre M, Delagado J, Carrillo-Carrasco N, Kobeh LG, Ruiz A, Cervantes R, Torres AP, Cabrera N, Gonzalez A, Maldonado C, Isibasi A. Leishmania lipophosphoglycan activates NK cells through toll-like receptor-2. Mol Biochem Parasitol. 2003;130:65–74. doi: 10.1016/s0166-6851(03)00160-9. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham AC. Parasitic adaptive mechanisms in infection by Leishmania. Exp Mol Pathol. 2002;72:132–141. doi: 10.1006/exmp.2002.2418. [DOI] [PubMed] [Google Scholar]
- 5.Davies CR, Cooper AM, Peacock C, Lane RP, Blackwell JM. Expresion of LPG and GP63 by different developmental stages of Leishmania major in the sandfly Phlebotomus papatasi. Parasitology. 1990;101:337–343. doi: 10.1017/s0031182000060522. [DOI] [PubMed] [Google Scholar]
- 6.de Veer MJ, Curtis JM, Baldwin TM, DiDonato JA, Sexton A, McConville MJ, Handman E, Schofield L. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and toll-like receptore 2 signalling. Eur J Immunol. 2003;33:2822–2831. doi: 10.1002/eji.200324128. [DOI] [PubMed] [Google Scholar]
- 7.Descoteaux A, Turco SJ. Functional aspects of the Leishmania donovani lipophosphoglycan during macrophage function. Microbes Infect. 2002;4:975–981. doi: 10.1016/s1286-4579(02)01624-6. [DOI] [PubMed] [Google Scholar]
- 8.Handman E, Bullen DV. Interaction of Leishmania with host macrophage. Trends Parasitol. 2002;18:332–334. doi: 10.1016/s1471-4922(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 9.Ilg T, Stierhof YD, Craik D, Simpson R, Handman E, Bacic A. Purification and structural characterization of a filamentous, mucinlike proteophosphoglycan secreted by Leishmania parasites. J Biol Chem. 1996;271:21583–21596. doi: 10.1074/jbc.271.35.21583. [DOI] [PubMed] [Google Scholar]
- 10.Ilg T, Stierhof YD, Wiese M, McConville MJ, Overath P. Characterization of phosphoglycan-containing secretory products of Leishmania. Parasitology. 1994;108:S63–S71. doi: 10.1017/s0031182000075739. [DOI] [PubMed] [Google Scholar]
- 11.Liew FY, Wei XQ, Proudfoot L. Cytokines and nitric oxide as effector molecules against parasitic infections. Philos Trans R Soc Lond B Biol Sci. 1997;352:1311–1315. doi: 10.1098/rstb.1997.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConville MJ, Bacic A. A family of glycoinositol phospholipids from Leishmania major. Isolation, characterization, and antigenicity. J Biol Chem. 1989;264:757–766. [PubMed] [Google Scholar]
- 13.McConville MJ, Turco SJ, Ferguson MA, Sacks DL. Developmental modification of lipophosphoglycan during the differentiation of Leishmania major promastigotes to an infectious stage. EMBO J. 1992;11:3593–3600. doi: 10.1002/j.1460-2075.1992.tb05443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miranda MK, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 15.Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev. 2005;18:293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proudfoot L, Nikoleav AV, Feng GJ, Wei WQ, Ferguson MA, Brimocombe JS, Liew FY. Regulation of the expression of nitric oxide synthase and leishmaniacidal activity by glycoconjugates of Leishmaina lipophosphoglycan in murine macrophages. Proc Natl Acad Sci USA. 1996;93:10984–10989. doi: 10.1073/pnas.93.20.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proudfoot L, O'Donnell CA, Liew FY. Glycoinositolphospholipids of Leishmania major inhibit nitric oxide synthesis and reduce leishmaniacidal activity in murine macrophages. Eur J Immunol. 1995;25:745–750. doi: 10.1002/eji.1830250318. [DOI] [PubMed] [Google Scholar]
- 18.Rao P, Pattabiraman TN. Reevaluation of the phenolsulfuric acid reaction for the estimation of hexoses and pentoses. Anal Biochem. 1989;181:18–22. doi: 10.1016/0003-2697(89)90387-4. [DOI] [PubMed] [Google Scholar]
- 19.Ropert C, Gazzinelli RT. Signaling of immune system cells by glycosylphosphatidylinositol (GPI) anchor and related structures derived from parasitic protozoa. Curr Opin Microbiol. 2000;3:395–403. doi: 10.1016/s1369-5274(00)00111-9. [DOI] [PubMed] [Google Scholar]
- 20.Sacks DL. Metacyclogenesis in Leishmania amastigotes. Exp Parasitol. 1989;69:100–103. doi: 10.1016/0014-4894(89)90176-8. [DOI] [PubMed] [Google Scholar]
- 21.Tonui WK, Mbati PA, Anjili CO, Orago AS, Turco SJ, Githure JI, Koech DK. Transmission blocking vaccine studies in leishmaniasis: I. Lipophosphoglycan is a promising transmission blocking vaccine molecule against cutaneous leishmaniasis. East Afr Med J. 2001;78:84–89. doi: 10.4314/eamj.v78i2.9094. [DOI] [PubMed] [Google Scholar]
- 22.Tsai CM, Frasch CE. A sensitive silver stain for detecting lipopolysaccharide in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 23.Turco SJ, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]