Abstract
Mycophenolic acid, an inhibitor of purine metabolism, was shown to block the replication of vaccinia virus in normal cell lines. This observation led to the development of a dominant one-step plaque selection system, based on expression of the Escherichia coli gpt gene, for the isolation of recombinant vaccinia viruses. Synthesis of xanthine-guanine phosphoribosyltransferase enabled only the recombinant viruses to form large plaques in a selective medium containing mycophenolic acid, xanthine, and hypoxanthine. To utilize the selection system efficiently, we constructed a series of plasmids that contain the E. coli gpt gene and allow insertion of foreign genes into multiple unique restriction endonuclease sites in all three reading frames between the translation initiation codon of a strong late promoter and synthetic translation termination sequences. The selection-expression cassette is flanked by vaccinia virus DNA that directs homologous recombination into the virus genome. The new vectors allow high-level expression of complete or partial open reading frames and rapid construction of recombinant viruses by facilitating the cloning steps and by simplifying their isolation. The system was tested by cloning the E. coli beta-galactosidase gene; in 24 h, this enzyme accounted for approximately 3.5% of the total infected-cell protein.
Full text
PDF
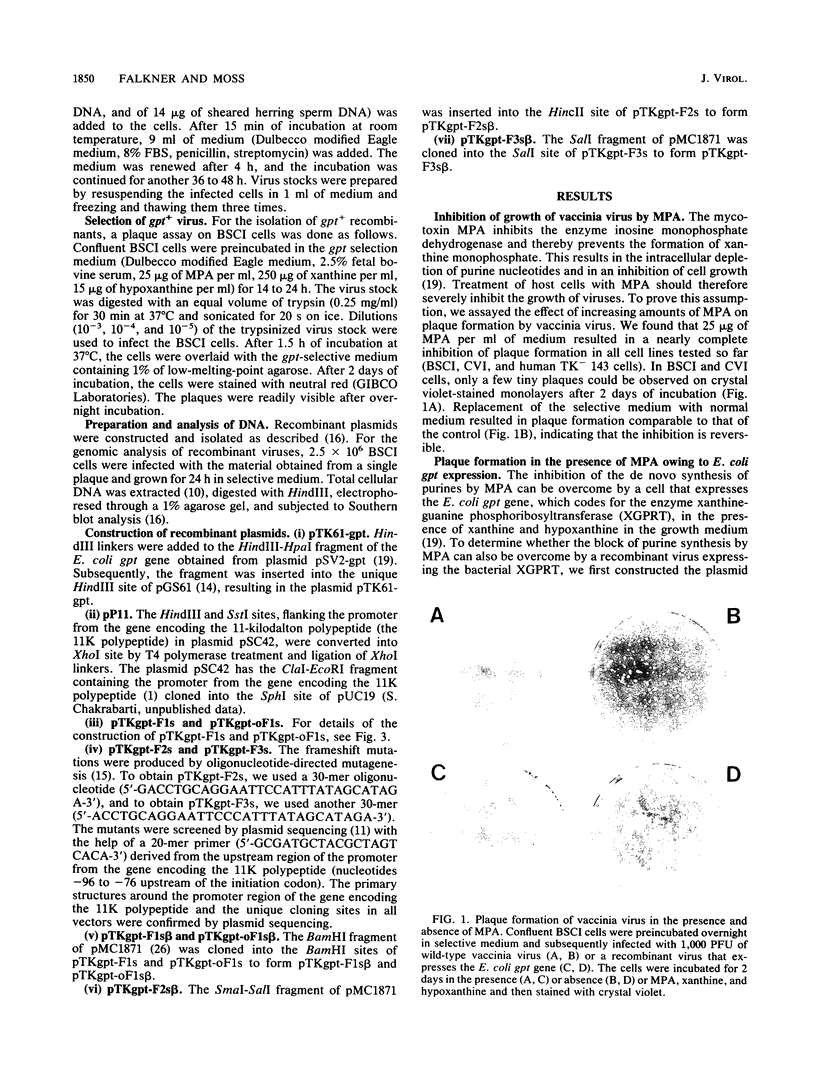

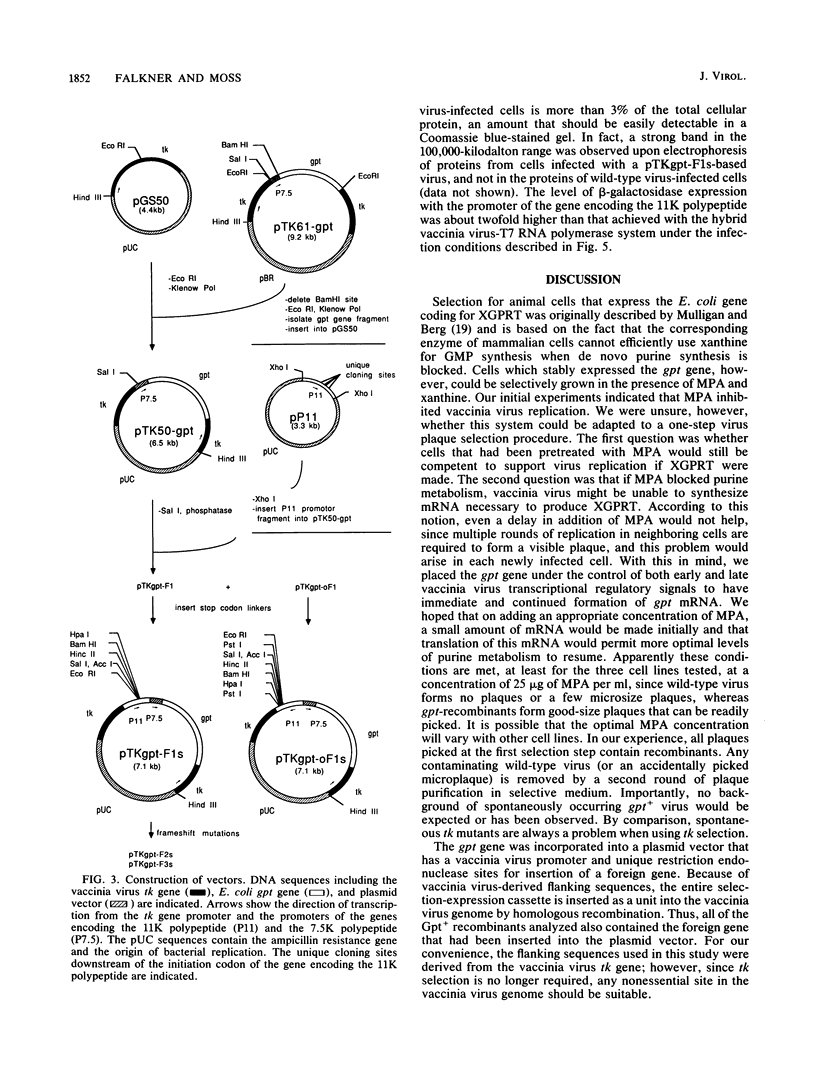

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertholet C., Drillien R., Wittek R. One hundred base pairs of 5' flanking sequence of a vaccinia virus late gene are sufficient to temporally regulate late transcription. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2096–2100. doi: 10.1073/pnas.82.7.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet C., Van Meir E., ten Heggeler-Bordier B., Wittek R. Vaccinia virus produces late mRNAs by discontinuous synthesis. Cell. 1987 Jul 17;50(2):153–162. doi: 10.1016/0092-8674(87)90211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Smith G. L., Cremer K., Notkins A. L., Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. 1985 Oct 31-Nov 6Nature. 317(6040):813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran M. A., Puckett C., Moss B. In vitro mutagenesis of the promoter region for a vaccinia virus gene: evidence for tandem early and late regulatory signals. J Virol. 1985 Apr;54(1):30–37. doi: 10.1128/jvi.54.1.30-37.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C. A., Rice C. M., Strauss J. H., Hruby D. E. Neomycin resistance as a dominant selectable marker for selection and isolation of vaccinia virus recombinants. Mol Cell Biol. 1985 Aug;5(8):1918–1924. doi: 10.1128/mcb.5.8.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Earl P. L., Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987 Jul;7(7):2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L. Vaccinia virus expression vectors. J Gen Virol. 1986 Oct;67(Pt 10):2067–2082. doi: 10.1099/0022-1317-67-10-2067. [DOI] [PubMed] [Google Scholar]
- Mandecki W. Oligonucleotide-directed double-strand break repair in plasmids of Escherichia coli: a method for site-specific mutagenesis. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7177–7181. doi: 10.1073/pnas.83.19.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Flexner C. Vaccinia virus expression vectors. Annu Rev Immunol. 1987;5:305–324. doi: 10.1146/annurev.iy.05.040187.001513. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicali D., Davis S. W., Weinberg R. L., Paoletti E. Construction of live vaccines by using genetically engineered poxviruses: biological activity of recombinant vaccinia virus expressing influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5364–5368. doi: 10.1073/pnas.80.17.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicali D., Grzelecki A., Huang C. Vaccinia virus vectors utilizing the beta-galactosidase assay for rapid selection of recombinant viruses and measurement of gene expression. Gene. 1986;47(2-3):193–199. doi: 10.1016/0378-1119(86)90063-6. [DOI] [PubMed] [Google Scholar]
- Reiss J. Detection of genotoxic properties of mycotoxins with the SOS chromotest. Naturwissenschaften. 1986 Nov;73(11):677–678. doi: 10.1007/BF00366694. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Cho H. Y., Huebner R. J. Non-producer human cells induced by murine sarcoma virus. Int J Cancer. 1975 Jan 15;15(1):23–29. doi: 10.1002/ijc.2910150104. [DOI] [PubMed] [Google Scholar]
- Rosel J. L., Earl P. L., Weir J. P., Moss B. Conserved TAAATG sequence at the transcriptional and translational initiation sites of vaccinia virus late genes deduced by structural and functional analysis of the HindIII H genome fragment. J Virol. 1986 Nov;60(2):436–449. doi: 10.1128/jvi.60.2.436-449.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B., Visca P., Vos J. C., Stunnenberg H. G. Discontinuous transcription or RNA processing of vaccinia virus late messengers results in a 5' poly(A) leader. Cell. 1987 Jul 17;50(2):163–169. doi: 10.1016/0092-8674(87)90212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira S. K., Chou J., Richaud F. V., Casadaban M. J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene. 1983 Nov;25(1):71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- Wittek R., Hänggi M., Hiller G. Mapping of a gene coding for a major late structural polypeptide on the vaccinia virus genome. J Virol. 1984 Feb;49(2):371–378. doi: 10.1128/jvi.49.2.371-378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. F., Moss B. In vitro synthesis of vaccinia virus late mRNA containing a 5' poly(A) leader sequence. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8883–8887. doi: 10.1073/pnas.84.24.8883. [DOI] [PMC free article] [PubMed] [Google Scholar]