Abstract
For treating Leishmania major infection in BALB/c mice, we used thalidomide in conjunction with glucantime. Groups of mice were challenged with 5 × 103 metacyclic promastigotes of L. major subcutaneously. A week after the challenge, drug treatment was started and continued for 12 days. Thalidomide was orally administrated 30 mg/kg/day and glucantime was administrated intraperitoneally (200 mg/kg/day). It was shown that the combined therapy is more effective than single therapies with each one of the drugs since the foot pad swelling in the group of mice received thalidomide and glucantime was significantly decreased (0.9 ± 0.2 mm) compared to mice treated with either glucantime, thalidomide, or carrier alone (1.2 ± 0.25, 1.4 ± 0.3, and 1.7 ± 0.27 mm, respectively). Cytokine study showed that the effect of thalidomide was not dependent on IL-12; however, it up-regulated IFN-γ and down-regulated IL-10 production. Conclusively, thalidomide seems promising as a conjunctive therapy with antimony in murine model of visceral leishmaniasis.
Keywords: Leishmania major, mice (BALB/c), thalidomide, glucantime, cytokine
INTRODUCTION
In recent years, thalidomide has drawn considerable amount of attention from the scientific community because of its immunomodulatory effects (Marriott et al., 1999). Thalidomide administration reduced the number of spread cells among resident macrophages in experimental murine tuberculosis (Arruda et al., 2004). In addition, thalidomide is now being considered as an adjuvant treatment for tuberculosis since it affects cytokine production and T lymphocyte proliferation. Thalidomide elevates the IFN-γ level and modulates several other cytokines as well, noteworthy IL-2 and IL-12. Thalidomide co-stimulates T lymphocytes, with greater effect on CD8+ than on CD4+ T cells. This finding is important, since CD8+ T cells have been shown to be contributory to the protective immune response to Mycobacterium tuberculosis infection (Fu et al., 2002).
The effects of combination therapy with thalidomide and leishmaniacidal drug, glucantime, on the course of disease induced by Leishmania major infection in BALB/c mice are unknown. It has been proposed that visceral leishmaniasis induced in BALB/c mice by the otherwise "dermatotropic" L. major may be a better model of human visceral leishmaniasis (Handman et al., 2001). We chose this model to investigate the effect of thalidomide on the course of visceral leishmaniasis induced by L. major in BALB/c mice. Patients suffering from visceral leishmaniasis come to clinic centers when the disease completely develops. Therefore, in the present study, the infected mice were treated 2 weeks after challenge to see whether thalidomide would affect the course of already established disease. The treatments were performed for 12 days, and the progression of disease was monitored based on measuring foot pad swellings.
MATERIALS AND METHODS
Parasites
Leishmania major promastigotes, MHROM/IR/75/ER, were grown in Schneider's medium supplemented with 10% heat-inactivated FBS, 292 μg/ml L-glutamine, 4.5 mg/ml glucose, 100 μg/ml streptomycin and 100 IU/ml penicilin at 23-25℃ as previously described (Alimohammadian et al., 2002). The parasites were kept in a virulent state by regular passage in susceptible BALB/c mice. Stationary phase promastigotes were harvested and centrifuged at 3,000 rpm for 10 min at 4℃. The pellet was washed 3 times in PBS (8 mM Na2HPO4, 1.75 mM KH2PO4. 0.25 mM KCl, 0.137 mM NaCl).
Mice
Female BALB/c mice (4-6 weeks old) were obtained from the Animal Breeding Stock Facility of Pasteur Institute of Iran, Karaj, Iran. The mice were divided into six groups (8 mice per group). The groups included Group C; control mice non-infected and non-treated, Group P; infected but non-treated, Goup OP; infected treated with sessami oil (carrier of thalidomide), Group TP; infected treated with thalidomide in sessami oil, Group GP; infected treated with glucantime, Group TGP; infected treated with thalidomide and glucantime.
Infection and disease development
The infected groups of mice received 5 × 103 infective stationary phase promastigotes in the hind foot pad subcutaneously (s.c). Every week after challenge the foot pad swelling was measured using a caliper. The data are expressed in units (1 unit = 10-2 cm of the net increase in foot pad thickness).
Drugs administration
Mice treated with glucantime (Marial, France) were received 100 mg/kg/day i.p. Mice treated with thalidomide (a kind gift from Laphal Laboratories, France) were received 30 mg/kg/day orally formulated in sessami oil. The drug administration was started 2 weeks after challenge. This period of time is enough for establishment of the infection. Consequently, administration of drugs was continued for 12 days.
Cell culture condition for cytokine assay
The draining lymph nodes (popliteal lymph nodes) were removed 4 and 7 weeks after challenge and total draining lymph node cells were isolated and washed 3 times with sterile PBS. The cells were resuspended in RPMI supplemented with 2 mM/L-glutamine, 100 U/ml penicillin, 1 mg/ml streptomycin (all from Sigma) and 10% human heat-inactivated fetal calf serum. Cells, 2 × 106 in number in 1 ml of culture medium were plated on flat-bottomed 24-well plates with or without Phorbol Myristate Acetate (PMA, 50 ng/ml tissue culture medium)/Ionomycin (1 μM). The supernatants were removed after 24 hr of incubation and stored at -20℃. We analyzed cytokine production by specific IL-10, IL-12 and IFN-γ enzyme-linked immunosorbent assay (ELISA) (Bender Med Systems, Austria) with a sensitivity of 10 pg/ml.
Statistical analysis
Statistical significance between groups was analyzed by student's t-test using SPSS version 10. Values of P < 0.05 were considered statistically significant.
RESULTS
Progression of disease
Fig. 1 shows the disease progression in different groups of mice. At the end of drug administration (4th week), there was no difference between groups of infected mice P and OP in terms of foot pad swelling (0.47 ± 0.1 mm and 0.43 ± 0.12 mm, respectively). Thickness of infected mice treated with glucantime was 0.2 ± 0.099 mm, thalidomide treated mice was 0.3 ± 0.1 mm, and mice treated with thalidomide and glucantime was 0.29 ± 0.11. At 5th week after challenge (one week after stopping drug administration) foot pad swelling in P and OP started to significantly increase (0.6 ± 0.1 mm and 0.92 ± 0.15 mm, respectively). However, no significant change in foot pad thickness of TP, GP and TGP groups was detected (0.34 ± 0.11 mm, 0.22 ± 0.099 mm and 0.31 ± 0.1 mm, respectively). From 5th week after challenge to the end of 7th week, the progression of foot pad swelling was observed in all groups of mice but TGP group showed mild progression compared to the other groups. The foot pad swelling of the groups of mice was 0.9 ± 0.2 mm for TGP (thalidomide + glucantime), 1.4 ± 0.3 mm for TP (thalidomide) and 1.2 ± 0.25 mm for GP (glucantime). The foot pad swelling of infected mice received only the drug carriers including P and OP groups was 1.7 ± 0.27 mm and 1.8 ± 0.25 mm, respectively.
Fig. 1.
Progression of foot pad swelling of different groups of BALB/c mice. The infected groups of mice received 5 × 103 infective stationary phase promastigotes in the hind foot pad (s.c). Every week after challenge the foot pad swelling was measured using a caliper. The data are expressed in units (1 unit = 10-2 cm of the net increase in foot pad thickness). The groups included Group P; infected but non-treated, Goup OP; infected treated with sessami oil (carrier of thalidomide), Group TP; infected treated with thalidomide in sessami oil, Group GP; infected treated with glucantime, Group TGP; infected treated with thalidomide and glucantime.
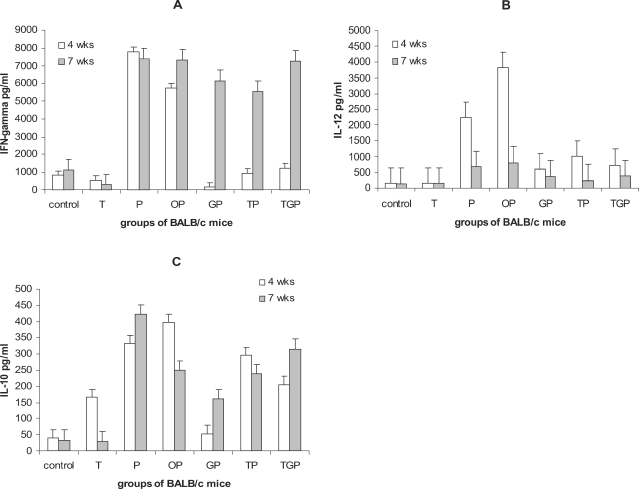
IFN-γ production
Fig. 2a shows the amount of IFN-γ production in the supernatants of draining lymph nodes cells stimulated with PMA/I at 4th (at the end of drug therapy) and 7th week after challenge (when the infection completely established). It was observed that infected mice without any treatment (Group P) produced significantly higher IFN-γ in response to PMA/I compared to non-infected control mice (7,800 ± 500 and 800 ± 100 pg/ml, respectively). Thalidomide treated mice without infection (Group T) showed slight decrease in IFN-γ production (550 ± 120 pg/ml). In Group P and TP no significant difference was observed in terms of IFN-γ production at 4th and 7th week after challenge. Infected mice received Tld, Glt or Tld + Glt produced much lower IFN-γ at 4th week after infection (at the end of drug administration). However, along with progression of disease, production of IFN-γ remarkably increased. Interestingly, infected mice treated with glucantime (Group GP) showed complete downregulation of IFN-γ production (158 ± 30 pg/ml) compared to groups TP and TGP (940 ± 50 and 1,240 ± 120 pg/ml, respectively).
Fig. 2.
Cytokine production in different groups of mice: cytokine production by draining lymph node cells of mice stimulated with PMA/I at the end of therapy (4 weeks after challenge) and three weeks later (7 weeks after challenge) were measured. Figures A-C shows the production of IFN-γ, IL-12 and IL-10, respectively. The groups included control mice; not-infected and non-treated, Group T; non-infected but treated with thalidomide, Group P; infected but non-treated, Goup OP; infected treated with sessami oil (carrier of thalidomide), Group TP; infected treated with thalidomide in sessami oil, Group GP; infected treated with glucantime, Group TGP; infected treated with thalidomide and glucantime.
IL-12 production
Fig. 2B shows the results of IL-12 production of the groups of mice. Similar to the IFN-γ results, IL-12 production significantly increased in infected mice (Groups P and OP) at 4th week after challenge (2,230 ± 530 and 3,830 ± 660 pg/ml, respectively) compared to non-infected mice (T = 150 ± 30 pg/ml and control = 145 ± 29 pg/ml). Infected mice with thalidomide treatment TP (1,010 ± 320 pg/ml) produced higher IL-12 than GP (600 ± 110 pg/ml) and TGP (740 ± 55 pg/ml) although no statistical significant difference was found. However along progression of disease (7th week after challenge), IL-12 production tended to decline. IL-12 results at week 7 are as follows: control = 140 ± 25, non-infected received thalidomide = 150 ± 45, parasite = 680 ± 78, GP = 380 ± 60, TP = 250 ± 50, TPG = 380 ± 35 pg/ml.
IL-10 production
Fig. 2C shows the results of IL-10 production. As expected, Thd induced IL-10 production in non-infected mice (Group T) at the end of drug administration (165 ± 39 pg/ml) compared to the control (40 ± 15 pg/ml). However, 3 weeks after the end of drug therapy, production of IL-10 significantly reduced in Group T (30 ± 9 pg/ml).
The presence of infection promoted IL-10 production by draining lymph node cells of infected mice without treatment (Group P = 330 ± 30 and group OP = 400 ± 55 pg/ml). IL-10 production by Groups TP and TGP mice at 4th week after challenge did not show statistical difference with untreated mice (300 ± 45 and 205 ± 30 pg/ml, respectively) but group of infected mice treated with glucantime showed significant reduction in IL-10 production (54 ± 15 pg/ml) compared to P (332 ± 40 pg/ml), OP (397 ± 55 pg/ml), TP (295 ± 35 pg/ml) and TGP (205 ± 35 pg/ml) groups at 4th week after challenge. Noteworthy, TP and TGP groups showed remarkable down-regulation in IL-10 production compared to P and OP groups At 7th week after challenge all groups showed up-regulation in IL-10 production except control group yet the glucantime treated group had the lowest IL-10 production.
DISCUSSION
Translating the host T-cell-mediated immune response into a feasible form of treatment has been a sought-after therapeutic objective in leishmaniasis, an intracellular protozoal infection which targets tissue macrophages. In disseminated (visceral) leishmaniasis, this aim has been pursued experimentally and clinically using 3 interrelated approaches: (i) identifying specific components of macrophage-activating, T-cell dependent pathways which can act alone as immunotherapy, (ii) understanding how this same Th1 cell-associated mechanism regulates in vivo responsiveness to chemotherapy, and (iii) blending immuno- and chemotherapy to optimize intracellular Leishmania killing in the tissues (Murray, 2000, 2001). Effective defense towards visceralizing strains, including Leishmania donovani, depends strictly upon T (Th1) cells, and acquired resistance is governed by T cell-and macrophage activating cytokines (Murray, 2000, 2001). Among the latter, IL-12 and IFN-γ play particularly prominent experimental roles. These cytokines initiate and/or drive the basic anti-leishmanial Th1 cell response and direct the assembly of tissue granulomas, structures within which intracellular parasites are killed by IFN-γ activated mononuclear phagocytes (Murray, 2000, 2001). In addition, along with host T cells, endogenous IL-12 and IFN-γ are also required for expression of the leishmanicidal effect of conventional chemotherapy, pentavalent antimony (Murrary and Delph-Etienne 2000; Murray et al., 2000). Not surprisingly, then, employing pro-host defense cytokines in an exogenous form, either alone or with antimony, represents the primary immunotherapeutic approach thus far tested experimentally and clinically in visceral infections (Sundar et al., 1995, 1997).
In the present study, it was shown that thalidomide and glucantime combined therapy significantly reduced the course of the disease compared to single therapy with each one of the drugs and this effect was dependent on IL-10 and IFN-γ production. As shown in Fig. 1, thalidomide + glucantime significantly reduced the course of disease up to 7th week after challenge (3 weeks after stopping the treatment) compared to mice treated with either glucantime or thalidomide. It is noteworthy that at 5th weeks after challenge (one week after stopping the treatment) the foot pad swelling of thalidomide treated mice was comparable to the glucantime treated mice which was remarkably lower than untreated groups. It has been reported that thalidomide could alter resistance of mice to Listeria monocytogenes (Karrow et al., 2003). Similarly, we showed that thalidomide is able to down-regulate progression of leishmaniasis although it did not completely inhibit the disease. In present report, glucantime therapy did not eradicate the infection which might be due to the short period of treatment. It might also be due to re-activation of infection since BALB/c mice is the most sensitive strain of mice to L. major infection. In addition, as is the case in several other infections, the clinically cured host organism still harbors small amounts of live L. major parasites (Strenger et al., 1996).
In order to see how the treatments affect cytokine production, draining lymph nodes of mice were excised and the isolated cells were cultured and stimulated with PMA/I. IL-10, IL-12 and IFN-γ secretion was measured in the supernatants harvested 24 hr after stimulation. Interestingly, infected mice without any treatment (Group P) produced significantly higher IFN-γ compared to non-infected control mice. This finding is in consonance with the previous reports demonstrating that IFN-γ production in BALB/c is not impaired even when the disease completely developed (Lang et al., 2003; Sommer et al., 1998). In addition, the disease severity would be affected by a mechanism independent of conventional helper T-cell responses since B6.C (lmr1/2) mice had similar cytokine levels to the parental C57BL/6 mice despite increased susceptibility and C.B6 (lmr1, lmr2) were similar to BALB/c despite increased resistance (Elso et al., 2004). In human visceral leishmaniasis it has also been found that plasma level of IFN-γ and IL-12 p40 is elevated (Hailu et al., 2004). In this study, infected mice received glucantime treatment failed to produce IFN-γ at the end of therapy but infected mice received thalidomide or combined T + G treatment (Groups TP and TGP) produced significantly higher IFN-γ than Group GP. This would explain why glucantime treatment of patients suffering from cutaneous leishmaniasis having negative skin test encounter relapse (Passos et al., 2000). Along progression of disease, however, IFN-γ production was upregulated again in GP, TP, and TGP groups.
Similar to the results of IFN-γ, IL-12 production was induced in infected-untreated mice at the end of therapy but along disease progression the draining lymph node cells showed comprehensive reduced IL-12 production. IL-12 production was very low at the end of therapy in GP, TP and TGP mice. No significant change was observed at 7th week after challenge.
At the end of the therapy, thalidomide induced much higher level of IL-10 production in uninfected mice compared to the control mice which is expectable. This finding is in agreement with previous report showing that thalidomide exerts its anti-inflammatory effect through IL-10 (Franks et al., 2004). Three weeks after stopping drug administration, IL-10 secretion was declined to the IL-10 level of control mice. Infection of L. major also induced IL-10 and along the progression of disease its level was unchanged. Nonetheless, thalidomide did not up-regulate IL-10 production in infected mice. The role of IL-10 in disease aggravation has been demonstrated (Noben-Trauth et al., 2003; Chatelain et al., 1999) which are in consistence with our results. Similar to IL-12 and IFN-γ, IL-10 production was decreased in infected mice received glucantime.
Up-regulation of IFN-γ and down-regulation of IL-10 in the group received glucantime and thalidomide is in agreement with the report studying cytokine production in patients suffering from cuaneous leishmaniasis received immuno-chemotherapy treated with glucantime and a vaccine against American cutaneous leishmaniasis (Toledo et al., 2001).
Conclusively, the results of cytokine assay showed that the effect of thalidomide on controlling the disease progression could be exerted through modulation of IFN-γ and IL-10 production. In addition, combined therapy with glucantime and thalidomide would be promising in visceral leishmaniasis, yet needs further investigations.
Footnotes
This work was financially supported by the grant No. 164, Pasteur Institute of Iran, Teheran, Iran.
References
- 1.Alimohammadian MH, Darabi H, Kariminia A, Rivier D, Bovay P, Mauel J, Ajdary S, Kharazmi A. Adjuvant Effect of Leishmania major promastigotes on the immune response of mice to ovalbumin. Iranian Biomed J. 2002;6:123–128. [Google Scholar]
- 2.Arruda MS, Richini VB, Oliveira SM, Vilani-Moreno FR. Experimental murine mycobacteriosis: evaluation of the functional activity of alveolar macrophages in Thalidomide-treated mice. Braz J Med Biol Res. 2004;37:485–492. doi: 10.1590/s0100-879x2004000400005. [DOI] [PubMed] [Google Scholar]
- 3.Chatelain R, Mauze S, Coffman RL. Experimental Leishmania major infection in mice: role of IL-10. Parasite Immunol. 1999;21:211–218. doi: 10.1046/j.1365-3024.1999.00224.x. [DOI] [PubMed] [Google Scholar]
- 4.Elso C, Kumar B, Smyth G, Foote S, Handman E. Dissociation of disease susceptibility, inflammation and cytokine profile in lmr1/2 congenic mice infected with Leishmania major. Genes Immun. 2004;5:188–196. doi: 10.1038/sj.gene.6364056. [DOI] [PubMed] [Google Scholar]
- 5.Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet. 2004;363:1802–1811. doi: 10.1016/S0140-6736(04)16308-3. [DOI] [PubMed] [Google Scholar]
- 6.Fu LM, Fu-Liu CS. Thalidomide and tuberculosis. Int J Tuberc Lung Dis. 2002;6:569–572. [PubMed] [Google Scholar]
- 7.Hailu A, van der Poll T, Berhe N, Kager PA. Elevated plasma levels of interferon (IFN)-gamma, IFN-gamma inducing cytokines, and IFN-gamma inducible CXC chemokines in visceral leishmaniasis. Am J Trop Med Hyg. 2004;71:561–567. [PubMed] [Google Scholar]
- 8.Handman E. Leishmaniasis: Current status of vaccine development. Clin Microbiol Rev. 2001;14:229–243. doi: 10.1128/CMR.14.2.229-243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karrow NA, Guoa TL, Zhanga LX, McCay JA, Musgrovea DL, Peacheea VL, Germolecb DR, White KL., Jr Thalidomide modulation of the immune response in female B6C3F1 mice: a host resistance study. Int Immunopharmacol. 2003;3:1447–1456. doi: 10.1016/S1567-5769(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 10.Karrow NA, McCay JA, Brown RD, Musgrove DL, Pettit DA, Munson AE, Germolec DR, White KL., Jr Thalidomide stimulates splenic antibody response and cytotoxic T lymphocyte activity and alters leukocyte subpopulation numbers in female B6C3F1 mice. Toxicol Appl Pharmacol. 2000;165:237–244. doi: 10.1006/taap.2000.8939. [DOI] [PubMed] [Google Scholar]
- 11.Lang T, Courret N, Colle JH, Milon G, Antoine JC. The levels and patterns of cytokines produced by CD4 T lymphocytes of BALB/c mice infected with Leishmania major by inoculation into the ear dermis depend on the infectiousness and size of the inoculum. Infect Immun. 2003;71:2674–2683. doi: 10.1128/IAI.71.5.2674-2683.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marriott JB, Muller G, Dalgleish AG. Thalidomide as an emerging immuno-therapeutic agent. Immunol Today. 1999;20:538–540. doi: 10.1016/s0167-5699(99)01531-5. [DOI] [PubMed] [Google Scholar]
- 13.Murray HW. Treatment of visceral leishmaniasis (kala-azar): a decade of progress and future approaches. Int J Infect Dis. 2000;4:158–177. doi: 10.1016/s1201-9712(00)90078-x. [DOI] [PubMed] [Google Scholar]
- 14.Murray HW. Clinical and experimental advances in treatment of visceral leishmaniasis. Antimicrob Agents Chemother. 2001;45:2185–2197. doi: 10.1128/AAC.45.8.2185-2197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray HW, Delph-Etienne S. Role of endogenous gamma interferon and macrophage microbicidal mechanisms in host response to chemotherapy in experimental visceral leishmaniasis. Infect Immun. 2000;68:288–293. doi: 10.1128/iai.68.1.288-293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray HW, Jungbluth A, Ritter E, Montelibano C, Marino MW. Visceral leishmaniasis in mice devoid of tumor necrosis factor and response to treatment. Infect Immun. 2000;68:6289–6293. doi: 10.1128/iai.68.11.6289-6293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noben-Trauth N, Lira R, Nagase H, Paul WE, Sacks DL. The relative contribution of IL-4 receptor signaling and IL-10 to susceptibility to Leishmania major. J Immunol. 2003;170:5152–5158. doi: 10.4049/jimmunol.170.10.5152. [DOI] [PubMed] [Google Scholar]
- 18.Passos VM, Barreto SM, Romanha AJ, Krettli AU, Volpini AC, Lima e Cosa MF. American cutaneous leishmaniasis: use of a skin test as a predictor of relapse after treatment. Bull World Health Organ. 2000;78:968–974. [PMC free article] [PubMed] [Google Scholar]
- 19.Sommer F, Meixner M, Mannherz M, Ogilvie AL, Rollinghoff M, Lohoff M. Analysis of cytokine patterns produced by individual CD4+ lymph node cells during experimental murine leishmaniasis in resistant and susceptible mice. Int Immunol. 1998;10:1853–1861. doi: 10.1093/intimm/10.12.1853. [DOI] [PubMed] [Google Scholar]
- 20.Stenger S, Donhauser N, Thuring H, Rollinghoff M, Bogdan C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J Exp Med. 1996;183:1501–1514. doi: 10.1084/jem.183.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundar S, Singh VP, Sharma S, Makharia MK, Murray HW. Response to interferon-gamma plus antimony in Indian visceral leishmaniasis. J Infect Dis. 1997;176:1117–1119. doi: 10.1086/516526. [DOI] [PubMed] [Google Scholar]
- 22.Sundar S, Murray HW. Effect of treatment with interferon-gamma alone in Indian visceral leishmaniasis. J Infect Dis. 1995;172:1627–1629. doi: 10.1093/infdis/172.6.1627. [DOI] [PubMed] [Google Scholar]
- 23.Toledo VP, Mayrink W, Gollob KJ, Oliveira MA, Costa CA, Genaro O, Pinto JA, Afonso LC. Immunochemotherapy in American cutaneous leishmaniasis: immunological aspects before and after treatment. Mem Inst Oswaldo Cruz. 2001;96:89–98. doi: 10.1590/s0074-02762001000100010. [DOI] [PubMed] [Google Scholar]