Abstract
To evaluate the protoscolicidal effects of various concentrations of hypertonic glucose, live protoscolices of sheep were exposed to 10%, 15%, 25% and 50% glucose solutions. Cetrimide (0.5%), silver nitrate (0.5%) and hypertonic saline (20%) were used as positive controls, while physiological saline was used as a negative control. After 1, 2 and 5 min, the protoscolicidal effects were determined by 1% eosin. A 25% glucose solution had no significant protoscolicidal effect. However, a 50% glucose solution revealed higher protoscolicidal effect than 0.5% silver nitrate but weaker effect than 0.5% cetrimide; the effect was comparable with that of 20% hypertonic saline. The results showed that hypertonic glucose solution is highly effective in killing protoscolices of Echinococcus granulosus in vitro.
Keywords: Echinococcus granulosus, hydatid cyst, protoscolices, protoscolicidal agents, hypertonic glucose solution
Hydatidosis is one of the most important parasitic zoonoses caused by Echinococcus granulosus. The disease has a worldwide distribution and is endemic in several countries including China, Kenya, Turkey and Iran (Mehrabani et al., 1999; Sadjjadi, 2006). Humans are infected by ingesting of parasite eggs leading to cyst formation mainly in the liver and lung (Muller, 2001).
The best treatment is considered surgery. During the surgery and before evacuation of the cyst, protoscolicidal agents are used to be injected into the cyst in order to prevent secondary cyst formations (Saidi, 1976). The most common protoscolicidal agents used are hypertonic saline, silver nitrate, cetrimide and formalin; each has a variety of dangerous complications such as biliary tract fibrosis and liver necrosis (Robinson and Arme, 1985; Abbasi Dezfuli et al., 1991; Prasad et al., 1991; Besim et al., 1998). In this regard, World Health Organization (WHO) purposed an urgent need to find new protoscolicidal agents which are more effective and with less complications (Powlowski et al., 2001).
Hypertonic glucose was reported to be a successful protoscolicidal agent for pericardial hydatid cyst (Ferrini et al., 1997), however, its protoscolicidal efficacy and the best concentration of hypertonic glucose was not tested by in vitro studies. Thus, this study was undertaken to evaluate the protoscolicidal effects of various concentrations of hypertonic glucose.
A 0.5% cetrimide, 0.9% and 20% sodium chloride, 0.5% silver nitrate, and 10%, 15%, 25% and 50% concentrations of glucose were used in this study. Sheep livers and lungs having hydatid cysts were transferred to Parasitology Laboratory within an hour after slaughter. Cyst surfaces were sterilized by heat and the cyst contents were evacuated completely and transferred into falcon tubes, where the protoscolices were precipitated and separated. A solution containing 30-40 × 103 protoscolices were provided and the viability of protoscolices was determined using eosin stain method. When the percentage of viable protoscolices was more than 90%, they were considered to be appropriate for our study.
In order to determine the viability of protoscolices, 0.01 ml of pooled protoscolices was transferred over a slide and mixed by 0.01 ml of 0.1% eosin and was evaluated by low power microscopy after one min. Dead protoscolices absorbed eosin and colored red but alive protoscolices remained colorless. When live protoscolices with enough volume were obtained, 0.1ml of pooled protoscolices was poured into a test tube and 1 ml of the agents was added. After 1 min of exposure, 10 ml of normal saline was added and centrifuged for 1 min with 300 rpm and the supernatant was discarded. The same procedure was repeated 2 times. These processes were repeated for each agent using 1, 2 and 5 min of exposures.
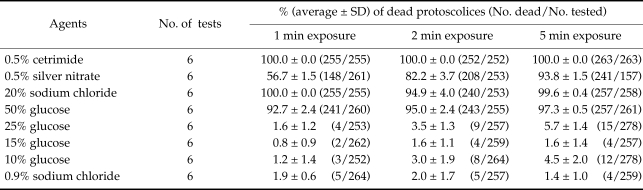
At least, 250 protoscolices were counted for each experiment. Each experiment was repeated 6 times and the average of dead and total protoscolices was measured (Table 1). It is notable that all the steps were done under sterilized condition and with sterilized materials. The ANOVA test was used for comparing the mean viability for different materials. A P-value of less than 0.05 were considered significant.
Table 1.
Protoscolicidal effects of different glucose concentrations in comparison with cetrimide, silver nitrate and sodium chloride solutions according to the time of exposure
The mean percentage of dead protoscolices and the agents used to the time of exposures were summarized in Table 1. After 1 min of exposure, 0.5% cetrimide with the potency of 100% killing of protoscolices could be considered as the best protoscolicidal agent (P = 0.00). A 50% glucose was in the second place after 0.5% cetrimide. The protoscolicidal effect of 0.05% cetrimide was the highest compared to other solutions after 2 min of exposure.
The ANOVA test showed that there was significant difference between the mean protoscolicidal effects of these solutions during 2 min of exposure (P = 0.003), however, a significant difference was not observed between 10%, 15% and 25% glucose solutions (P = 0.14) and even physiological saline (P = 0.23). It is worth to say that, after 1 and 2 min of exposure, the mean percentage of dead protoscolices with 10%, 15% and 25% glucose and 0.9% sodium chloride was so low to ignore their protoscolicidal effects.
After 5 min of exposure, cetrimide remained in the first rank, 20% sodium chloride was the second and 50% glucose was the third. Here, the protoscolicidal effects of 10%, 15% and 25% glucose were more than control in physiological saline (P = 0.00).
Hypertonic glucose was used as a protoscolicidal agent in pericardial hydatid cyst just one time (Ferrini et al., 1997); however, there were neither in vitro nor in vivo prior studies evaluating the protoscolicidal effects of this solution. Previous researches revealed that 0.05% cetrimide was a potent protoscolicidal agent even in very low concentration (Powlowski et al., 2001). Therefore, it is reasonable for WHO to introduce it as a protoscolicidal agent to be compared to other agents for evaluation of their potency (Anonymous, 1996). Also in the present study, a 100% protoscolicidal effect was observed with 0.5% cetrimide after 1 min of exposure.
In 1963, protoscolicidal effects of silver nitrate were studied. It was shown that 0.05% silver nitrate had higher protoscolicidal effect than 1% concentration (Meymerian et al., 1963). The protoscolicidal effect of 0.5% silver nitrate was confirmed in in vivo through injection of exposed protoscolices into the mice peritoneum (Saidi, 1976). In our study, 0.5% silver nitrate could kill 94% of protoscolices after 5 min of exposure in vitro.
Hypertonic sodium chloride with production of osmotic gradient was able to destroy protoscolices (Abbasi Dezfuli et al., 1991). Other studies showed that 3% and 10% concentrations didn't have any protoscolicidal effect. However, 20% concentration could kill all protoscolices after 5 min of exposure (Abbasi Dezfuli et al., 1991; Ghazanfari et al., 1998). In the present study, 20% sodium chloride could kill almost all protoscolices (99.6%) after 5 min. As the first experience in our study, hypertonic glucose up to 25% concentration had a negligible protoscolicidal effect even after 5 min of exposure while in 50% concentration after 1 and 2 min of exposure, had higher protoscolicidal effect than 20% sodium chloride and 0.5% silver nitrate. After 5 min of exposure, it was less effective than 20% sodium chloride, yet it was more effective than 0.5% silver nitrate.
We recommend future studies on 50% glucose concentration to be designed in a longer period (over 5 min) to determine the essential time of exposure that is needed to gain 100% protoscolicidal effect. Also it is recommended for other concentration of glucose up to 25% to detect whether they have acceptable protoscolicidal effect or not.
As the viability of protoscolices with the criteria used in in vitro studies is doubtful, it should be reminded that exposed protoscolices have to be injected into the mice peritoneum for confirmation of its protoscolicidal effect (Robinson and Arme, 1985). In addition, before usage of 50% glucose as a protoscolicidal agent, its probable complications over the internal body organs and biliary system should be evaluated.
ACKNOWLEDGMENTS
The authors would like to thank the Center for Development of Clinical Research Study of Nemazee Hospital for typing and editorial assistance.
References
- 1.Abbassi Dezfuli A, Shishineh P, Shadmehr MB, Ghaffarnejad MH. Early and latest effects of scolicidal agents on liver and bile ducts, an experimental study. Iranian J Med Sci. 1991;16:36–39. [Google Scholar]
- 2.WHO informal working group on echinococcosis. Guidelines for treatment of cystic and alveolar echinococcosis in humans. Bull World Health Organ. 1996;74:231–242. [PMC free article] [PubMed] [Google Scholar]
- 3.Besim H, Karayalcin K, Hamamci O, Gungor C, Korkmaz A. Scolicidal agents in hydatid cyst surgery. HPB Surg. 1998;10:347–351. doi: 10.1155/1998/78170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrini JB, Pichard L, Domergue J, Maurel P. Longterm primary cultures of adult human hepatocytes. Chem Biol Interact. 1997;107:31–45. doi: 10.1016/s0009-2797(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 5.Ghazanfari T, Hassan ZM, Yaraii R. The in vitro effects of aqueous garlic extract and garlic fractions on the growth of Leishmania major. Kowsar Med J. 1998;5122:117–122. (in Persian) [Google Scholar]
- 6.Mehrabani D, Oryan A, Sadjjadi SM. Prevalence of Echinococcus granulosus in stray dogs and herbivores in Shiraz. Vet Parasitol. 1999;86:217–220. doi: 10.1016/s0304-4017(99)00151-x. [DOI] [PubMed] [Google Scholar]
- 7.Meymerian E, Luttermoser GW, Frayha GJ, Schwabe CW, Prescott B. Host-parasite relationships in echinococcosis: X. Laboratory evaluation of chemical scolicides as adjuncts to hydatid surgery. Ann Surg. 1963;158:211–215. doi: 10.1097/00000658-196308000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller R. Worms and Human Disease. Oxon, UK: CAB International; 2001. pp. 85–86. [Google Scholar]
- 9.Pawlowski ZS, Eckert J, Vuitton DA, Ammann RW. Echinococcosis in human and clinical aspects, diagnosis and treatment. In: Eckert J, et al., editors. Manual on Echinococcosis in Human and Animal: A Public Health Problem of Global Concern. France: WHO/OIE; 2001. pp. 20–71. [Google Scholar]
- 10.Prasad J, Bellamy PR, Stubbs RS. Instillation of scolicidal agents into hepatic hydatid cysts: can it any danger be justified. New Zeal Med J. 1991;104:336–337. [PubMed] [Google Scholar]
- 11.Robinson RD, Arme C. Echinococcus granulosus: failure of the eosin exclusion test to demonstrate death of protoscolices. Ann Trop Med Parasitol. 1985;79:117. doi: 10.1080/00034983.1985.11811897. [DOI] [PubMed] [Google Scholar]
- 12.Sadjjadi SM. Present situation of echinococcosis in the Middle East and Arabic North Africa. Parasitol Int. 2006;55(suppl):S197–S202. doi: 10.1016/j.parint.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Saidi F. Surgery of Hydatid Disease. 1st ed. London, England: WB Saunders; 1976. [Google Scholar]