Abstract
Toxoplasma gondii has been shown to result in life-threatening encephalitis in immunocompromised patients after reactivation of dormant parasites. In order to obtain information on immune responses related to this phenomenon, BALB/c mice were infected with 25 cysts of the 76K strain of T. gondii, then, treated orally with dexamethasone (Toxo/Dexa-treated group) in order to reactivate the chronic toxoplasmosis. None of the T. gondii-infected mice died during the experimental periods, whereas the Toxo/Dexa-treated mice evidenced a significant attenuation of survival periods. Toxoplasma-specific IgG2a, IgA and IgM titers in sera were significantly depressed in the Toxo/Dexa-treated mice; however, the IgG1 sera titers were similar to those seen in the Toxoplasma-infected mice. The percentages of CD4+ and CD8α+ T cells in the Toxo/Dexa-treated mice were significantly reduced 2 weeks after dexamethasone treatment. IFN-γ and IL-10 production levels in the Toxo/Dexa-treated mice were depressed significantly, whereas IL-4 production was increased temporarily. The expression levels of the Toxoplasma-specific P30 and B1 genes were found to have been increased in the Toxo/Dexa-treated mice in comparison with the Toxoplasma-infected mice. Collectively, the findings of this study demonstrate that reactivation of murine toxoplasmosis as the result of dexamethasone treatment induced a depression in Th1 immune responses, whereas Th2 immune responses were not significantly influenced.
Keywords: Toxoplasma gondii, dexamethasone, cytokine, antibody, mouse, Th1, Th2 response
INTRODUCTION
Toxoplasma gondii is an obligate intracellular protozoan parasite, which occurs globally among both humans and animals. Transmission to humans occurs either through the ingestion of T. gondii oocysts shed into the environment by cats, or by eating the meat of infected animals. After the acute stage, the parasite forms cysts (latent stage) in a variety of organs, particularly the brain, heart, and skeletal muscle, thereby establishing a chronic infection. Immunosuppression may result in reactivation of a latent infection, and such T. gondii reactivation normally presents as a major opportunistic infectious disease within central nervous systems of AIDS patients (Kasper, 2004). However, little information is currently available regarding the reactivation of chronic toxoplasmosis.
Cell-mediated immunity performs a primary role in resistance against T. gondii, although humoral immunity also appears to be involved. CD8+ T cells are the major afferent limb of cellular immunity against acute infection, but CD4+ T cells are also involved (Denkers and Gazzinelli, 1998; Casciotti et al., 2002; Filisetti and Candolfi, 2004; Wang et al., 2005). Among the CD4+ T cells, Th1 cells generate IL-2, interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) and -β (TNF-β) (Romagnani, 1996). IFN-γ is the central cytokine associated with resistance against T. gondii during both the early and late (toxoplasmic encephalitis) stages of infection (Denkers and Gazzinelli, 1998; Suzuki, 2002a, b). Th2 cells synthesize a number of cytokines, including IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, and IL-14 (Romagnani, 1996). IL-4 is the main promoter of type-2 responses and is classically reported as counter-regulating type-1 immunity (Heinzel et al., 1989; Nickdel et al., 2004). Cytokines have been shown to play an important role in the pathogenesis of toxoplasmosis, and there is a change in the levels of cytokines during the reactivation of T. gondii infection. However, there were not many reports about Th1/Th2 cytokine responses during the reactivation of chronic toxoplasmosis.
B cells are also principal components of protection in vaccinated mice challenged with highly virulent strains of T. gondii. The administration of antisera to T. gondii effects a reduction of mortality in these animals (Kang et al., 2000; Chen et al., 2003). These results indicate that antibody generation by B cells may be important with regard to the control of latent persistent infections. Resistance is operative under collaboration between T and B cells. Among immune system effecter molecules, antibodies are unequivocally crucial for pathogen control. At present, there are numerous studies related to molecular and cellular events involved in T cell responses; however, little is known about cellular and molecular events of B cell responses.
In immunocompromised patients, T. gondii can result in life-threatening toxoplasmic encephalitis after reactivation of dormant parasites. Currently, little information is available regarding the immune responses inherent to reactivated toxoplasmosis, particularly the production patterns of Th1/Th2 cytokines and of various antibodies. In order to clarify this phenomenon, BALB/c mice were administered with 25 cysts of a 76K strain of T. gondii and/or dexamethasone in order to induce primary and reactivated toxoplasmic encephalitis, after which the mice were killed serially. Then, the survival time, serum antibody titers, splenic T cell subsets, Th1/Th2 cytokine production, and parasite burdens were examined.
MATERIALS AND METHODS
Mice and Toxoplasma strains
Female BALB/c mice were obtained from the Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea). All mice used were of 10-12 weeks of age, and were documented as specific-pathogen-free animals. RH and 76K strains of T. gondii were utilized. The RH strain was employed in the preparation of Toxoplasma lysate antigen (TLA), and this strain was maintained in vitro on human foreskin fibroblasts in an atmosphere of 5% CO2, at 37℃. The 76K strain was used to infect BALB/c mice for a study on immunological effects of reactivated toxoplasmosis.
Preparation of Toxoplasma lysate antigen
Infected fibroblasts were scraped, forcibly passed through a 27-gauge needle, and centrifuged for 10 min at 900 g using Percoll (Sigma Chemical Co., St. Louis, Missouri, USA) to pellet the parasites. Then, the parasites were sonicated on ice and centrifuged for 40 min at 100,000 g. The supernatants were pooled and sterile filtered (Gelman Sciences, Ann Arbor, Michigan, USA) and the protein contents were determined via the Bradford method, using bovine serum albumin as the standard. Parasite antigens (TLA) were stored in aliquots at -20℃ until use.
Mice infection with T. gondii and immunosuppression
The 76K strain cysts of T. gondii were prepared by homogenization of the brain tissue in saline, and 25 cysts were intragastrically administered to mice (Toxo-infected group). In accordance with the procedure described by Nicoll et al. (1997), the mice were administered 10 mg/L of dexamethasone (Sigma) in drinking water 4-8 weeks after infection, in order to induce the reactivation of cerebral toxoplasmosis (Toxo/Dexa-treated group). In parallel with the Toxo/Dexa-treated group, uninfected mice were also treated with dexamethasone (Dexa-treated group). All groups receiving dexamethasone were administered 1 mg/L oxytetracycline (Sigma) in drinking water for the duration of the study, in order to reduce contraction of other opportunistic infections. Control mice (uninfected and untreated) were treated in similar manners with tap water. Mice were serially killed after infection, and survival days, antibody titers, Th1/Th2 cytokine production, and parasite burdens were evaluated. To decrease the bias of each mouse, mice were selected evenly at indicated time points.
Quantification of tissue parasite burdens by Southern blot
Southern blots for quantification of tissue parasite burdens were conducted in accordance with the procedure described by Meyer et al. (2000). DNA was prepared from mouse brains by homogenization of 100 mg tissue in 2 ml 0.1 M NaCl, 0.2 M sucrose, 0.01 M EDTA and 0.3 M Tris (pH 8.0). The solution was then incubated for 2 hr at 65℃ after addition of 10% SDS and proteinase K (100 µg/ml). Potassium acetate (8 M) was then added, followed by 1 hr of precipitation of the solution at 4℃ and 10 min of centrifugation at 5,000 g. The aqueous phase was extracted with phenol/chloroform and precipitated with ethanol. The oligonucleotide primers used to initiate DNA amplification were complementary to the segments of the B1 gene (5'GGAACTGCATCCGTTCATGAG and 5'-TCTTTAAAGCGTTCGTGGTC) or P30 gene (5'ATGTCGGTTTCGCTGCACTTC and 5'TCACGCGACACAAGCTGCGATAGAGCC) of T. gondii DNA that had been PCR amplified in a solution containing 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, 200 µM of each deoxynucleotide, and 0.4 µM of primers. A positive control (DNA extracted directly from tachyzoites of RH strain) and a negative control (distilled water) were tested for each reaction. We applied the same volume of DNA already adjusted to the same optical density, such that the applied volume of each lane was identical in all experiments. The reaction was conducted for 35 cycles, each consisting of 60 sec at 94℃, 90 sec at 55℃, and 60 sec at 72℃. After migration on 2% agarose gel, the PCR products were passively transferred onto nylon membranes. The membranes were fixed for 15 min at 120℃. The blots were pre-hybridized for 3 hr at 42℃. For hybridization, a non-isotopic 5'-GGCGACCAATCTGCGAATACACC probe (B1 gene) or 5'CTCCCACTCTTGCGTCACC (P30 gene) was used. For development, the probe was labeled at the end with digoxigenin (Boehringer Mannheim, Berlin, Germany). After rinsing, the membrane was hybridized with an anti-digoxigenin antibody, which was labeled with alkaline phosphatase. After further rinsing, the membrane was developed using the chemiluminescent substrate, CSPD [isodium 3-(4-methoxyspirofl,2-dioxetane-3,2'-(5'-chloro)tricyclo [3.3.1.13,7]decan)4-yl) phenyl phosphate] (DIG Luminescent Detection Kit, Roche Molecular Biochemicals, Berlin, Germany), and detected by exposure on X-ray film. DNA was quantified using an imaging densitometer.
Titration of serum antibodies by ELISA
Each well of a 96-well plate was coated with TLA (10 µg/ml), then incubated overnight at 4℃. After blocking, the serum samples were diluted to 1:100 in 0.1% BSA/PBS containing 0.05% Tween 20 and added at 100 µl per well. After 2 hr, HRP-conjugated goat anti-mouse IgG, IgG1, IgG2a, IgA or IgM (Southern Biotechnology Associates Inc., Birmingham, Alabama, USA) was applied. After washing, freshly prepared o-phenylenediamine dihydrochloride was added, and the reaction was halted via the addition of 4N H2SO4 Optical density was read at 492 nm, using a Titertek Multiscan ELISA reader (Laboratory Systems, Helsinki, Finland).
Splenocyte preparation and culture
Spleens from mice were homogenized, and erythrocytes were lysed with Tris-NH4Cl (pH 7.2). After washing with RPMI 1640 (Sigma), splenocytes were resuspended in complete RPMI 1640 medium containing 10% FBS, 1 mM sodium pyruvate (Sigma), 100 U/ml penicillin, and 0.1 mg/ml streptomycin. The splenocytes were cultured with TLA (20 µl/ml) in 96-well flat-bottom tissue culture plates (200 µl of 4 × 106 cells/ml) for 48 hr at 37℃ in 5% CO2. Cell-free supernatants were harvested and stored at -20℃ until assayed for cytokines.
Cytokine ELISA of splenocyte culture supernatants
Mouse IFN-γ, IL-2, IL-4, IL-5, and IL-10 levels were determined using a cytokine detection ELISA kit (Genzyme, Cambridge, Massachusetts, USA) in accordance with the manufacturer's protocols. Each well of a 96-well microtiter plate was coated with 100 µl of hamster anti-mouse IFN-γ, IL-2, IL-4, IL-5, or IL-10, diluted in 0.05 M carbonate buffer (pH 9.5), and incubated overnight at 4℃. The plates were washed 3 times, and blocked with 1% BSA/PBS. Culture supernatants or recombinant mouse IFN-γ, IL-2, IL-4, IL-5 or IL-10 standards were then applied to the plates, and incubated for 1 hr at 37℃. After washing, biotinylated goat anti-mouse IFN-γ, IL-2, IL-4, IL-5 or IL-10 was added, and the plates were incubated for an additional 1 hr at 37℃. One hundred µl of HRP-conjugated streptavidin was then added to each microtiter well. Binding was visualized using tetramethylbenzidine containing H2O2. The reaction was halted with 2N H2SO4, and the absorbance was read at 450 nm on a Titertek Multiscan plate reader. IFN-γ, IL-2, IL-4, IL-5 and IL-10 levels were quantitated via reference to standard curves generated using rIFN-γ, IL-2, IL-4, IL-5, or IL-10.
Phenotypic analysis via flow cytometry
Splenocytes containing 1 × 106 cells were incubated with either 50 µl of FITC-conjugated anti-mouse CD4+ and CD8α+ monoclonal antibodies (1:100 dilution in 0.1% BSA/PBS; PharMingen, San Diego, California, USA) or with an isotype-specific control (PharMingen) for 60 min at 4℃. The cells were washed 3 times in 0.1% BSA/PBS via centrifugation, fixed with 1% paraformaldehyde, and analyzed by flow cytometry (FACScan, Becton Dickinson, Franklin Lakes, New Jersey, USA). Data were analyzed using the Cell-Quest program (Becton Dickinson).
Statistical analysis
Results are expressed as the mean ± standard deviation (SD) for each group. Statistical evaluations of differences in survival days, antibody titers, cytokine levels, and T cell subset percentages were conducted using 2-tailed Mann-Whitney U tests and Student's t-tests. Differences were considered significant at a P-value of < 0.05.
RESULTS
Decrease in the survival day of mice after dexamethasone treatment
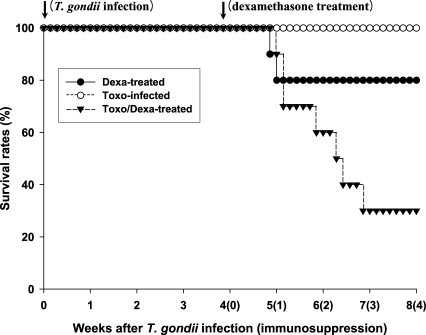
As shown in Fig. 1, none of the Toxoplasma-infected BALB/c (Toxo-infected) mice died after infection throughout the experimental period. However, survival times (in days) of the dexamethasone-treated uninfected and Toxoplasma-infected BALB/c (Dexa-treated and Toxo/Dexa-treated group, respectively) mice were shortened. The mean survival days of the Toxo/Dexa-treated group were significantly lower than those of the Toxo-infected group (44.6 ± 8.9 days vs. 56.0 ± 0.0 days, P = 0.0029), and we also detected a significant difference between the survival days of the Toxo/Dexa-treated and Dexa-treated groups (44.6 ± 8.9 days vs. 51.5 ± 9.5 days, P = 0.027). However, the survival time of the Dexa-treated group was similar to that of the Toxo-infected group (51.5 ± 9.5 days vs. 56.0 ± 0.0 days, P = 0.16).
Fig. 1.
Survival days of BALB/c mice either infected with T. gondii or treated orally with dexamethasone. Each group contained 10 mice. The Dexa-treated group of BALB/c mice were treated with 10 mg/L of dexamethasone in drinking water, 4~8 weeks after the beginning of the experiment. The Toxo-infected group of BALB/c mice were orally infected with 25 cysts of a 76K strain of T. gondii, and then evaluated for 8 weeks. The Toxo/Dexa-treated group, consisting of Toxoplasma-infected BALB/c mice, was treated with 10 mg/L of dexamethasone in drinking water for 4-8 weeks, beginning at 4 weeks PI. The data are representative of 1 of 2 separate experiments.
Increase of the parasite load in the brain after dexamethasone treatment
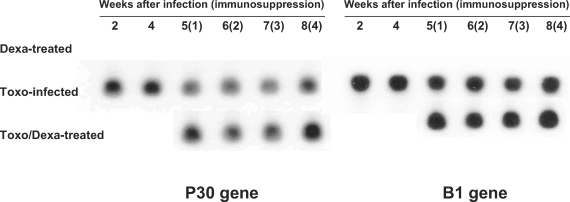
Toxoplasma-specific P30 and B1 genes were not detected in the Dexa-treated mice; however, Toxo-infected mice evidenced expression of both the Toxoplasma-specific P30 and B1 genes (Fig. 2). In the Toxo-infected mice, parasite burdens in the brain reached a maximum at approximately 4 weeks after infection, and thereafter the number of parasites in the brain stabilized. After dexamethasone treatment in the Toxo-infected mice, the expression of the Toxoplasma P30 and B1 genes in the brains of the Toxo/Dexa-treated mice increased as compared to the Toxo-infected mice, and peaked 4 weeks after dexamethasone treatment, i.e., 8 weeks postinfection (PI).
Fig. 2.
Detection of T. gondii P30 and B1 genes in the brains of mice either infected with T. gondii or orally treated with dexamethasone. P30 and B1 gene expression levels were analyzed via Southern blot hybridization. The data shown are representative of 1 of 2 separate experiments.
Depression of Toxoplasma-specific IgG2a, IgA and IgM titers after dexamethasone treatment
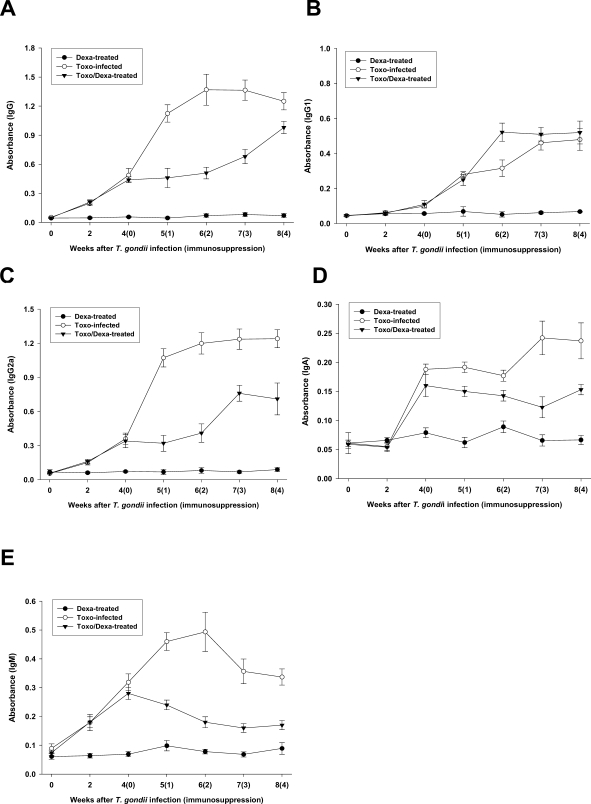
Serum samples from the Toxo-infected, Dexa-treated, and Toxo/Dexa-treated mice were assayed for IgG, IgG1, IgG2a, IgA, and IgM titers against TLA using ELISA (Figs. 3A, 3B, 3C, 3D, and 3E, respectively). We detected no significant increases of any type of antibodies in the Dexa-treated mice as compared with the uninfected mice (day 0). The specific IgG, IgG2a, and IgM titers were found to have increased immediately after T. gondii infection, in comparison to that of the uninfected mice (Figs. 3A, 3C and 3E, respectively), whereas the IgG2a and IgA titers increased significantly 2 weeks after infection (Figs. 3B and 3D, respectively).
Fig. 3.
Time course of the IgG (A), IgG1 (B), IgG2a (C), IgA (D) and IgM (E) antibody titers of sera from mice either infected with T. gondii or orally treated with dexamethasone. The data are expressed as the means ± SD of 5 mice.
From 4 weeks after infection, the Toxoplasma-infected mice were treated with dexamethasone in their drinking water (Toxo/Dexa-treated group), after which the antibody titers were serially checked. The specific IgG, IgG2a, IgA, and IgM titers of the Toxo/Dexa-treated mice were depressed significantly after dexamethasone treatment. In particular, the titers of IgG2a and IgM were decreased markedly (Figs. 3C and 3E, respectively). We detected no specific difference in IgG1 titers between the Toxo-infected and Toxo/Dexa-treated mice (Fig. 3B).
Temporary decrease in the proportions of CD4+ and CD8α+ T cells after dexamethasone treatment
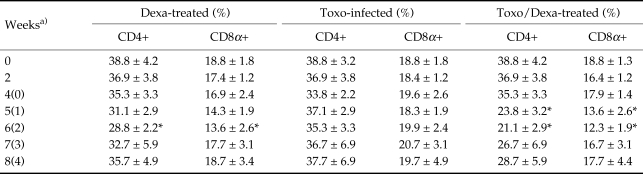
The proportions of each T cell subset in the Toxo-infected, Dexa-treated, and Toxo/Dexa-treated mice are shown in Table 1. In the uninfected mice, the relative percentages of CD4+ and CD8α+ T cells in the spleen were approximately 38.8 ± 4.2% and 18.8 ± 1.8%, respectively. The percentages of CD4+ T cells of splenocytes from the Toxo-infected mice were not significantly altered in comparison to those observed in the uninfected mice, although we did observed a temporary reduction. In the Dexa- and Toxo/Dexa-treated mice, the percentages of CD4+ or CD8α+ T cells were reduced significantly 2 weeks after dexamethasone treatment (38.8 ± 4.2% vs. 21.1 ± 2.9% in CD4+ T cells of Toxo/Dexa-treated mice, P = 0.003; 18.8 ± 1.8% vs. 12.3 ± 1.9% in CD8+ T cells, P = 0.024), and then slowly recovered to original levels thereafter.
Depression of IFN-γ and IL-10, but not IL-4, productions after dexamethasone treatment
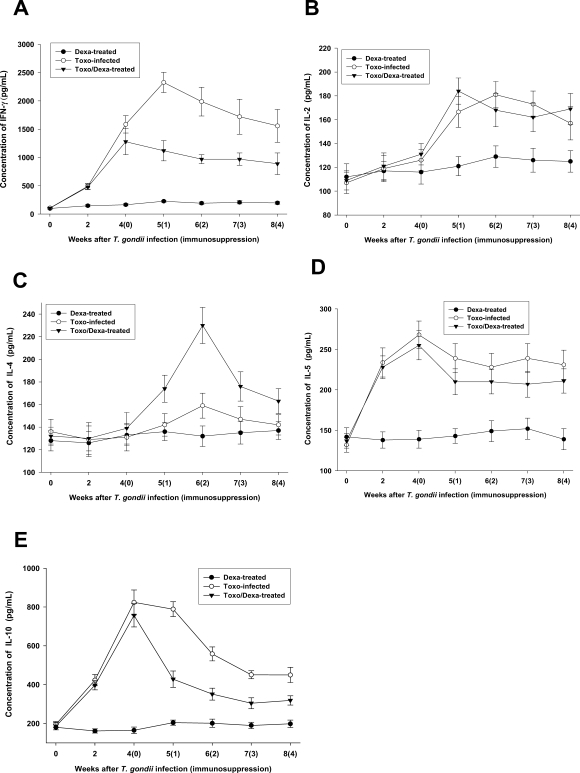
We assessed the production of Th1 and Th2 cytokines from the culture supernatants from splenocytes. The spleen cells from the Toxo-infected, Dexa-treated, and Toxo/Dexa-treated mice were stimulated for 48 hr with TLA, after which the levels of IFN-γ, IL-2, IL-4, IL-5, and IL-10 were assessed using ELISA (Figs. 4A, 4B, 4C, 4D and 4E, respectively). IFN-γ, IL-2, IL-4, IL-5, and IL-10 production levels in the Dexa-treated mice were similar to those seen in the uninfected mice (P > 0.05). In the Toxo-infected mice, IFN-γ, IL-5 and IL-10 productions increased immediately after T. gondii infection (P < 0.05), whereas IL-2 production was increased significantly after 2 weeks PI. At 5 weeks PI, the IFN-γ production levels of the Toxo-infected mice increased by more than 20-fold those of the uninfected mice. However, IL-2 and IL-5 productions increased only by approximately 2 fold. IL-4 production in the Toxo-infected mice remained at basal levels until 4 weeks PI, after which IL-4 production increased slightly (Fig. 4C). After immunosuppression, IFN-γ and IL-10 productions were depressed significantly (Figs. 4A and 4E, respectively), whereas IL-4 production increased markedly 2 weeks after immunosuppression (i.e., 6 weeks PI) (Fig. 4C). IL-2 and IL-5 productions in the Toxo/Dexa-treated mice evidenced no significant differences in comparison to the Toxo-infected mice (Figs. 4B and 4D).
Fig. 4.
In vitro production of IFN-γ (A), IL-12 (B), IL-4 (C), IL-5 (D) and IL-10 (E) from splenocytes of mice either infected with T. gondii or orally treated with dexamethasone. Splenocytes were cultured for 48 hr at 37℃ with Toxoplama lysate antigen, after which the cytokine concentrations in the supernatants were assayed via ELISA. The data are expressed as the means ± SD of 5 mice.
DISCUSSION
The profile of T cell cytokine synthesis is directly relevant to regulation of the immune response, as well as determination of relative resistance or susceptibility to T. gondii (Denkers and Gazzinelli, 1998; Lieberman and Hunter, 2002). In our study, BALB/c mice infected with T. gondii (Toxo-infected group) evidenced a predominantly Th1-polarized response, whereas the dexamethasone-treated Toxoplasma infected mice (Toxo/Dexa-treated group) were significantly depressed with regard to IgG2a, IgA, IgM, IFN-γ and IL-10 productions, as compared to the Toxo-infected mice. However, IgG1, IL-4, IL-2, and IL-5 productions in the Toxo/Dexa-treated mice were similar to or higher than what were observed in the Toxo-infected mice. These findings demonstrate that reactivation of toxoplasmosis by dexamethasone treatment induces depression of Th1 immune responses, but does not significantly influence Th2 immune responses.
We used a T. gondii-resistant BALB/c strain in this study. This may be the reason why susceptible strains (e.g., C57BL/6) spontaneously induce necrotizing toxoplasmic encephalitis during the late stage of infection, which means that resistant strains of mice are more suitable than susceptible strains with regard to the maintenance of chronic latent infections (Suzuki, 1999, 2002b). In our study, some mice evidenced classical locomotor signs of cerebral toxoplasmosis after treatment with dexamethasone, and displayed focal necrosis, an accumulation of inflammatory cells, and foci of gliosis in the brain (data not shown), which are typical findings of toxoplasmic encephalitis (Nicoll et al., 1997). The mean survival time (in days) of the Toxo/Dexa-treated mice were significantly decreased, whereas the parasite burdens in the brain were elevated. These data demonstrate that the immunosuppression of chronically infected mice may result in the reactivation of a latent infection, which is initiated by the disruption of cysts and followed by the proliferation of tachyzoites (Suzuki, 2002b).
Many reports have asserted that immune cytokines are essential with regard both to regulatory and effecter actions in determining the outcome of intracellular infection. IFN-γ has been demonstrated to be crucial both for the early control of tachyzoite expansion, and for the prevention of the reactivation of dormant parasite stages (Denkers and Gazzinelli, 1998; Suzuki, 2002a, b). IL-10 performs a vital function in the control of inflammatory responses during acute T. gondii infection (Wilson et al., 2005), and IL-4 is the primary promoter of type-2 immune responses (Nickdel et al., 2004). The results of the present study showed that IFN-γ and IL-10 productions were markedly increased immediately after T. gondii infection. However, the production of IL-4 in the Toxo-infected mice remained at basal levels until 3 weeks PI, after which IL-4 production increased slightly. These results were similar to those of a previous report, which demonstrated that IFN-γ, TNF-α, IL-2, IL-6, IL-10 and IL-12 mRNA in the brains and spleens of Toxoplasma-infected animals were induced to a significant extent, but that only low levels of IL-4 mRNA were detectable (Deckert-Schluter et al., 1995). These results show that T. gondii infection can basically elicit responses tending toward a Th1 immune response. After dexamethasone treatment, IFN-γ and IL-10 productions were depressed significantly, whereas the IL-4 production was significantly increased. These findings revealed that reactivation of murine toxoplasmosis induced a depression of Th1 immune responses; however, Th2 immune responses were activated. In the present study, IL-4 production was markedly increased in Toxo/Dexa-treated mice after dexamethasone treatment, whereas the productions of other cytokines were decreased or similar to Toxoplasma-infected mice. These phenomena indicate that dexamethasone induced the immunosuppression of Toxoplasma-infected mice; therefore, IL-4 production was significantly increased as a main promoter of Th2 immune response and as a counter-regulating Th1 immunity (Heinzel et al., 1989; Nickdel et al., 2004).
Protective immunity against T. gondii is dependent on a potent, cell-mediated, T cell-dependent immune response (Denkers and Gazzinelli, 1998; Filisetti and Candolfi, 2004). CD8+ T cells are of principal importance in generation of protective immunity against toxoplasmosis (Casciotti et al., 2002). In particular, the CD8+ subset of Vβ8+ T cells constitutes a major afferent limb of IFN-γ-mediated resistance against toxoplasmic encephalitis in BALB/c mice (Wang et al., 2005). Recent studies have shown that NK cells can perform an important function in the induction of primary CD8+ T-cell immunity against intracellular infection in the absence of CD4+ T cells (Combe et al., 2005). According to our results, the proportions of CD8α+ T cells in the Toxo-infected mice were similar to those of the control group; however, the percentages of CD8α+ T cells and the production of IFN-γ in the Toxo/Dexa-treated mice were significantly reduced after the administration of dexamethasone. These results show that decreased percentages of CD8α+ T cells in Toxo/Dexa-treated mice may depress IFN-γ production, followed by an increment of Toxoplasma parasites within the brain (Casciotti et al., 2002; Wang et al., 2005).
B cells or antibodies are also important components of acquired resistance against T. gondii. However, there have been few reports regarding B cell responses or antibody titers in a toxoplasmosis model. Guk et al. (2005) reported that the intracellular infection of murine splenic lymphocytes with T. gondii tachyzoites impaired their capacity to generate cytokine and immunoglobulin secretions. In the present study, antibody titers of serum IgG, IgG2a, and IgM increased immediately after T. gondii infection, whereas IgG1 titers increased significantly after 5 weeks PI. After dexamethasone treatment, serum IgG2a and IgM titers of the Toxo/Dexa-treated mice were depressed significantly, whereas serum IgG1 titers were not significantly altered. These data indicate that T. gondii infection basically elicits a tendency toward a Th1 immune response; however, Th1 immune responses were depressed after immunosuppression, but not Th2 immune responses. Finally, our results may have important roles to the control and management of immunosuppressive individuals, including those with human immunodeficiency virus infection.
Table 1.
The kinetics of phenotype changes of splenocytes from BALB/c mice either infected with T. gondii or treated with dexamethasone orally
a)Weeks after T. gondii infection (dexamethasone treatment).
* Statistically significant differences as compared with control group (day 0). Data are presented as the mean ± SD of 5 mice. Splenocytes were stained with FITC-conjugated CD4+ or CD8α+ mAb, and then analyzed by a FACScan.
Footnotes
This work was supported by a Korea Research Foundation Grant (KRF-2003-002-E00047).
References
- 1.Casciotti L, Ely KH, Williams ME, Khan IA. CD8+-T-cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4+ T cells. Infect Immun. 2002;70:434–443. doi: 10.1128/IAI.70.2.434-443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen M, Mun HS, Piao LX, Aosai F, Norose K, Mohamed RM, Belal US, Fang H, Ahmed AK, Kang HK, Matsuzaki G, Kitamura D, Yano D. Induction of protective immunity by primed B-1 cells in Toxoplasma gondii-infected B cell-deficient mice. Microbiol Immunol. 2003;47:997–1003. doi: 10.1111/j.1348-0421.2003.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 3.Combe CL, Curiel TJ, Moretto MM, Khan IA. NK cells help to induce CD8+-T-cell immunity against Toxoplasma gondii in the absence of CD4+ T cells. Infect Immun. 2005;73:4913–4921. doi: 10.1128/IAI.73.8.4913-4921.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deckert-Schluter M, Albrecht S, Hof H, Wiestler OD, Schluter D. Dynamics of the intracerebral and splenic cytokine mRNA production in Toxoplasma gondii-resistant and -susceptible congenic strains of mice. Immunology. 1995;85:408–418. [PMC free article] [PubMed] [Google Scholar]
- 5.Denkers EY, Gazzinelli RT. Regulation of function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filisetti D, Candolfi E. Immune response to Toxoplasma gondii. Ann Ist Super Sanita. 2004;40:71–80. [PubMed] [Google Scholar]
- 7.Guk SM, Kook J, Jeon YH, Choi JH, Han ET, Shin EH, Chai JY. Suppressed cytokine and immunoglobulin secretions by murine splenic lymphocytes infected in vitro with Toxoplasma gondii tachyzoites. J Parasitol. 2005;91:467–470. doi: 10.1645/GE-404R. [DOI] [PubMed] [Google Scholar]
- 8.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang H, Remington JS, Suzuki Y. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-γ, TNF-α and inducible nitric oxide synthase. J Immunol. 2000;164:2629–2634. doi: 10.4049/jimmunol.164.5.2629. [DOI] [PubMed] [Google Scholar]
- 10.Kasper LH. Harrison's Principles of Internal Medicine. 16th. New York, USA: McGraw-Hill company; 2004. Toxoplasma infection; pp. 1222–1227. [Google Scholar]
- 11.Lieberman LA, Hunter CA. The role of cytokines and their signaling pathways in the regulation of immunity to Toxoplasma gondii. Int Rev Immunol. 2002;21:373–403. doi: 10.1080/08830180213281. [DOI] [PubMed] [Google Scholar]
- 12.Meyer DJ, Allan JE, Beaman MH. Distribution of parasite stages in tissues of Toxoplasma gondii infected SCID mice and human peripheral blood lymphocyte-transplanted SCID mice. Parasite Immunol. 2000;22:567–579. doi: 10.1046/j.1365-3024.2000.00338.x. [DOI] [PubMed] [Google Scholar]
- 13.Nickdel MB, Lyons RE, Roberts F, Brombacher F, Hunter CA, Alexander J, Roberts CW. Intestinal pathology during acute toxoplasmosis is IL-4 dependent and unrelated to parasite burden. Parasite Immunol. 2004;26:75–82. doi: 10.1111/j.0141-9838.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- 14.Nicoll S, Wright S, Maley SW, Burns S, Buxton D. A mouse model of recrudescence of Toxoplasm gondii infection. J Med Microbiol. 1997;46:263–266. doi: 10.1099/00222615-46-3-263. [DOI] [PubMed] [Google Scholar]
- 15.Romagnani S. Understanding the role of Th1/Th2 cells in infection. Trends Microbiol. 1996;471:470–473. doi: 10.1016/s0966-842x(97)82906-x. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y. Genes, cells and cytokines in resistance against development of toxoplasmic encephalitis. Immunobiology. 1999;201:255–271. doi: 10.1016/S0171-2985(99)80066-7. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki Y. Host resistance in the brain against Toxoplasma gondii. J Infect Dis. 2002a;185(Suppl 1):S58–S65. doi: 10.1086/337999. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki Y. Immunopathogenesis of cerebral toxoplasmosis. J Infect Dis. 2002b;186(Suppl 2):S234–S240. doi: 10.1086/344276. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Claflin J, Kang H, Suzuki Y. Importance of CD8+Vbeta8+ T cells in IFN-gamma-mediated prevention of toxoplasmic encephalitis in genetically resistant BALB/c mice. J Interferon Cytokine Res. 2005;25:338–344. doi: 10.1089/jir.2005.25.338. [DOI] [PubMed] [Google Scholar]
- 20.Wilson EH, Wille-Reece U, Dzierszinski F, Hunter CA. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J Neuroimmunol. 2005;165:63–74. doi: 10.1016/j.jneuroim.2005.04.018. [DOI] [PubMed] [Google Scholar]