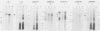Abstract
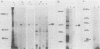
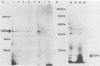
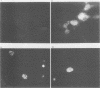
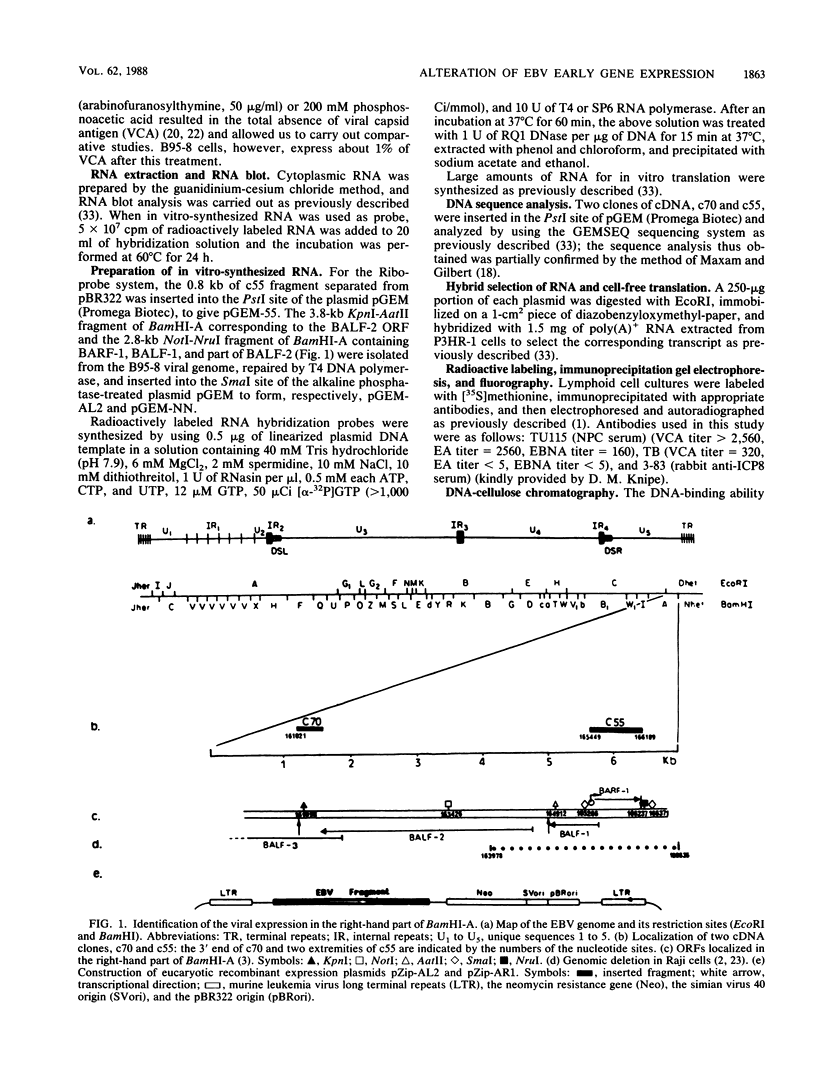
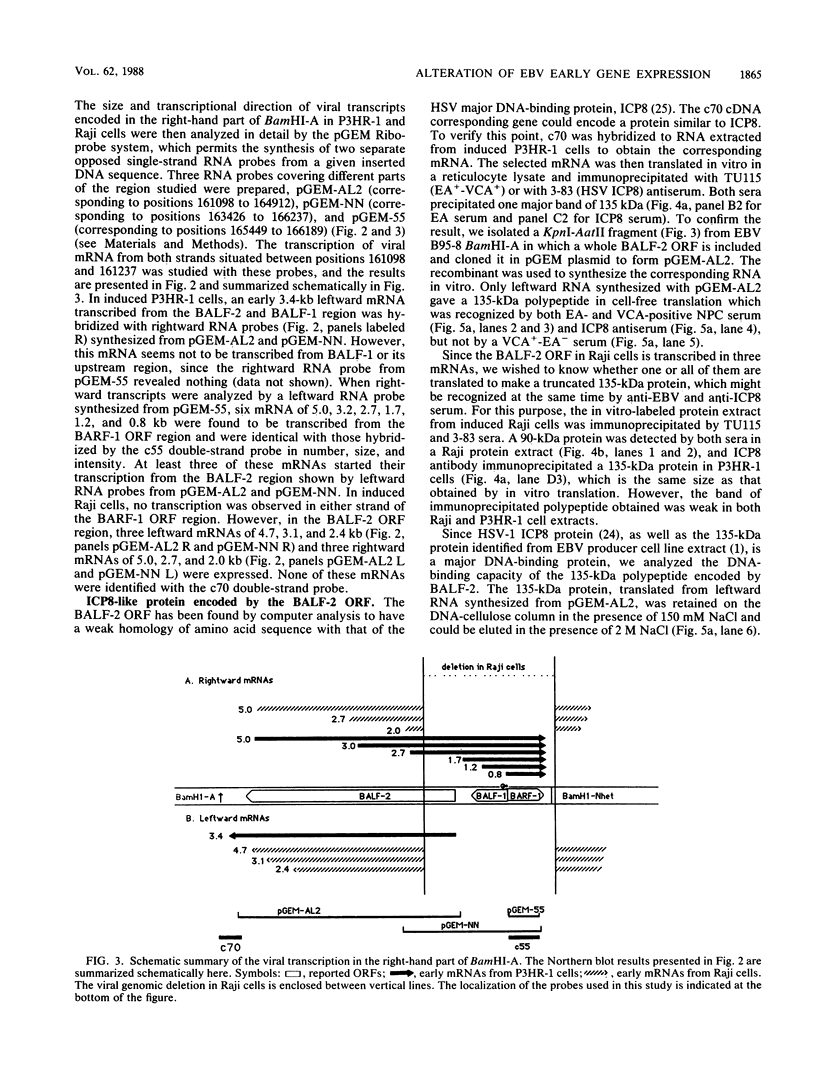
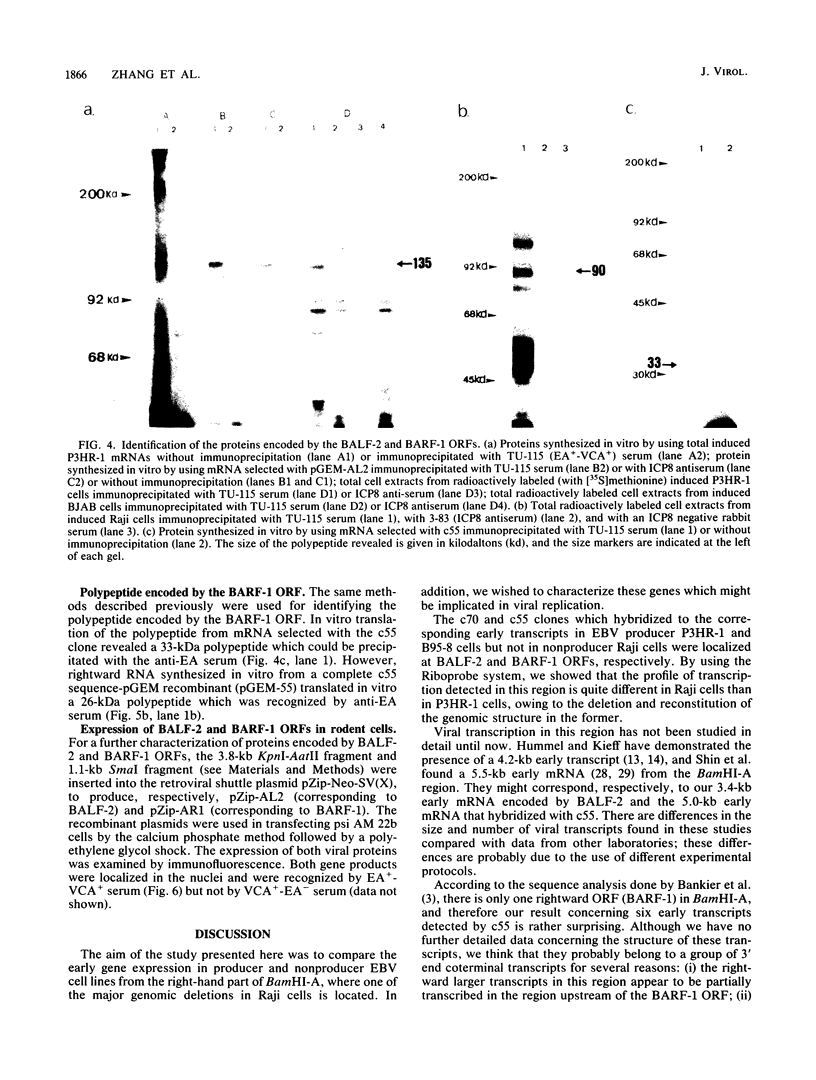
The Epstein-Barr virus-carrying lymphoblastoid cell line Raji has two major genomic deletions and is incapable of virus production. Two cDNA clones, c70 and c55, were constructed from early mRNA of P3HR-1 cells and localized, respectively, in BALF-2 and BARF-1 open reading frames where one of the major genomic deletion in Raji cells is situated. These were used to search the different early viral transcripts in producer P3HR-1 and nonproducer Raji lines. c70 and c55 hybridized with their corresponding mRNAs only in producer lines. Analysis with in vitro-synthesized RNA probes showed quite a different transcriptional profile in Raji cells than in P3HR-1 cells. In the P3HR-1 line, BALF-2 encodes a 3.4-kilobase (kb) mRNA during the early phase and a 3.3-kb mRNA during the late phase, and in the Raji line, the probe corresponding to BALF-2 hybridized with three mRNAs of 5.0, 3.1, and 2.4 kb; in P3HR-1 cells, BARF-1 encodes a group of 3'-conterminal transcripts (0.8, 1.2, 1.7, 2.7, 3.2, and 5.0 kb) during both the early and late stages; in Raji cells, however, 0.8-, 1.2-, and 1.7-kb mRNAs are absent, the only mRNAs transcribed being upstream of the deletion and of 5.0, 2.6, and 2.0 kb in size. In vivo and in vitro experiments demonstrated that the BALF-2 open reading frame encodes an early 135-kilodalton (kDa) protein which possesses DNA-binding ability and can be recognized by a herpes simplex virus ICP-8 antiserum. The BARF-1 open reading frame encodes in vitro a 26- to 33-kDa early protein recognized by anti-EA serum. The proteins of both two genes expressed in psi AM 22b cells were localized in nuclei. According to their properties, both proteins, particularly the BALF-2-encoded 135-kDa DNA-binding protein, could play a role in virus replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bankier A. T., Deininger P. L., Satchwell S. C., Baer R., Farrell P. J., Barrell B. G. DNA sequence analysis of the EcoRI Dhet fragment of B95-8 Epstein-Barr virus containing the terminal repeat sequences. Mol Biol Med. 1983 Nov;1(4):425–445. [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Cohen L. K., Speck S. H., Roberts B. E., Strominger J. L. Identification and mapping of polypeptides encoded by the P3HR-1 strain of Epstein-Barr virus. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4183–4187. doi: 10.1073/pnas.81.13.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. D., Mulligan R. C. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6349–6353. doi: 10.1073/pnas.81.20.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson T. J., Barrell B. G., Farrell P. J. Coding content and expression of the EBV B95-8 genome in the region from base 62,248 to base 82,920. Virology. 1986 Jul 15;152(1):136–148. doi: 10.1016/0042-6822(86)90379-x. [DOI] [PubMed] [Google Scholar]
- Godowski P. J., Knipe D. M. Identification of a herpes simplex virus function that represses late gene expression from parental viral genomes. J Virol. 1985 Aug;55(2):357–365. doi: 10.1128/jvi.55.2.357-365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski P. J., Knipe D. M. Mutations in the major DNA-binding protein gene of herpes simplex virus type 1 result in increased levels of viral gene expression. J Virol. 1983 Sep;47(3):478–486. doi: 10.1128/jvi.47.3.478-486.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski P. J., Knipe D. M. Transcriptional control of herpesvirus gene expression: gene functions required for positive and negative regulation. Proc Natl Acad Sci U S A. 1986 Jan;83(2):256–260. doi: 10.1073/pnas.83.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M., Ooka T., Matsuo T., Kieff E. Epstein-Barr virus glycoprotein homologous to herpes simplex virus gB. J Virol. 1987 Feb;61(2):499–508. doi: 10.1128/jvi.61.2.499-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Hummel M., Kieff E. Epstein-Barr virus RNA. VIII. Viral RNA in permissively infected B95-8 cells. J Virol. 1982 Jul;43(1):262–272. doi: 10.1128/jvi.43.1.262-272.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M., Kieff E. Mapping of polypeptides encoded by the Epstein-Barr virus genome in productive infection. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5698–5702. doi: 10.1073/pnas.79.18.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallin B., Klein G. Epstein-Barr virus carried by Raji cells: a mutant in early functions? Intervirology. 1983;19(1):47–51. doi: 10.1159/000149336. [DOI] [PubMed] [Google Scholar]
- Littler E., Purifoy D., Minson A., Powell K. L. Herpes simplex virus non-structural proteins. III. Function of the major DNA-binding protein. J Gen Virol. 1983 May;64(Pt 5):983–995. doi: 10.1099/0022-1317-64-5-983. [DOI] [PubMed] [Google Scholar]
- Manet E., Chevallier A., Zhang C. X., Ooka T., Chavrier P., Daillie J. Construction and use of cDNA clones for the mapping and identification of Epstein-Barr virus early P3HR-1 mRNAs. J Virol. 1985 May;54(2):608–614. doi: 10.1128/jvi.54.2.608-614.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Frame M. C. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the exonuclease gene and neighbouring genes. Nucleic Acids Res. 1986 Apr 25;14(8):3435–3448. doi: 10.1093/nar/14.8.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka T., Calender A. Effects of arabinofuranosylthymine on Epstein-Barr virus replication. Virology. 1980 Jul 15;104(1):219–223. doi: 10.1016/0042-6822(80)90379-7. [DOI] [PubMed] [Google Scholar]
- Ooka T., Calender A., de Turenne M., Daillie J. Effect of arabinofuranosylthymine on the replication of Epstein-Barr virus and relationship with a new induced thymidine kinase activity. J Virol. 1983 Apr;46(1):187–195. doi: 10.1128/jvi.46.1.187-195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka T., Lenoir G. M., Decaussin G., Bornkamm G. W., Daillie J. Epstein-Barr virus-specific DNA polymerase in virus-nonproducer Raji cells. J Virol. 1986 May;58(2):671–675. doi: 10.1128/jvi.58.2.671-675.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack A., Delius H., Zimber U., Bornkamm G. W. Two deletions in the Epstein-Barr virus genome of the Burkitt lymphoma nonproducer line Raji. Virology. 1984 Feb;133(1):146–157. doi: 10.1016/0042-6822(84)90433-1. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Littler E., Purifoy D. J. Nonstructural proteins of herpes simplex virus. II. Major virus-specific DNa-binding protein. J Virol. 1981 Sep;39(3):894–902. doi: 10.1128/jvi.39.3.894-902.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. P., McGeoch D. J. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the genes for DNA polymerase and the major DNA binding protein. Nucleic Acids Res. 1985 Nov 25;13(22):8143–8163. doi: 10.1093/nar/13.22.8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan W. T., Weir A. C. Interaction with nucleic acids and stimulation of the viral DNA polymerase by the herpes simplex virus type 1 major DNA-binding protein. J Virol. 1984 Dec;52(3):727–733. doi: 10.1128/jvi.52.3.727-733.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y. M., Hirschhorn R. R., Mercer W. E., Surmacz E., Tsutsui Y., Soprano K. J., Baserga R. Gene transfer: DNA microinjection compared with DNA transfection with a very high efficiency. Mol Cell Biol. 1982 Sep;2(9):1145–1154. doi: 10.1128/mcb.2.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S., Donovan J., Nonoyama M. Phosphonoacetic acid-resistant RNA of Epstein-Barr virus in productively infected cells. Virology. 1983 Jan 15;124(1):196–200. doi: 10.1016/0042-6822(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Shin S., Tanaka A., Nonoyama M. Transcription of the Epstein-Barr virus genome in productively infected cells. Virology. 1983 Jan 15;124(1):13–20. doi: 10.1016/0042-6822(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Vaughan P. J., Banks L. M., Purifoy D. J., Powell K. L. Interactions between herpes simplex virus DNA-binding proteins. J Gen Virol. 1984 Nov;65(Pt 11):2033–2041. doi: 10.1099/0022-1317-65-11-2033. [DOI] [PubMed] [Google Scholar]
- Weigel R., Miller G. Major EB virus-specific cytoplasmic transcripts in a cellular clone of the HR-1 Burkitt lymphoma line during latency and after induction of viral replicative cycle by phorbol esters. Virology. 1983 Mar;125(2):287–298. doi: 10.1016/0042-6822(83)90202-7. [DOI] [PubMed] [Google Scholar]
- Weller S. K., Lee K. J., Sabourin D. J., Schaffer P. A. Genetic analysis of temperature-sensitive mutants which define the gene for the major herpes simplex virus type 1 DNA-binding protein. J Virol. 1983 Jan;45(1):354–366. doi: 10.1128/jvi.45.1.354-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. X., Decaussin G., de Turenne Tessier M., Daillie J., Ooka T. Identification of an Epstein-Barr virus-specific desoxyribonuclease gene using complementary DNA. Nucleic Acids Res. 1987 Mar 25;15(6):2707–2717. doi: 10.1093/nar/15.6.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]