Abstract
Aminomethyl polystyrene resin was reacted with 4-(5′-formyl-2′-hydroxyphenyl)benzoic acid and 4-(5′-formyl-2′-hydroxyphenyl)phenyl propionic acid, respectively, in the presence of 1-hydroxybenzotriazole and 1,3-diisopropylcarbodiimide to yield polymer-bound benzaldehydes. The phenolic group in resins was acetylated with acetic anhydride to afford two polymer-bound 4-acetoxybenzaldehydes. The reductive amination of polymer-bound linkers by amines and sodium triacetoxyborohydride, followed by sulfonylation with alkyl or arylsulfonyl chloride derivatives in the presence of pyridine and the cleavage with TFA/DCM, produced pure sulfonamides.
Solid-phase organic synthesis has emerged as a powerful tool to generate large molecular libraries1-3 of small-sized molecules and to accelerate lead discovery and optimization processes. The challenge now is to extend the ability of solid-phase chemistry to generate a large number of structurally diversified compounds by developing suitable polymer-bound reagents4,5 that accommodate the synthesis of various compounds.
The reagents have to be attached to the polymer through a linker. Linkers that can generate different compounds depending on the attached compounds and the cleavage conditions are needed. A number of polymer-bound linkers have been used for the synthesis of different compounds.5-29 In some cases, the purification of final products is still needed, because of the instability of polymer-bound linkers in different reaction conditions and/or the leakage of different materials upon cleavage. Therefore, the polymer-bound linkers that have application in producing pure final products are preferred. Stable polymer-bound linkers are needed that provide the concomitant cleavage of pure and various products and removal of the linker group without any loss in overall synthetic efficiency. Furthermore, for the synthesis of diverse number of compounds, such as organosulfur and organophosphorus compounds, polymer-bound linkers have not been developed extensively.
To explore further the synthetic utility of the polymer-bound linkers as tools for the synthesis of diverse number of compounds without the need of purification in the final cleavage step, two new polymer-bound linkers of p-acetoxybenzaldehyde, 1a and 1b, were synthesized (Figure 1).
Figure 1.
Polymer-bound linkers of p-acetoxybenzaldehyde, 1a and 1b.
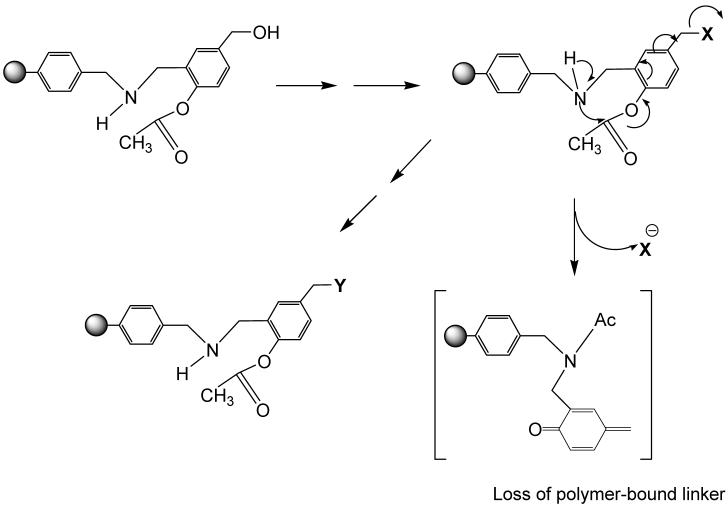
Because of the proximity of the amino group and p-acetoxy group in the previous reported polymer-bound linkers of p-acetoxybenzyl alcohol20,23,24, the intramolecular reactions caused uncontrolled partial release of some intermediates before further modifications on attached moieties (X) (Scheme 1). Therefore, the yield of final products was lower than expected.
Scheme 1.
The intramolecular reaction in polymer-bound intermediates derived from polymer-bound p-acetoxybenzyl alcohol reduces the efficiency in solid-phase synthesis, because of the partial loss and release of some of the unmodified intermediates.
Polymer-bound p-acetoxybenzaldehydes 1a and 1b offered several advantages compared to other polymer-bound linkers reported by us and others.20,23,24 First, a large separation between the nitrogen atom in amide and p-acetoxy group was introduced in new polymer-bound linkers 1a and 1b. The presence of the nitrogen atom in amide form and the large distance between amide bond and p-acetoxy group minimized the intramolecular reaction between these functional groups. The polymer-bound intermediates were stable even in basic conditions. Second, 1a and 1b were designed to produce final products without the need for purification. The final products were synthesized in a short period, high yields, and parallel format, and were easily separated from the resin-bound linkers. Finally, 1a and 1b were stable in basic conditions (e.g., pyridine). This allows the potential use of 1a and 1b for the synthesis of diverse classes of compounds by converting them to polymer-bound amines through reductive amination and further substitution of amino functional groups. To demonstrate this, their application in solid-phase organic synthesis of sulfonamides is shown here. Presumably, 1a and 1b can be reduced to polymer-bound benzyl alcohols and then be used for the synthesis of monophosphorylated compounds as shown previously for other polymer-bound linkers of p-acetoxybenzyl alcohol.21-25
For the synthesis of polymer-bound p-acetoxybenzaldehydes 1a and 1b, building block linkers, 4-(5′-formyl-2′-hydroxyphenyl)benzoic acid (5a) and 4-(5′-formyl-2′-hydroxyphenyl)phenyl propionic acid (5b), were required for the attachment to aminomethyl polystyrene resin. The reaction of 3-bromo-4-hydroxybenzaldehyde (4) in toluene/ethanol/water with 4-(4,4,5,5-tetramethyl-1,3,2-bioxaborolan-2-yl)benzoic acid (3a) and [4-(2-carboxyethyl)phenyl]boronic acid (3b), respectively, in the presence of tetrakis(triphenylphosphine)palladium (Pd(Ph3P)4) at 110 °C for 13 h afforded (5a, 83%) and (5b, 95%) (Scheme 2). Compound 3a was synthesized quantitatively by the reaction between 2,3-dimethyl-2,3-butanediol (pinacol) and 4-carboxylphenylboronic acid (2) in THF/toluene (Scheme 2). Although compound 2 can be used directly in reaction with 4, we used protected form of boronic acid 3a. It appears that the protection of boronic acid is not essential, since 5b was produced from unprotected 3b in a slightly higher yield when compared to that of 5a from protected 3a.
Scheme 2.
Synthesis of Building Block Linkers 5a and 5b.
Building block linkers 5a and 5b were used for the synthesis of 1a and 1b, respectively (Scheme 3). The reaction of aminomethyl polystyrene resin 6 with 5a and 5b, respectively, in the presence of 1-hydroxybenzotriazole (HOBt) and 1,3-diisopropylcarbodiimide (DIC) afforded 7a-b. Polymer-bound p-acetoxybenzaldehydes, 1a-b, were synthesized by the reaction of 7a-b with acetic anhydride in the presence of pyridine (Scheme 3).
Scheme 3.
Synthesis of Solid-Phase Polymer-Bound Linkers of 4-Acetoxy-3-phenylbenzaldehyde (1a-b).
We investigated whether developed polymer-bound linkers can have potential application for the synthesis of sulfonamides. Sulfonamides are currently used as therapeutic agents, such as sulfa antibiotics (e.g., sulfathiazole) or serotonin antagonists (e.g., antimigraine Sumatriptan).30 Isoquinoline sulfonamides inhibit protein kinases by competing with ATP.31-33 Although sulfonamides can be synthesized through solution-phase methods, the synthesis of large library of sulfonamides using these strategies is cumbersome, because a large number of final products need to be purified. Several solid-phase routes have been reported for the synthesis of sulfonamides.34,35 We examined polymer-bound linkers of p-acetoxybenzaldehyde (1a-b) for the synthesis of sulfonamides to demonstrate their general application.
The solid-phase synthetic strategy of sulfonamides consisted of three steps (Scheme 4): reductive amination, sulfonylation, and cleavage. The reductive amination of a polymer-bound aldehydes 1a-b by amines (e.g., aniline, 4-chloroaniline, benzylamine, 4-methoxybenzylamine,) and sodium triacetoxyborohydride (NaBH(OAc)3) afforded 9-12a and 9-12b. Sulfonylation of resin-bound amines 9-12a and 9-12b with alkyl or arylsulfonyl chloride derivatives (e.g., p-toluenesulfonyl chloride, 3,5-dimethoxybenzene-1-sulfonyl chloride, 3-nitrobenzenesulfonyl chloride) in the presence of pyridine gave polymer-bound sulfonamides 13-24a and 13-24b. The sulfonamide remained bound to the polymer-bound linker under basic conditions. The activation step by hydrolysis of the acetyl group and cleavage of the products from the resins in TFA/DCM/H2O (74:24:2 v/v/v) produced pure sulfonamides 26-37 (>98%) (Scheme 4). In total, by using different combinations of amines and sulfonyl chlorides, 12 compounds were synthesized using both polymer-bound linkers (overall yield 60-83% calculated from 1a-b). Compounds 27, 30, 33, and 36 are novel compounds.
Scheme 4.
Synthesis of Sulfonamides.
The cleavage mechanism of final sulfonamides from 13-24a and 13-24b is shown in Scheme 4. The multi-step cleavage mechanism is shown in one step here for simple demonstration. The linkers remained covalently bound on the resins using both polymer-bound linkers, which facilitated the separation of the final products by filtration.
Figure 2 shows the chemical structures of the synthesized compounds. The final products were characterized by nuclear magnetic resonance spectra (1H NMR and 13C NMR), and high-resolution time-of-flight electrospray mass spectrometry.
Figure 2.
Structures of Synthesized Sulfonamides.
Products were compared for yield and purity. There were no significant differences in the purity of the final products using resin-bound linkers 1a and 1b (>98%), but most compounds were produced from 1a in higher yields than those from 1b (Table 1). The compounds did not need any purification (>98% pure) compared with the previously reported solid-phase methods for the synthesis of sulfonamides,34,35 and were produced in comparable or higher yields.
Table 1.
Overall Isolated Yields and Final Cleavage Yields of Products for Sulfonamides (26-37)
| No. | Overall yield (%) calcd from 1a | Overall yield (%) calcd from 1b | Final Cleavage Yield (%) from 13-24a | Final Cleavage Yield (%) from 13-24b |
|---|---|---|---|---|
| 26 | 83 | 72 | 94 | 89 |
| 27 | 78 | 73 | 91 | 86 |
| 28 | 80 | 74 | 96 | 89 |
| 29 | 82 | 76 | 88 | 93 |
| 30 | 81 | 74 | 89 | 89 |
| 31 | 72 | 60 | 84 | 78 |
| 32 | 70 | 78 | 85 | 90 |
| 33 | 77 | 74 | 90 | 87 |
| 34 | 62 | 74 | 80 | 89 |
| 35 | 67 | 74 | 88 | 88 |
| 36 | 76 | 79 | 95 | 90 |
| 37 | 67 | 72 | 88 | 86 |
In conclusion, two stable polymer-bound linkers were synthesized and used for the solid-phase synthesis of sulfonamides. The amines and sulfonyl chlorides were mixed with the polymer-bound linkers, respectively, and were thereby “captured” as immobilized compounds. Washing the support guaranteed that no unreacted starting amines or sulfonyl chlorides remained. In the final cleavage reaction, the linkers remained covalently bound on the resins, which facilitated the separation of the final products by filtration. This solid-phase strategy allowed the synthesis of sulfonamides in a short synthetic route without the need for purification of intermediates and final products. Furthermore, this strategy offered the advantages of facile isolation and recovery of pure final products. These polymer-bound linkers can be used for solid-phase preparation of other biologically important compounds.
Experimental Section
As a representative example, resin 1a (1.62 g, 0.78 mmol/g) was swelled in 1,2-dichloroethane (30 mL) and was shaken at room temperature for 15 min. Aniline (0.6 mL, 6.4 mmol) was added to the swelled resin. The mixture was shaken for 1 h at room temperature. NaBH(OAc)3 (1.35 g, 6.4 mmol) was added to the reaction mixture, After 6 h shaking at room temperature, the resin was collected by filtration and washed with water (2 × 30 mL), DCM (2 × 30 mL), and MeOH (2 × 30 mL), respectively, and dried under vacuum to give 9a (1.71 g, 94%, 0.70 mmol/g). Polymer-bound aniline 9a (550 mg, 0.70 mmol/g) was swelled in cold dry pyridine (30 mL). To the swelled resin p-toluenesulfonyl chloride (1.0 g, 5.26 mmol) was added and the reaction was stirred at room temperature for 6 h. The resin was washed with water (2 × 30 mL), DCM (2 × 30 mL) and MeOH (2 × 30 mL), and dried under vacuum for 24 h to give 13a (606 mg, 95%, 0.60 mmol/g). Polymer-bound sulfonamide 13a (606 mg, 0.60 mmol/g) was suspended in TFA/DCM/H2O (74:24:2 v/v/v, 5 mL). After 30 min shaking of the mixture at room temperature, the resin was collected by filtration and washed with DCM (5 mL), THF (5 mL), and MeOH (5 mL). The filtrate was evaporated at room temperature and the residue was washed with water (10 mL) and extracted with ethyl acetate (2 × 10 mL). The organic phase was dried with anhydrous sodium sulfate and evaporated to afford pure 4-methyl-N-phenylbenzenesulfonamide (26, 84 mg, 94%; overall yield calculated from 1a, 83%). 1H NMR (CDCl3, 400 MHz, δ ppm): 7.70 (d, J = 8.30 Hz, 2H), 7.30-7.21 (m, 4H), 7.19 (br s, 1H), 7.14-7.06 (m, 3H), 2.37 (s, 3H); 13C NMR (CDCl3, 100 MHz, δ ppm): 144.3, 137.0, 136.4, 130.1, 129.7, 127.7, 125.6, 121.9, 22.0; HR-MS (ESI-TOF) (m/z) for C13H13NO2S: calcd, 247.0667; found, 248.2760 [M + H]+.
Supplementary Material
Acknowledgement
We acknowledge the financial support from National Center for Research Resources, NIH, Grant Number 1 P20 RR16457
References
- (1).Gallop MA, Barret RW, Dower WJ, Fodor SPA, Gordon EM. J. Med. Chem. 1994;37:1233–1251. doi: 10.1021/jm00035a001. [DOI] [PubMed] [Google Scholar]
- (2).Gordon EM, Barret RW, Dower WJ, Fodor SP, Gallop MA. J. Med. Chem. 1994;37:1385–1401. doi: 10.1021/jm00036a001. [DOI] [PubMed] [Google Scholar]
- (3).Terrett NK, Gardner M, Gordon DW, Kobylecki RJ, Steele J. Tetrahedron. 1995;51:8135–8173. [Google Scholar]
- (4).James IW. Tetrahedron. 1999;55:4855–4946. [Google Scholar]
- (5).Backes BJ, Virgilio AA, Ellman JA. J. Am. Chem. Soc. 1996;118:3055–3056. [Google Scholar]
- (6).Kenner GW, McDermott JR, Sheppard RC. Chem. Commun. 1971;12:636–637. [Google Scholar]
- (7).Backes BJ, Ellman JA. J. Am. Chem. Soc. 1994;116:11171–11172. [Google Scholar]
- (8).Backes BJ, Ellman JA. J. Org. Chem. 1999;64:2322–2330. doi: 10.1021/jo990271w. [DOI] [PubMed] [Google Scholar]
- (9).Backes BJ, Dragoli DR, Ellman JA. J. Org. Chem. 1999;64:5472–5478. doi: 10.1021/jo990271w. [DOI] [PubMed] [Google Scholar]
- (10).Link A, van Calenbergh S, Herdewijn P. Tetrahedron Lett. 1998;39:5175–5176. [Google Scholar]
- (11).Golisade A, Herforth C, Wieking K, Kunick C, Link A. Bioorg. Med. Chem. Lett. 2001;11:1783–1786. doi: 10.1016/s0960-894x(01)00296-7. [DOI] [PubMed] [Google Scholar]
- (12).Routledge A, Abell S, Balasubramanian S. Tetrahedron Lett. 1997;38:1227–1230. [Google Scholar]
- (13).Lee HB, Balasubramanian S. J. Org. Chem. 1999;64:3454–3460. doi: 10.1021/jo981987e. [DOI] [PubMed] [Google Scholar]
- (14).Wade WS, Yang F, Sowin TJ. J. Comb. Chem. 2000;2:266–275. doi: 10.1021/cc990077n. [DOI] [PubMed] [Google Scholar]
- (15).Hulme B, Peng J, Morton G, Salvino JM, Herpin T, Labaudiniere R. Tetrahedron Lett. 1998;39:7227–7230. [Google Scholar]
- (16).Panke G, Frank R. Tetrahedron Lett. 1998;39:17–18. [Google Scholar]
- (17).Nicolaou KC, Winssinger N, Hughes R, Smethurst C, Cho SY. Angew. Chem., Int. Ed. 2000;39:1084–1088. doi: 10.1002/(sici)1521-3773(20000317)39:6<1084::aid-anie1084>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- (18).Scicinski JJ, Congreve MS, Ley SV. J. Comb. Chem. 2004;6:375–384. doi: 10.1021/cc0499791. [DOI] [PubMed] [Google Scholar]
- (19).Estep KG, Neipp CE, Stramiello LMS, Adam MD, Allen MP, Robinson S, Roskamp EJ. J. Org. Chem. 1998;63:5300–5301. [Google Scholar]
- (20).Chitkul B, Atrash B, Bradley M. Tetrahedron Lett. 2001;42:6211–6214. [Google Scholar]
- (21).Parang K, Fournier EJ-L, Hindsgaul O. Org. Lett. 2001;3:307–309. doi: 10.1021/ol0069498. [DOI] [PubMed] [Google Scholar]
- (22).Parang K. Bioorg. Med. Chem. Lett. 2002;12:1863–1866. doi: 10.1016/s0960-894x(02)00266-4. [DOI] [PubMed] [Google Scholar]
- (23).Ahmadibeni Y, Parang K. J. Org. Chem. 2005;70:1100–1103. doi: 10.1021/jo048113e. [DOI] [PubMed] [Google Scholar]
- (24).Ahmadibeni Y, Parang K. Org. Lett. 2005;7:5589–5592. doi: 10.1021/ol0521432. [DOI] [PubMed] [Google Scholar]
- (25).Ahmadibeni Y, Parang K. J. Org. Chem. 2006;71:5837–5839. doi: 10.1021/jo0610107. [DOI] [PubMed] [Google Scholar]
- (26).Ahmadibeni Y, Parang K. Org. Lett. 2005;7:1955–1958. doi: 10.1021/ol050385w. [DOI] [PubMed] [Google Scholar]
- (27).Kan J, Toy P. J. Sulfur Chem. 2005;26:509–540. [Google Scholar]
- (28).McAllister LA, McCormick RA, Procter DJ. Tetrahedron. 2005;61:11527–11576. [Google Scholar]
- (29).Ahmadibeni Y, Parang K. J. Org. Chem. 2006 doi: 10.1021/jo0611115. Web Release Date: 11-Jul-2006; DOI: 10.1021/jo0611115. [DOI] [PubMed] [Google Scholar]
- (30).Meng CQ. Curr. Med. Chem. 1997;4:385–404. [Google Scholar]
- (31).Xu RM, Carmel G, Kuret J, Cheng X. Proc. Nat. Acad. Sci. U. S. A. 1996;93:6308–6313. doi: 10.1073/pnas.93.13.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hidaka H, Inagaki M, Kawamoto S, Sasaki Y. Biochemistry. 1984;23:5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- (33).Ricouart A, Gesquiere JC, Tartar A, Sergheraert C. J. Med. Chem. 1991;34:73–78. doi: 10.1021/jm00105a012. [DOI] [PubMed] [Google Scholar]
- (34).Fivush AM, Willson TM. Tetrahedron Lett. 1997;38:7151–7154. [Google Scholar]
- (35).Raju A, Kogan TP. Tetrahedron Lett. 1997;38:3373–3376. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.