Abstract

Olefin metathesis in aqueous solvents is sought for applications in green chemistry and with the hydrophilic substrates of chemical biology, such as proteins and polysaccharides. Most demonstrations of metathesis in water, however, utilize exotic complexes. We have examined the performance of conventional catalysts in homogeneous water–organic mixtures, finding that the second-generation Hoveyda–Grubbs catalyst has extraordinary efficiency in aqueous dimethoxyethane and aqueous acetone. High (71–95%) conversions are achieved for ring-closing and cross metathesis of a variety of substrates in these solvent systems.
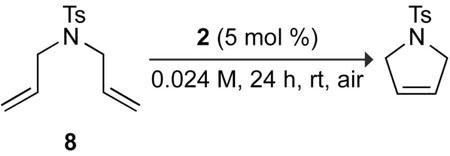
As a highly effective means for creating carbon–carbon bonds, olefin metathesis is a privileged reaction in the armamentarium of synthetic and polymer chemists.1 Readily available and highly active ruthenium catalysts 1–4 have popularized metathesis chemistry in organic solvents (Figure 1).2,3 On the other hand, metathesis in aqueous solvents largely remains the domain of catalysts designed expressly for use in water (Figure 2).4,5,6 Despite the allure of aqueous olefin metathesis for biological applications7 and green chemistry,8 the synthesis of these water-soluble ligands and complexes imposes barriers to their wider use. Developing aqueous reaction conditions suitable for catalysts such as 1–4 is an alternative approach. In the initial work with well-defined catalysts in aqueous systems, Grubbs and coworkers performed ring-openingmetathesis polymerization (ROMP) with 1 in aqueous emulsions,9 a method that allowed Kiessling and coworkers to synthesize biologically active glycopolymers.10 For ring-closing metathesis (RCM) and cross metathesis (CM), Blechert and coworkers employed commercially-available 2 and complex 6c, a relative of commercially-available 4, in water–methanol and water–DMF mixtures.6 Although they achieved high conversion in the ring-closure of N-tosyldiallylamine (8) in 3:1 water/methanol and 3:1 water/DMF over an extended reaction time of 12 h, these reactions were not accomplished in homogenous systems: neither the substrate nor the catalyst was dissolved completely in the aqueous phase. In most cases detailed in their report, the ruthenium complex was only sparingly soluble in the aqueous reaction mixture.
Figure 1.
Conventional ruthenium olefin metathesis catalysts.
Figure 2.
Metathesis catalysts adapted for aqueous solvents.
Metathesis in homogeneous aqueous systems would likely be faster and more versatile than in these heterogeneous systems. An effective system for metathesis with commercially available catalysts in homogeneous aqueous media would not only make this chemistry more accessible, but also highlight the limitations of the standard catalysts in water, informing catalyst-design efforts. Yet, reports of the use of common metathesis catalysts in an aqueous context are limited. For these reasons, we chose to test the capabilities of catalysts 1–4 in homogeneous aqueous media, and we report the results of our exploration herein. First, we screened various organic solvents as co-solvents for RCM of 8 in a homogeneous aqueous solution (Table 1). The solvents typically used for olefin metathesis reactions, such as CH2Cl2, 1,2-dichloroethane, and toluene, are immiscible with water, so we resorted to water-miscible solvents. Tetrahydrofuran (THF), used previously as a solvent for ROMP and acyclic diene metathesis with varying success,11 failed as a cosolvent for RCM. On the other hand, the ethylene glycol ether-based solvents dimethoxyethane (DME or glyme) and poly(ethylene glycol) (PEG) were excellent co-solvents. Their improved results with respect to THF and dioxane could relate to their better coordinating ability.12 Able to coordinate the tetracoordinate ruthenium complexes of the metathesis catalytic cycle, these ethers could more ably protect them from detrimental coordination by water. Grubbs and coworkers suggest that decomposition of metathesis intermediates in water results from water coordination at ruthenium in the methylidene-propagating species.13 Interestingly, these results show that the protective environment of the PEG-bearing ligand in 6a can also be provided by ethylene glycol ethers in the bulk solvent.
Table 1.
RCM catalyzed by 2 in aqueous media
 | |
|---|---|
| solventa | conversion (%) |
| 4:1 THF/H2O | 3 |
| 4:1 1,4-dioxane/H2O | 5 |
| 4:1 DMF/H2O | 75 |
| 4:1 (CH3)2CO/H2O | >95 |
| 4:1 DME/H2O | >95 |
| 3:1 PEG-500 dimethyl ether/H2O | >95 |
Mass:mass ratio.
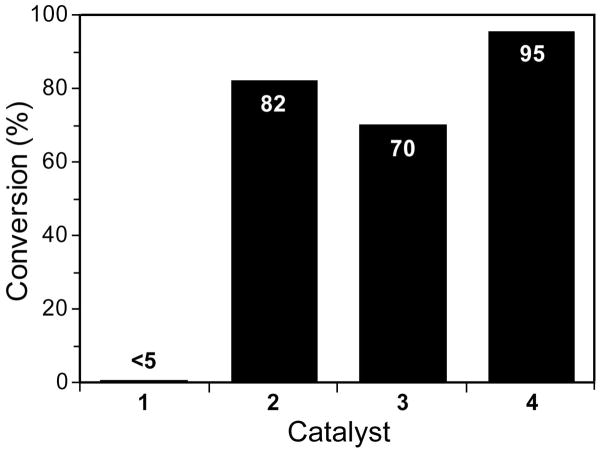
Next, we studied the performance of different commercially available catalysts in the DME–water solvent system with substrate 8 (Figure 3). Phosphine-free complex 4 displayed the highest turnover, with both N-heterocyclic carbene (NHC) complexes outperforming the 1st-generation catalysts. The more σ-donating NHC ligand could aid olefin coordination over attack by water, favoring metathesis with the 2nd-generation catalysts as opposed to decomposition. The advantage of 4 over 2 is more difficult to rationalize because both complexes produce the same propagating species. The improved performance of 4 could be due to the presence of the chelating isoproxy moiety, which could potentially protect the catalyst from decomposition in water prior to entry into the catalytic cycle, as suggested by Blechert and coworkers for metathesis in organic solvents.14 Although the ether ligand binds more loosely than does the phosphine of 2 (4 initiates >800-fold faster at 25 °C),15 its rebinding to ruthenium prior to metathesis is unimolecular. As a result, the ether ligand is more likely to rebind the coordinatively unsaturated intermediate, protecting it from water to preserve the complex in solution.14 In addition to helping select known catalysts for use in water–organic systems, these results can inform the design of water-soluble catalysts. The combination of a chelate and an NHC produces a more effective catalyst for aqueous metathesis, as borne out by complexes 6a, 6b, and 7. Future catalyst designs should incorporate these features.
Figure 3.
RCM conversion of 0.05 M substrate 8 catalyzed by complexes 1–4: 1 mol % [Ru], 2:1 DME/water, 24 h, rt, under air.
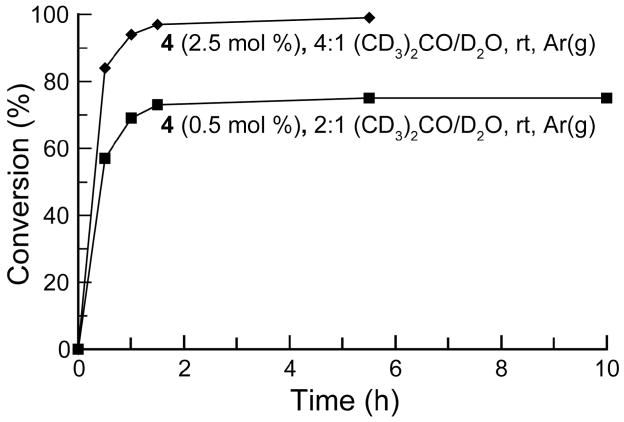
Following the identification of 4 as the superior catalyst in the water–DME system, the progress of RCM in acetone–water was monitored to evaluate the rate of the reaction and the lifetime of catalytically-active species (Figure 4). The majority of RCM occurred within 90 min of the beginning of the reaction, as is typical for RCM of 8 with this catalyst and temperature.16 Notably, this reaction is far faster than the 12-h reaction time reported by Blechert and coworkers for their heterogeneous systems.10 Nevertheless, catalyst decomposition also proceeds quickly in the aqueous solvent, with the results of the trial with 0.5 mol % catalyst indicating that the majority of the catalytically-active species is destroyed within 90 min. In organic solvents, on the other hand, complex 4 can be recovered after RCM reactions requiring several hours.3
Figure 4.
Kinetics of RCM of 0.05 M substrate 8 catalyzed by complex 4 in acetone–water monitored by 1H NMR spectroscopy.
With optimized reaction conditions established, we probed the RCM of a variety of dienes using complex 4 in aqueous DME and acetone solutions. Under these homogeneous conditions, substrates with a variety of substituents were metathesized to form five-, six-, and seven-membered cyclic products (Table 2). High conversion was consistently achieved with traditional non-polar substrates, except for diallyldiphenylsilane (14). Water-soluble substrates such as 11 and 16—a putative model for peptide substrates—were more challenging, requiring increased catalyst loading and organic cosolvent concentrations for high conversion. As it has with many other metathesis systems, diallyldimethylammonium chloride (12) resisted RCM.5,17 Complex 4 is also capable of homodimerization of allyl alcohol (18) in acetone–water, achieving good conversion over several hours (Table 3). Nevertheless, CM of other substrates in aqueous solvents has proven elusive for us as well as others.5,18
Table 2.
RCM of representative dienes catalyzed by 4 in aqueous media under air
| substrate | solvent ([substrate], M) | mol %4 | conversion (%) (time, h) |
|---|---|---|---|

9 |
2:1 (CD3)2CO/D2O (0.05) | 3 | >95 (2) |
| 2:1 DME/H2O (0.05) | 3 | 85 (3) | |

10 |
2:1 (CD3)2CO/D2O (0.05) | 3 | 90 (2) |
| 2:1 DME/H2O (0.05) | 3 | >95 (3) | |

8 |
2:1 (CD3)2CO/D2O (0.05) | 3 | >95 (2) |
| 2:1 DME/H2O (0.05) | 1 | 95 (24) | |

11 |
2:1 (CD3)2CO/D2O (0.01) | 10 | >95 (48) |
| 4:1 DME/H2O (0.05) | 10 | 77 (24) | |

12 |
2:1 (CD3)2CO/D2O (0.05) | 3 | 0 (2) |

13 |
2:1 (CD3)2CO/D2O (0.05) | 3 | >95 (2) |

14 |
2:1 (CD3)2CO/D2O (0.05) | 3 | 0 (2) |
| 2:1 DME/H2O (0.05) | 3 | 0 (3) | |

15 |
2:1 (CD3)2CO/D2O (0.05) | 3 | >95 (2) |

16 |
4:1 DME/H2O (0.02) | 40 | >95 (24) |

17 |
2:1 (CD3)2CO/D2O (0.05) | 3 | >95 (2) |
| 2:1 DME/H2O (0.05) | 3 | 75 (4) |
Table 3.
CM catalyzed by 4 in an aqueous medium under air
 | |
|---|---|
| time (h) | conversion (%) |
| 2 | 71 |
| 6 | 75 |
The effectiveness of 4 for RCM and CM in aqueous–organic solution is comparable to that of complex 6b, which is among the best catalysts for metathesis in pure water. Two conclusions can be drawn: homogeneous aqueous–organic mixtures could be advantageous for some olefin metathesis reactions, and the principle advantage of current aqueous metathesis catalysts is merely water solubility. Conventional catalysts such as 4 are active in aqueous solvents, and hence can be used for metathesis of polar molecules if the substrate is amenable to aqueous DME, PEG, or acetone. For instance, some enzymes and polysaccharides are compatible with aqueous DME,19 suggesting that these biomolecules might be suitable for a metathesis strategy that avoids the synthesis of specialized complexes. Moreover, we have found that ribonuclease A is >90% soluble in 3:2 DME/phosphate-buffered saline (PBS). In this solution, complex 4 is not only soluble, but also catalyzes the quantitative RCM of N-tosyldiallylamine (8), even in the presence of ribonuclease A.20 Thus, apart from its insolubility in pure water, 4 is nearly as effective in the presence of water as are specialized complexes like 6b. Hence, additional advances in aqueous metathesis will require enhanced ligands that not only provide water solubility but also improve upon the NHC and isopropoxy chelate in protecting the complex from water.
Supplementary Material
Procedure for the preparation of compound 16 and for performing metathesis reactions, and related analytical data. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We are grateful to A. Choudary (University of Wisconsin–Madison) for critical review of this manuscript. J.B.B. was supported by an NDSEG Fellowship sponsored by the Air Force Office of Scientific Research. This work was supported by grant GM44783 (NIH). NMRFAM was supported by grant P41RR02301 (NIH).
References
- 1.(a) Grubbs RH. Handbook of Metathesis. Wiley-VCH; Weinheim, Germany: 2003. [Google Scholar]; Nicolaou KC, Bulger PG, Sarlah D. Angew Chem, Int Ed. 2005;44:4490–4527. doi: 10.1002/anie.200500369. [DOI] [PubMed] [Google Scholar]; (b) Grubbs RH. Angew Chem, Int Ed. 2006;45:3760–3765. doi: 10.1002/anie.200600680. [DOI] [PubMed] [Google Scholar]
- 2.(a) Schwab P, Grubbs RH, Ziller JW. J Am Chem Soc. 1996;118:100–110. [Google Scholar]; (b) Kingsbury JS, Harrity JPA, Bonitatebus PJ, Jr, Hoveyda AH. J Am Chem Soc. 1999;121:791–799. [Google Scholar]; (c) Scholl M, Ding S, Lee CW, Grubbs RH. Org Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 3.Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J Am Chem Soc. 2000;122:8168–8179. [Google Scholar]
- 4.(a) Mohr B, Lynn DM, Grubbs RH. Organometallics. 1996;15:4317–4325. [Google Scholar]; (b) Binder JB, Guzei IA, Raines RT. Adv Synth Catal. 2007;349:395–404. doi: 10.1002/adsc.200600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Hong SH, Grubbs RH. J Am Chem Soc. 2006;128:3508–3509. doi: 10.1021/ja058451c. [DOI] [PubMed] [Google Scholar]; (b) Jordan JP, Grubbs RH. Angew Chem, Int Ed. 2007;46:5152–5155. doi: 10.1002/anie.200701258. [DOI] [PubMed] [Google Scholar]
- 6.Connon SJ, Rivard M, Zaja M, Blechert S. Adv Synth Catal. 2003;345:572–575. [Google Scholar]
- 7.(a) Banasiak DS. J Mol Catal A: Chem. 1985;28:107–115. [Google Scholar]; (b) Mortell KH, Gingras M, Kiessling LL. J Am Chem Soc. 1994;116:12053–12054. [Google Scholar]; (c) Miller SJ, Blackwell HE, Grubbs RH. J Am Chem Soc. 1996;118:9606–9614. [Google Scholar]; (c) Jenkins CL, Vasbinder MM, Miller SJ, Raines RT. Org Lett. 2005;7:2619–2622. doi: 10.1021/ol050780m. [DOI] [PubMed] [Google Scholar]; (d) Stymiest JL, Mitchell BF, Wong S, Vederas JC. J Org Chem. 2005;70:7799–7809. doi: 10.1021/jo050539l. [DOI] [PubMed] [Google Scholar]
- 8.(a) Streck R. J Mol Catal. 1992;76:359–372. [Google Scholar]; (b) Yao Q, Zhang Y. Angew Chem, Int Ed. 2003;42:3395–3398. doi: 10.1002/anie.200351222. [DOI] [PubMed] [Google Scholar]; Cornils B, Herrmann W. Aqueous-Phase Organometallic Catalysis. 2. Wiley-VCH; Weinheim, Germany: 2004. [Google Scholar]; (d) Clavier H, Grela K, Kirschning A, Mauduit M, Nolan SP. Angew Chem, Int Ed. 2007;46:6786–6801. doi: 10.1002/anie.200605099. [DOI] [PubMed] [Google Scholar]
- 9.(a) Fraser C, Grubbs RH. Macromolecules. 1995;28:7248–7255. [Google Scholar]; (b) Lynn DM, Kanaoka S, Grubbs RH. J Am Chem Soc. 1996;118:784–790. [Google Scholar]; (c) Mecking S, Held A, Bauers FM. Angew Chem, Int Ed. 2002;41:544–561. [Google Scholar]
- 10.(a) Manning DD, Hu X, Beck P, Kiessling LL. J Am Chem Soc. 1997;119:3161–3162. [Google Scholar]; (b) Kanai M, Mortell KH, Kiessling LL. J Am Chem Soc. 1997;119:9931–9932. [Google Scholar]
- 11.(a) Jin W, Fukushima T, Kosaka A, Niki M, Ishii N, Aida T. J Am Chem Soc. 2005;127:8284–8285. doi: 10.1021/ja051859p. [DOI] [PubMed] [Google Scholar]; (b) Tastard CY, Hodge P, Ben-Haida A, Dobinson M. React Funct Polym. 2006;66:93–107. [Google Scholar]
- 12.Smid J. In: Ions and Ion-Pairs in Organic Reactions. Szwarc M, editor. Vol. 1. Wiley Interscience; New York: 1972. pp. 85–151. [Google Scholar]; (b) Seddon WA, Fletcher JW, Catterall R, Sopchyshyn FC. Chem Phys Lett. 1977;48:584–586. [Google Scholar]
- 13.Towards a second-generation aqueous Grubbs metathesis catalyst: Understanding ruthenium methylidene decomposition in the presence of water (Jordan, J. P.; Hong, S. H.; Grubbs, R. H. Presented at the 229th National Meeting of the American Chemical Society, San Diego, CA, March 2005; Paper INOR-619).
- 14.Maechling S, Zaja M, Blechert S. Adv Synth Catal. 2005;347:1413–1422. [Google Scholar]
- 15.Love JA, Morgan JP, Trnka TM, Grubbs RH. Angew Chem, Int Ed. 2002;41:4035–4037. doi: 10.1002/1521-3773(20021104)41:21<4035::AID-ANIE4035>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Zaja M, Connon SJ, Dunne AM, Rivard M, Buschmann N, Jiricek J, Blechert S. Tetrahedron. 2003;59:6545–6558. [Google Scholar]
- 17.Lynn DM. PhD Thesis. California Institute of Technology; Pasadena, CA: 1999. [Google Scholar]
- 18.Connon SJ, Blechert S. Bioorg Med Chem Lett. 2002;12:1873–1876. doi: 10.1016/s0960-894x(02)00260-3. [DOI] [PubMed] [Google Scholar]
- 19.(a) Miller JF, Graves DJ. Biochem Biophys Res Commun. 1981;99:1377–1383. doi: 10.1016/0006-291x(81)90771-3. [DOI] [PubMed] [Google Scholar]; (b) van Tol JBA, Stevens RMM, Veldhuizen WJ, Jongejan JA, Duine JA. Biotechnol Bioeng. 1995;47:71–81. doi: 10.1002/bit.260470109. [DOI] [PubMed] [Google Scholar]; (c) van Langen LM, Oosthoek NHP, van Rantwijk F, Sheldon RA. Adv Synth Catal. 2003;345:797–801. [Google Scholar]; (d) Yoon JH, McKenzie D. Enzyme Microb Technol. 2005;36:439–446. [Google Scholar]
- 20.Reaction conditions: 4 mol % 4, 0.025 M substrate 8, 0.2 mg/mL ribonuclease A, 3:2 DME/PBS, 24 h, rt, under air. It is noteworthy that water comprises 80% of the molecules in this reaction mixture.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Procedure for the preparation of compound 16 and for performing metathesis reactions, and related analytical data. This material is available free of charge via the Internet at http://pubs.acs.org.