Abstract
Sensory experiences contribute to the development and specialization of signal processing capacities in the mammalian auditory system during a “critical period” of postnatal development. Earlier studies have shown that passive exposure to tonal stimuli during this postnatal epoch induces a large-scale expansion of the representations of those stimuli within the primary auditory cortex (A1) (Zhang et al., 2001). Here, we show that exposing rat pups through the normal critical period epoch and beyond to continuous, un-modulated, moderate-level tones induces no such representational distortion, and in fact disrupts the normal development of frequency response selectivity and tonotopicity all across area A1. The area of cortex responding selectively to continuously exposed sound frequencies was actually reduced, when compared to rats reared in normal environments. Strong exposure-driven plasticity characteristic of the critical period could be induced well beyond the normal end of the critical period, by simply modulating the tonal stimulus. Thus, continuous tone exposure, like continuous noise exposure (Chang and Merzenich, 2003), ineffectively induces critical period plasticity, and indefinitely blocks the closure of a normally-brief critical period window. These findings again demonstrate the crucial role of temporally structured inputs for inducing the progressive cortical maturational changes that result in the closure of the critical period window.
Keywords: cortical map, critical period, development, environment, frequency receptive field, plasticity
Introduction
Earlier studies have documented an early, several-day-long postnatal epoch – a “critical period” – during which the rat primary auditory cortex (A1) can be plastically remodeled on a large scale by merely exposing the pup to repeated sounds. Beyond this period, passive sound exposure has less influence on the progressive development of frequency tuning and tonotopicity in A1 (de Villers-Sidani et al., 2007; Nakahara et al., 2004; Zhang et al., 2001, 2002; Zhou and Merzenich, 2007). Studies have also indicated that exposure-based cortical remodeling during the critical period strongly depends on the modulation of sounds in the environment. For example, exposing rat pups to pulsed tones of a specific frequency results in an over-representation of that frequency in A1 (Zhang et al., 2001; de Villers-Sidani et al., 2007; Han et al., 2007). On the other hand, if rat pups are exposed to temporally modulated white noise, the tonotopic organization of A1 is degraded while, paradoxically, there is a premature closure of the critical period (Zhang et al., 2002; Zhou and Merzenich, 2007). If rat pups are exposed to continuous white noise, the spectral and temporal response selectivity and cortical inhibition is sustained in a degraded and immature state, and the critical period window is maintained open, indefinitely (Chang and Merzenich, 2003; Chang et al., 2005). Here, we extend those studies, by documenting the consequences of rearing pups through this critical period epoch and beyond in the presence of continuous, moderate-level tonal stimuli.
Experimental procedures
All experiment procedures used in this study were approved by the Animal Care and Use Committees at the University of California, San Francisco.
Tone exposure
Rat pups (Sprague-Dawley) and their mothers were placed in sound-shielded test chambers from P7 to P35 under continuous pure tone stimuli (either 3.5 or 7 kHz). Note that this exposure extends for about 22 days beyond the postnatal time of normal closure of the “critical period” in A1 in the rat (de Villers-Sidani et al., 2007). The sound signal was generated by a function generator (BK Precision Dynascan Corp., Chicago, IL) and amplified to a calibrated level in the free sound field of ~65-dB SPL. No significant harmonic signals or other sound distortions were recorded via wide sampling in the chamber in which the tonal stimulus was delivered. Animals were give access to water and food ad libitum under an 8-h light/16-h dark cycle. No abnormalities in the behavior of either the mother or pups could be detected during sound exposure. The weights of all pups and mothers were continuously monitored, and there was no weight loss compared with naïve rats, indicating normal lactation. Activities during waking and the sleep behaviors of these rats indicated that the sound stimuli were not stressful. After the end of the period of continuous-tone exposure at P35, the tonal stimulus (a 7-kHz tone) in a subset of rats was continuously modulated (50-ms duration tone pips with 5-ms ramps at ~65-dB SPL, delivered at 5 pulses per second (pps), followed by a 1-second silent interval to minimize adaptation effects) for two weeks (i.e., from P36–P49). In addition, one group of naïve rats was also exposed to a pulsed 7-kHz tone, from P36–P49. These secondarily-sound-exposed animals were then returned to standard housing conditions for a subsequent 2-week period (i.e., P50–P63) before neural response recording in A1.
Electrophysiological recording
Electrophysiological recording of cortical responses was conducted at ~P35 and ~P63 for matched groups of exposed rats and naïve control rats. Rats were initially anesthetized with an i.p. injection of sodium pentobarbital (50 mg/kg body weight). Throughout the surgical procedures and during the recording session, a state of areflexia was maintained with supplemental doses of 8 mg/ml dilute pentobarbital injected i.p. The trachea was cannulated to ensure adequate ventilation, and the cisterna magnum was drained of cerebrospinal fluid to minimize cerebral edema. The skull was secured in a head holder leaving the ears unobstructed. After reflecting the right temporalis muscle, the auditory cortex was exposed and the dura resected. The cortex was maintained under a thin layer of viscous silicone oil to prevent desiccation.
Cortical responses were recorded with parylene-coated tungsten microelectrodes (1–2 megohms at 1 kHz; FHC Inc., Bowdoinham, ME). Recording sites were chosen to evenly sample from the auditory cortex while avoiding blood vessels, and were marked on a magnified digital image of the cortical surface vasculature. At each recording site the microelectrode was lowered orthogonally into the cortex to a depth of 480–550 µm (layers 4 and 5), where vigorous stimulus-driven responses were recorded. Acoustic stimuli were generated using TDT System III (Tucker-Davis Technology, Alachua, FL) and delivered to the left ear through a calibrated earphone (STAX 54) with a sound tube positioned inside the external auditory meatus. A software package (SigCal, SigGen, and Brainware; Tucker-Davis Technologies, Inc.) was used to calibrate the earphone, generate acoustic stimuli, monitor cortical response properties online, and store data for offline analysis. The evoked spikes of a neuron or a small cluster of neurons were collected at each site.
Cortical mapping and data analysis
Frequency tuning curves were reconstructed by presenting pure tones of 50 frequencies (1–30 kHz, 25-ms duration, 5-ms ramps) at eight sound intensities (0- to 70-dB SPL in 10-dB increments) to the contralateral ear in a random, interleaved sequence at a rate of 2 pps. The CF of a cortical site was defined as the frequency at the tip of the V-shaped tuning curve. For flat-peaked tuning curves, CF was defined as the midpoint of the plateau at threshold. For tuning curves with multiple peaks, CF was defined as the frequency at the most sensitive tip (i.e., with lowest threshold). Response bandwidths (BW20s) of tuning curves were measured for all sites. The response latency was defined as the time from stimulus onset to the earliest response, using peri-stimulus time histograms of responses to all tone pips.
The area of A1 was defined as previously described (Zhang et al., 2002). To generate A1 maps, Voronoi tessellation (a Matlab routine) was performed to create tessellated polygons, with electrode penetration sites at their centers. Each polygon was assigned the characteristics (i.e., CF) of the corresponding penetration site. In this way, every point on the surface of the auditory cortex was linked to the characteristics experimentally derived from a sampled cortical site that was closest to this point.
We used a previously defined index to quantitatively describe the precision of tonotopicity in A1 (Zhang et al., 2001, 2002). The line connecting the two most anterior and posterior penetrations within A1 was used as a reference for the tonotopic axis. We then rotated each map to orient the tonotopic axis horizontally. After rotation, new x coordinates of penetrations in each rat were normalized to be within a range from 0.0 to 1.0, and penetration sites were plotted according to their CFs and x coordinates. The logarithmic frequency range (1–30 kHz) was converted to a linear range (0–1). We defined the index as the average minimal distance from each data point to the line connecting (0,0) and (1,1). The larger the index, the more disordered the tonotopicity of the tonotopic map.
Results
Effects of continuous tone exposure on frequency representations in A1
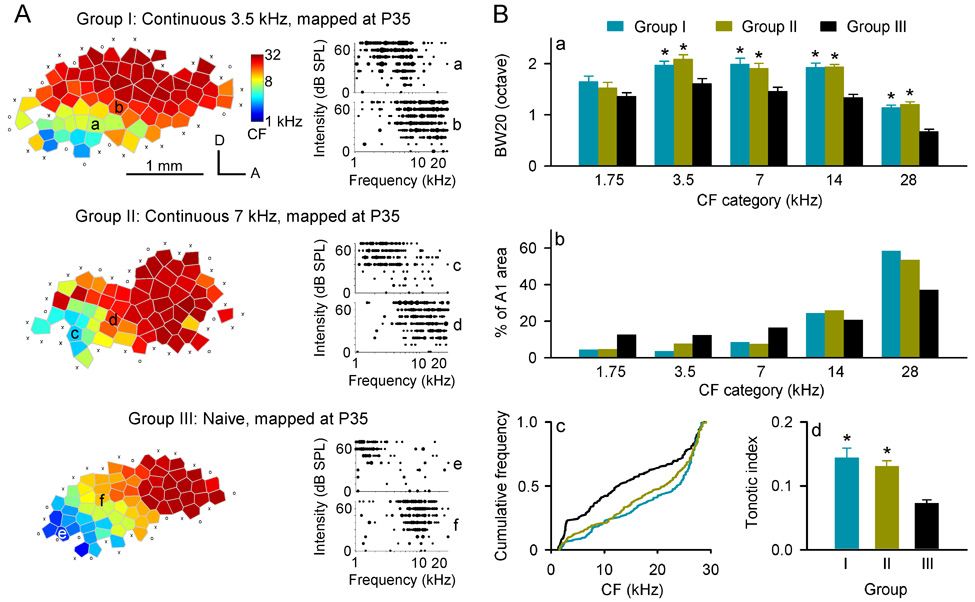
Rat pups were reared in continuous pure tones (3.5- or 7-kHz; n=3 for each group) from P7–P35. A1 was subsequently electrophysiologically mapped in fine detail immediately after sound exposure cessation. Naïve rats (n=4) reared under standard housing conditions were also mapped at the same ages. As shown in Fig. 1A (left; Group III), A1 in naïve rats consisted of iso-frequency bands oriented approximately orthogonal to a rostro-caudal frequency-representation gradient. Cortical neuron tuning curves recorded in most sites (93%) within A1 were V-shaped, with a clear CF defined at the low threshold peak (Fig. 1Ae,f). In exposed rats, by contrast, tuning curves recorded from a large number of sites (49% for 3.5-kHz exposed rats and 46% for 7-kHz exposed rats) within A1 were flat-peaked or multipeaked (Fig. 1Aa–d). Comparison of bandwidths measured at 20 dB above threshold (BW20) showed that tuning curves recorded in exposed rats were more broadly tuned than were those of naïve rats (Fig. 1Ba; ANOVA with post hoc Student-Newman-Keuls test, all p<0.05–0.001), except for lowest CF category centered at 1.75-kHz (ANOVA, p>0.1). However, there was no significant difference in BW20s between rats exposed to 3.5- and 7-kHz (ANOVA with post hoc Student-Newman-Keuls test, all p>0.05).
Fig. 1.
(A) Representative characteristic frequency (CF, kHz) maps and tonal receptive fields recorded from A1 of rats exposed to continuous 3.5-kHz (Group I) or 7-kHz (Group II) tones during the critical period, and from naïve rats (Group III). The color of each polygon in these maps indicates the CF for neurons recorded at that site (see color scales). Polygons are Voronoi tessellations representing each neuronal middle-cortical-layer response sample site, generated so that every point on the cortical surface was assumed to have the characteristics of its closest neighbors. For each example of tonal receptive field recorded from the sites marked in maps, responses are represented by dots in the response area with dot size proportional to the number of spikes evoked by tonal stimuli at each frequency & intensity. a, multipeaked tuning curve. b–d, flat-peaked tuning curves. e and f, V-shaped tuning curves. A, anterior; D, dorsal. ○, non-A1 site; ×, unresponsive cortical site. (B) Average receptive field bandwidth at 20 dB above threshold (BW20) (a), percentage of A1 area that was tuned to different frequency ranges (b), CF distributions (c), and average tonotopic indices (d) of different groups of rats. Bin size =1 octave. *, p<0.05–0.001. Error bars represent means ± SE.
At first glance, the A1 area tuned to higher frequencies in exposed rats appeared to be larger, while the area tuned to lower frequencies appeared smaller, when compared to naïve rats (Fig.1A, left; Group I or II vs. III). To verify that observation, we compared the percentage of A1 area that was tuned to different frequencies in each group. As shown in Fig. 1Bb, the percentage of A1 area in exposed rats were larger than in naïve rats at CF categories centered at 14- and 28-kHz, but was smaller at CF categories centered at 1.75-, 3.5-, and 7-kHz (χ2=21.7, p<0.006). This result was further confirmed by quantitative comparisons of distribution for all CFs obtained from different groups (Fig. 1Bc). Significant rightward shift of CF distribution for exposed rats compared to naïve rats (Kruskal-Wallis with post hoc Bonferroni’s test, both p<0.001) indicates decreased areas of representation for lower frequencies, but increased representational areas of higher frequencies, induced by continuous pure-tone exposure. Again, there were no significant differences in the percentages of A1 areas tuned to different frequencies and in CF distribution between rats exposed to 3.5- and 7-kHz (Kruskal-Wallis with post hoc Bonferroni’s test, p>0.05).
We also quantified the precision of A1 tonotopicity for different groups by calculating a tonotopic index. As shown in Fig. 1Bd, average indices in exposed rats were significantly larger than in naïve rats (ANOVA with post hoc Student-Newman-Keuls test, both p<0.01), showing that less ordered tonotopic maps resulted from early sound exposure. Although the average index in rats exposed to 3.5-kHz tones was also larger than in rats exposed to 7-kHz tones, that difference did not quite reach statistical significance (ANOVA with post hoc Student-Newman-Keuls test, p>0.05).
Cortical response thresholds across all frequencies (Fig. 2A) and the latencies (Fig. 2B) recorded from exposed rats, however, were not significantly different from that recorded from naïve rats (ANOVA, all p>0.05), indicating normal peripheral hearing of these rats after moderate sound exposure.
Fig. 2.
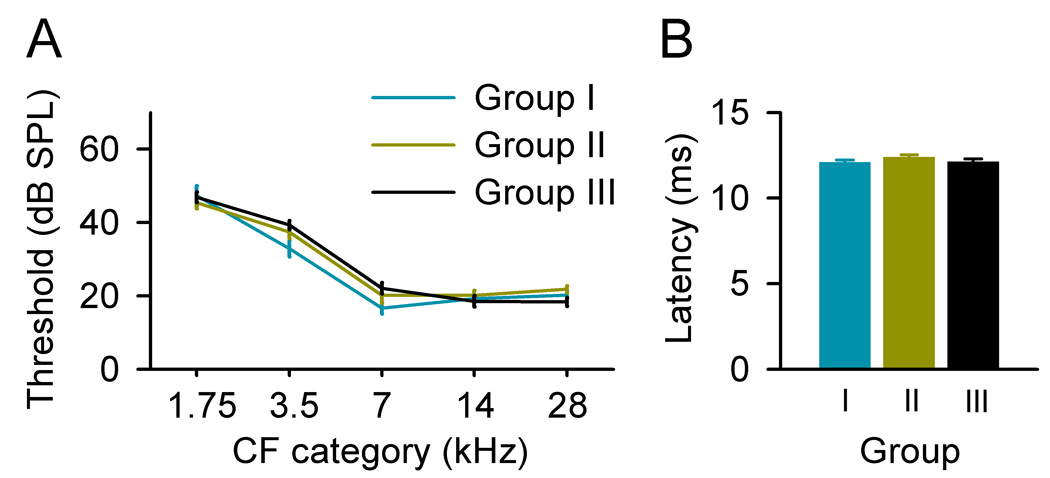
Average response thresholds at different CF categories (A) and average latencies (B) recorded from different groups of rats. Bin size =1 octave. Error bars represent means ± SE.
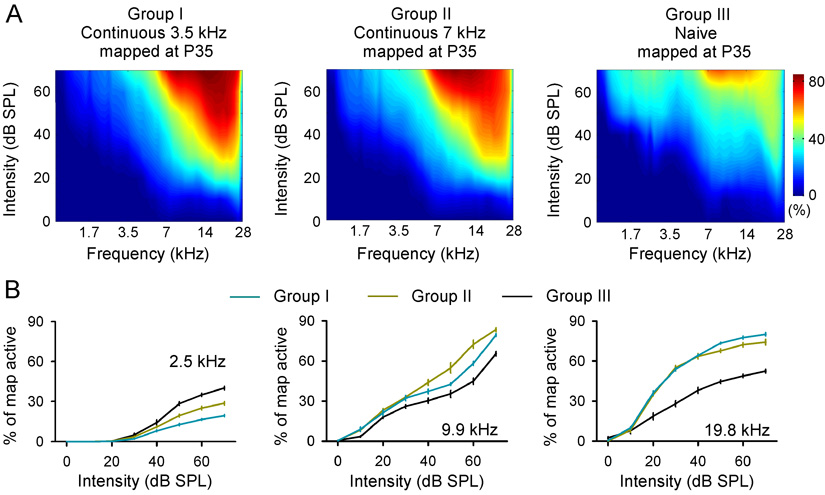
We further compared A1 recruitment functions for different groups of rats. These recruitment functions measure the percentage of the map area activated by a tonal stimulus at a specific frequency and intensity, rather than solely focusing on the preferred frequency determined at the threshold (i.e., CF) for each recording site. It is clear that A1 recruitment functions obtained from exposed rats were very substantially different from that obtained from naïve rats, and marked by an increase in the percentages of A1 area activated by high frequency but a decrease in the percentage of A1 areas activated by low frequency at intensity ranges of ~20–70 dB SPL (Fig. 3A, Groups I or II vs. III). Fig. 3B presents the average percentage of each map active for ¼-octave-wide range of frequencies centered on low- (2.5-kHz), middle- (9.9-kHz), and high- frequency (19.8-kHz) tones of increasing intensity. Two-way ANOVA showed that the proportional area of A1 activated by low frequencies was smaller in exposed rats, but was larger in middle and high frequency domains, when compared to naïve animals (all p<0.0001).
Fig. 3.
(A) Average percentage of A1 that was activated by tones of varying frequency and intensity, in rats exposed to continuous 3.5-kHz or 7-kHz tones, and in naïve rats. (B) Average percentage of each map active for a ¼-octave-wide range of frequencies centered on low- (2.5-kHz), middle- (9.9-kHz), and high-frequency (19.8-kHz) tones of increasing intensity.
Delayed closure of the critical period resulting from continuous tone exposure
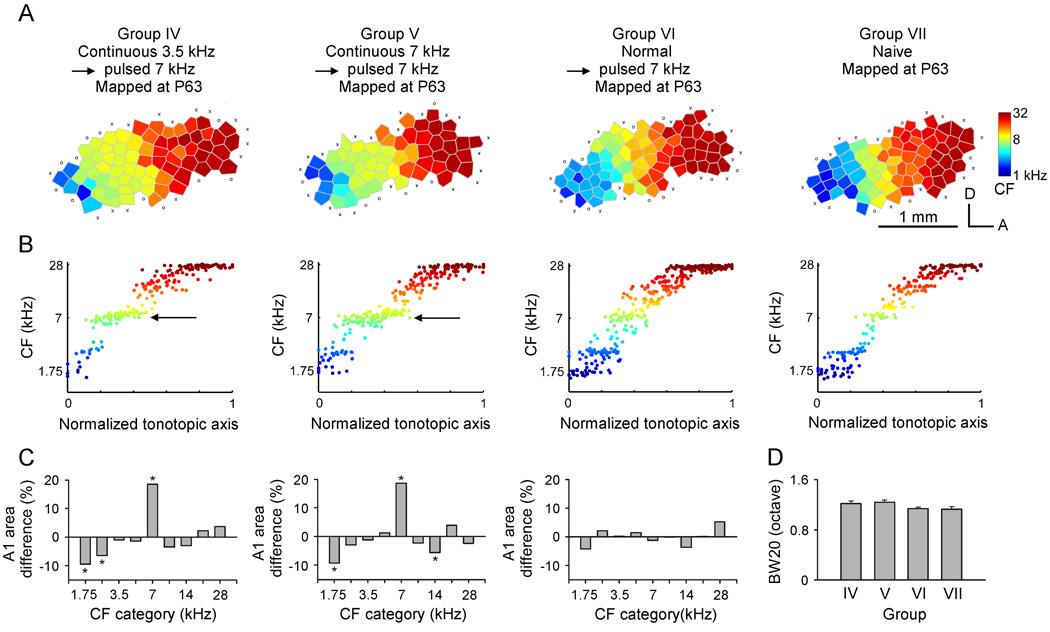
An earlier study from our laboratory showed that continuous broad-band noise exposure delayed the end of the critical period for A1 development (Chang and Merzenich, 2003). To determine the impacts of continuous pure-tone exposure on the critical period window, a subset of previously continuous-tone-exposed rats were exposed to pulsed 7-kHz tone pips for two weeks, i.e., rats experienced an immediately shift in exposure from continuous tones to modulated tones at P35. These double-exposed subgroups of rats were defined as Group IV (n=3, exposed to 3.5-kHz pure tones during the critical period) or V (n=3, exposed to 7-kHz pure tones during the critical period), respectively. In addition, another group of age-matched naïve rats was also exposed to pulsed 7-kHz tones over the same epoch (P36–P49; Group VI, n=5). Cortical fields of Group IV, V and VI rats were then mapped and compared with age-matched non-sound-exposed rats (Group VII, n=3).
In the CF maps for rats in Groups IV or V, the area tuned to 7-kHz frequency was enlarged, when compared to that of age-matched naïve rats (Fig. 4A, Group IV or V vs. VII). Such tone-specific enlargement resulting from mere exposure to sound stimuli characterizes “critical period” plasticity. This distortion is also illustrated in Fig. 4B, where CFs of all recording sites from each groups of rats are plotted against a normalized tonotopic axis. Examination of the CF distribution reveals an over-representation of sites tuned to 7-kHz, and a relative under-representation of sites tuned to immediately lower or higher frequencies, in both Groups IV and V, when compared to either naïve-exposed (presumably no longer in the critical period) or age-matched naïve non-exposed rats (Fig. 4B, Group IV or V vs. VI or VII). Note that the maps and CF distributions in Group VI rats were substantially like that of naïve rats (Fig. 4A and B, Group VI vs. VII).
Fig. 4.
(A) Representative CF maps obtained from Groups IV, V, VI and VII rats. Group IV rats were exposed to continuous 3.5-kHz tones from P7–P35, then pulsed 7-kHz tones for a subsequent 2-week-long period (P36–P49). Group V, rats were exposed to continuous 7-kHz tones, then after P35, to pulsed 7-kHz tones. Group VI naïve rats were exposed to pulsed 7-kHz tones from P36 to P49. Group VII were non-sound-exposed naïve rats. (B) Distributions of CFs plotted against a normalized tonotopic axis in different groups of rats. Note that there was increased A1 area that was tuned to 7-kHz in both Groups IV and V (arrows), but not in Groups VI and VII. (C) Differences in percentages of the A1 area tuned to different frequencies in all four rat groups. Note the large-scale growth in the proportional area of representation of 7-kHz-tuned neurons in Group IV or V vs. Group VI or VII rats. *, p<0.001. (D) Average BW20 for all recording sites in different groups of rats. Error bars represent means ± SE.
To quantitatively characterize effects of pulsed tone exposure on A1 frequency representation of different rat groups, the percentages of A1 areas representing each frequency range were averaged within the same experimental group, and the differences in percentages between exposed and naïve rats were plotted (Fig. 4C). Average percentages of A1 areas tuned to 7 kHz ± 0.25 octave in Groups IV and V were very significantly increased compared to naïve rats (unpaired t test, both p <0.001). Average percentages of A1 areas tuned to lower or higher frequencies in these two groups, however, were reduced although the differences at some CF categories did not reach the statistical significance (Fig. 4C, Groups IV and V). As expected, the distribution of average percentage of A1 area tuned to different frequency ranges in Group VI was not different from that of naïve rats (Fig. 4C, Group VI; unpaired t test, all p> 0.05).
We also compared average BW20s of tuning curves recorded from different rat groups. Although average BW20s in groups IV and V appeared to be larger than in naïve rats, no significant difference was found among different groups (Fig. 4D; ANOVA, p>0.05).
Discussion
In this study, we determined the consequences, for the functional maturation of cortical field A1, of rearing rat pups in the presence of continuous, moderate-level pure-tone stimuli. Continuous tone rearing had markedly different impacts on the response selectivity and the frequency representations of A1 than did rearing in the presence of pulsed tones (de Villers-Sidani et al., 2007; Han et al., 2007; Zhang et al., 2001). i) Frequency receptive fields recorded in rats exposed to continuous tones were typically flat-peaked or multipeaked, and were far less selective for sound frequency when compared to naïve animals. In pulsed tone-exposed rats, however, receptive fields were substantially like that of naïve rats. A characteristic frequency was almost always easily determinable, and they were almost always continuous and single-peaked. ii) Continuous tone exposure disrupted the normal progressive refinement of A1 tonotopic maps, and it distorted map topographies, resulting in larger-than-normal areas tuned to higher-frequencies and smaller-than-normal areas tuned to lower-frequencies. There was no clear relationship between observed changes and the frequency of the continuous tones that these rat pups were exposed to. By contrast, A1 tonotopic reorganization in pulsed tone exposure cases was highly frequency-dependent, and always resulted in an over-representation of exposed frequency and a reduction in the representation of immediately adjacent frequencies. iii) Continuous tone exposure delayed the closure of the critical period window for A1 development. Pulsed tone exposure accelerated A1 maturation.
It has long been argued that the temporal patterns of sensory inputs are critically important for cortical development (Buonomano and Merzenich, 1998; Katz and Shatz, 1996; Rauschecker, 1999; Weliky and Katz, 1997; Merzenich, 2001). Earlier studies have shown that the postnatal sharpening of frequency receptive fields and the formation of an orderly, continuous frequency representation in A1 require exposure to normally variable auditory inputs during the critical period (Chang and Merzenich, 2003; Chang et al., 2005; de Villers-Sidani et al., 2007; Keuroghlian and Knudsen, 2007; Nakahara et al., 2004; Reale et al., 1987; Stanton and Harrison, 1996; Zhang et al., 2001, 2002; Zhou and Merzenich, 2007). Selective strengthening and elimination of neuronal connections through activity-dependent synaptic plasticity contribute significantly to the establishment of feature-selective cortical response properties (Zhang et al., 2002). We speculate that inappropriate activity-dependent modifications on cortical excitatory and inhibitory connections under continuous tone exposure might contribute to the degraded response selectivity and disrupted tonotopic organization observed in this study, as well as to those reported in early studies (Kenet et al., 2007; Zhang et al., 2002; Zhou and Merzenich, 2007). On the other hand, experience-dependent changes can also be induced in the sub-cortical central auditory system (Chiu et al, 2003; Lorke et al., 2003; Poon and Chen, 1992; Sanes and Constantine-Paton, 1983, 1985; Yu et al., 2007). In the mouse inferior colliculus, for example, click stimuli during development was shown to block the normal sharpening of auditory receptive fields (Sanes and Constantine-Paton, 1983, 1985). Early sound exposure also resulted in a cluster of neurons tuned to the exposed tone frequency in the rat inferior colliculus (Poon and Chen, 1992). It is probable that observed post-exposure changes in A1 are also partly the result of feed-forward responses reflecting experience-dependent changes in various sub-cortical structures.
It should be noted that earlier studies have shown that loud tone (~120-dB SPL) exposure, which typically results in a peripheral hearing loss, also induces a large-scale reorganization of frequency map in the auditory cortex of juvenile and adult cats (Eggermont and Komiya, 2000; Norena et al., 2003). Exposure to a 120-dB SPL pure tone (5- or 6-kHz), for example, induced a shift in cortical CF toward lower frequency and a broadening of the tuning curve, due to changes in the balance between excitation and inhibition at both cortical and subcortical levels after sound exposure (Norena et al., 2003). In this study, we applied moderate-level tone stimuli (~65-dB SPL) and no elevation in cortical response threshold has been recorded in exposed rats compared to naïve animals, indicating normal peripheral function after sound exposure. We also observed larger-than-normal areas which were tuned to higher frequencies in A1 of exposed rats, in addition to less-selective tuning curves.
The duration of the critical period varies by sensory modality and in different animal species. While critical period extends for ~2 weeks in the rat visual cortex (Fagiolini et al., 1994), it only lasts a couple of days (P11–P13) for rat A1 when rats are reared with their mother and siblings in a normal cage environment (de Villers-Sidani et al., 2007). The relatively brief normal critical period window is comparable in length of the critical period for rat somatosensory cortex (S1 barrel-field) (Rice and Van der Loos, 1977; Schlaggar and O'Leary, 1993). In addition, the duration of the critical period is substantially influenced by early sensory experiences. Early dark-rearing, for example, results in an indefinite prolongation of the critical period window for mammalian visual cortex (cats, Cynader, 1983; Mower, 1991; mice, Iwai et al., 2003; rats, Fagiolini et al., 1994). Continuous noise rearing also retards the critical period development in the rat A1 (Chang and Merzenich, 2003). We show here that early continuous tone exposure also delays the closure of the critical period for A1 development. Thus when these exposed rats were subsequently exposed to pulsed tone stimuli even beyond the normal cortical period window, substantial exposure-driven plasticity was still recorded in the A1. One surprise in these studies was the impacts that tone exposure appeared to have on the progressive functional maturation of A1. We had anticipated that continuous tone exposure might block A1 maturation, but only with those regions most directly engaged by the pure-tone stimulus. To the contrary, on these functional grounds, virtually all of A1 appears to have been affected.
It is possible that the observed experience-induced delay closure of the critical period window may be related to the release of the brain-derived neurotrophic factor (BDNF) (Chang and Merzenich, 2003; Chang et al., 2005), which is the primary agent that enables the final cortical changes that result in critical period closure (Maffei, 2002). The results are also consistent with the finding that strengthening of locally correlated cortical activity results in the release of BDNFs that promotes the postnatal maturation of the visual cortex (Castren et al., 1992; Hanover et al., 1999). Together, our findings indicate that patterned inputs play a crucial role for driving changes across the critical period that account for its contributions to early post-natal specialization of cortical processing, and for the ultimate closure of this powerful, early epoch of cortical plasticity.
Acknowledgements
This work was supported by National Institutes of Health Grant NS-10414, the Sandler Fund, and the Coleman Fund. It is respectfully dedicated to a world leader in the auditory neuroscience community, Professor Kirsten Osen.
Abbreviations
- A1
primary auditory cortex
- BDNF
brain-derived neurotrophic factor
- BW20
bandwidth measured at 20 dB above threshold
- CF
characteristic frequency
- Pn
postnatal day n
- pps
pulses per second
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Castren E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci USA. 2005;102:16460–16465. doi: 10.1073/pnas.0508239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu TW, Poon PW, Chan WY, Yew DT. Long-term changes of response in the inferior colliculus of senescence accelerated mice after early sound exposure. J Neurol Sci. 2003;216:143–151. doi: 10.1016/s0022-510x(03)00230-2. [DOI] [PubMed] [Google Scholar]
- Cynader M. Prolonged sensitivity to monocular deprivation in dark-reared cats: effects of age and visual exposure. Brain Res. 1983;284:155–164. doi: 10.1016/0165-3806(83)90002-0. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ, Komiya H. Moderate noise trauma in juvenile cats results in profound cortical topographic map changes in adulthood. Hear Res. 2000;142:89–101. doi: 10.1016/s0378-5955(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Han YK, Kover H, Insanally MN, Semerdjian JH, Bao S. Early experience impairs perceptual discrimination. Nat Neurosci. 2007;10:1191–1197. doi: 10.1038/nn1941. [DOI] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19:RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y, Fagiolini M, Obata K, Hensch TK. Rapid critical period induction by tonic inhibition in visual cortex. J Neurosci. 2003;23:6695–6702. doi: 10.1523/JNEUROSCI.23-17-06695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kenet T, Froemke RC, Schreiner CE, Pessah IN, Merzenich MM. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc Natl Acad Sci USA. 2007;104:7646–7651. doi: 10.1073/pnas.0701944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuroghlian AS, Knudsen EI. Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol. 2007;82:109–121. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Lorke DE, Wong LY, Lai HW, Poon PW, Zhang A, Chan WY, Yew DT. Early postnatal sound exposure induces lasting neuronal changes in the inferior colliculus of senescence accelerated mice (SAMP8): a morphometric study on GABAergic neurons and NMDA expression. Cell Mol Neurobiol. 2003;23:143–164. doi: 10.1023/A:1022993704617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L. Plasticity in the visual system: role of neurotrophins and electrical activity. Arch Ital Biol. 2002;140:341–346. [PubMed] [Google Scholar]
- Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Brain Res Dev Brain Res. 1991;58:151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Zhang LI, Merzenich MM. Specialization of primary auditory cortex processing by sound exposure in the "critical period". Proc Natl Acad Sci USA. 2004;101:7170–7174. doi: 10.1073/pnas.0401196101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norena AJ, Tomita M, Eggermont JJ. Neural changes in cat auditory cortex after a transient pure-tone trauma. J Neurophysiol. 2003;90:2387–2401. doi: 10.1152/jn.00139.2003. [DOI] [PubMed] [Google Scholar]
- Poon PW, Chen X. Postnatal exposure to tones alters the tuning characteristics of inferior collicular neurons in the rat. Brain Res. 1992;585:391–394. doi: 10.1016/0006-8993(92)91243-8. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Auditory cortical plasticity: a comparison with other sensory systems. Trends Neurosci. 1999;22:74–80. doi: 10.1016/s0166-2236(98)01303-4. [DOI] [PubMed] [Google Scholar]
- Reale RA, Brugge JF, Chan JC. Maps of auditory cortex in cats reared after unilateral cochlear ablation in the neonatal period. Brain Res. 1987;431:281–290. doi: 10.1016/0165-3806(87)90215-x. [DOI] [PubMed] [Google Scholar]
- Rice FL, Van der Loos H. Development of the barrels and barrel field in the somatosensory cortex of the mouse. J Comp Neurol. 1977;171:545–560. doi: 10.1002/cne.901710408. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Constantine-Paton M. Altered activity patterns during development reduce neural tuning. Science. 1983;221:1183–1185. doi: 10.1126/science.6612332. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Constantine-Paton M. The sharpening of frequency tuning curves requires patterned activity during development in the mouse, Mus musculus. J Neurosci. 1985;5:1152–1166. doi: 10.1523/JNEUROSCI.05-05-01152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, O'Leary DD. Patterning of the barrel field in somatosensory cortex with implications for the specification of neocortical areas. Perspect Dev Neurobiol. 1993;1:81–91. [PubMed] [Google Scholar]
- Stanton SG, Harrison RV. Abnormal cochleotopic organization in the auditory cortex of cats reared in a frequency augmented environment. Audi Neurosci. 1996;7:97–107. [Google Scholar]
- Weliky M, Katz LC. Disruption of orientation tuning in visual cortex by artificially correlated neuronal activity. Nature. 1997;386:680–685. doi: 10.1038/386680a0. [DOI] [PubMed] [Google Scholar]
- Yu X, Sanes DH, Aristizabal O, Wadghiri YZ, Turnbull DH. Large-scale reorganization of the tonotopic map in mouse auditory midbrain revealed by MRI. Proc Natl Acad Sci USA. 2007;104:12193–12198. doi: 10.1073/pnas.0700960104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4:1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Disruption of primary auditory cortex by synchronous auditory inputs during a critical period. Proc Natl Acad Sci USA. 2002;99:2309–2314. doi: 10.1073/pnas.261707398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Merzenich MM. Intensive training in adults refines A1 representations degraded in an early postnatal critical period. Proc Natl Acad Sci USA. 2007;104:15935–15940. doi: 10.1073/pnas.0707348104. [DOI] [PMC free article] [PubMed] [Google Scholar]