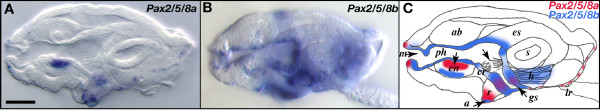
Abstract
Background
Gene duplication provides opportunities for lineage diversification and evolution of developmental novelties. Duplicated genes generally either disappear by accumulation of mutations (nonfunctionalization), or are preserved either by the origin of positively selected functions in one or both duplicates (neofunctionalization), or by the partitioning of original gene subfunctions between the duplicates (subfunctionalization). The Pax2/5/8 family of important developmental regulators has undergone parallel expansion among chordate groups. After the divergence of urochordate and vertebrate lineages, two rounds of independent gene duplications resulted in the Pax2, Pax5, and Pax8 genes of most vertebrates (the sister group of the urochordates), and an additional duplication provided the pax2a and pax2b duplicates in teleost fish. Separate from the vertebrate genome expansions, a duplication also created two Pax2/5/8 genes in the common ancestor of ascidian and larvacean urochordates.
Results
To better understand mechanisms underlying the evolution of duplicated genes, we investigated, in the larvacean urochordate Oikopleura dioica, the embryonic gene expression patterns of Pax2/5/8 paralogs. We compared the larvacean and ascidian expression patterns to infer modular subfunctions present in the single pre-duplication Pax2/5/8 gene of stem urochordates, and we compared vertebrate and urochordate expression to infer the suite of Pax2/5/8 gene subfunctions in the common ancestor of olfactores (vertebrates + urochordates). Expression pattern differences of larvacean and ascidian Pax2/5/8 orthologs in the endostyle, pharynx and hindgut suggest that some ancestral gene functions have been partitioned differently to the duplicates in the two urochordate lineages. Novel expression in the larvacean heart may have resulted from the neofunctionalization of a Pax2/5/8 gene in the urochordates. Expression of larvacean Pax2/5/8 in the endostyle, in sites of epithelial remodeling, and in sensory tissues evokes like functions of Pax2, Pax5 and Pax8 in vertebrate embryos, and may indicate ancient origins for these functions in the chordate common ancestor.
Conclusion
Comparative analysis of expression patterns of chordate Pax2/5/8 duplicates, rooted on the single-copy Pax2/5/8 gene of amphioxus, whose lineage diverged basally among chordates, provides new insights into the evolution and development of the heart, thyroid, pharynx, stomodeum and placodes in chordates; supports the controversial conclusion that the atrial siphon of ascidians and the otic placode in vertebrates are homologous; and backs the notion that Pax2/5/8 functioned in ancestral chordates to engineer epithelial fusions and perforations, including gill slit openings.
Background
Ohno's classical model to explain the fate of genes after gene duplication [1] predicts that one gene duplicate preserves the original gene function while its paralog either disappears by accumulation of detrimental mutations (called nonfunctionalization [2]) or occasionally acquires beneficial mutations that confer novel, positively selected functions (called neofunctionalization [2]). The duplication-degeneration-complementation (DDC) model predicts a third alternative, in which the two duplicate genes become permanently preserved as a consequence of complementary, degenerative mutations that result in partitioning of ancestral subfunctions, so that the sum of the functions of two paralogs equals the functions of the original gene prior to the duplication [2,3]. The DDC model also predicts that after the initial preservation of the two duplicates, whether by subfunctionalization or from neofunctionalization, further partitioning of redundant subfunctions may occur. Studies show that functional constraints on genes duplicated in whole-genome duplications are relaxed, compared with singletons, for tens of millions of years [4]. In addition, novel functions may originate over time, their evolution facilitated by the relaxation of pleiotropy occasioned by fewer tasks in each descendent gene duplicate compared with its single copy gene ancestor [2,5-9].
Understanding the evolution of duplicated genes is important because of the hypothesis that gene duplicates provide opportunities for the evolution of reproductive barriers that lead to lineage divergence [10], and for the origin of evolutionary novelties [1,11,12]. In vertebrates, many sets of paralogous genes arose in two rounds of genome duplication (R1 and R2) that took place in early vertebrate evolution [12-18]. An additional round of genome duplication (R3) occurred in the teleost lineage after ray-fin fish diverged from lobe-fin fish, and provided additional gene family members observed in many fish models [19-23]. It has been suggested that the R1 and R2 genome amplifications facilitated the origin of vertebrate innovations [24], and the R3 event may have facilitated the teleost species radiation [9,25].
Non-vertebrate chordates often have single copies of vertebrate gene families because their lineages diverged from the vertebrate lineage before the R1 and R2 genome duplication events. Recent phylogenomic analyses converge on the conclusion that the chordate subphylum Urochordata, which includes the classes Larvacea and Ascidiacea, are the closest living relatives of the vertebrates, constituting the group Olfactores (vertebrates + urochordates), while the subphylum Cephalochordata, including the amphioxus, diverged basally among chordates ([7,26-30], reviewed in [9]). As gene duplication is pervasive [31,32], non-vertebrate chordates sometimes have genes that duplicated independently in the cephalochordate [33-35] or urochordate lineages [35-41].
We propose that the comparative analysis of gene expression patterns in a gene family that experienced lineage-specific, independent duplication events, interpreted in a phylogenetic context and with respect to subfunction partitioning, can help in the inference of ancient gene functions and in the identification of gene functions that arose by neofunctionalization, and thus may be important for lineage divergence and the origin of developmental novelties. To test this proposition, we examined the Pax2/5/8 gene family. Pax2/5/8 genes encode transcription factors with conserved motifs, including a paired domain, homeodomain, and octapeptide, and are associated with mechanosensory development in mammals [42], frogs [43], fish [44,45], flies [46,47], ascidians [48] and in mollusks [49], while in a cnidarians, the apparent Pax2/5/8 homolog is associated with nerve and sensory cell differentiation [50,51].
Pax2/5/8 genes duplicated independently in different chordate lineages. Within the vertebrates, tetrapods have three members of the Pax2/5/8 gene family (Pax2, Pax5 and Pax8), and teleosts additionally have duplicate pax2 genes ([45] and references therein). In contrast to vertebrates, the basally diverging cephalochordate amphioxus possesses a single Pax2/5/8 gene that is equally related to Pax2, Pax5 and Pax8 [52], and urochordates have two Pax2/5/8 genes (Pax2/5/8a and Pax2/5/8b) [38,53,54] that originated in a duplication event prior to the divergence of larvacean and ascidian lineages [38]. The chordate Pax2/5/8 family embodies a full spectrum of gene evolutionary events: non-duplication (in amphioxus); independent duplications (within urochordates and vertebrates); gene loss (for example, tetrapods have just three of the four paralogs expected from two rounds of genome duplication); neofunctionalization (for example, vertebrate Pax5 in lymphocyte development [55]); and ancestral subfunctions appear to have been partitioned between paralogs within a lineage and, further, differently partitioned between lineages (for example, Pax2 and Pax8 in fish and mammal thyroids [56]).
The independent duplication of the stem olfactores' Pax2/5/8 gene in vertebrate and urochordate lineages provides replicate evolutionary experiments to explore the principles of subfunction partitioning and the origin of novel functions. To exploit this opportunity, we provide here a detailed description of expression patterns for Pax2/5/8 paralogs during development of the larvacean urochordate Oikopleura dioica. We then compare our results with expression patterns of Pax2/5/8 orthologs in ascidians, with independently duplicated Pax2/5/8 paralogs in vertebrates, and with the non-duplicated Pax2/5/8 gene in amphioxus as an outgroup. We discuss the expression of the Pax2/5/8 gene family during development of the heart, endostyle, pharynx, and sensory organs, and provide new insights that reconcile previous conflicting hypotheses about the homology of the ascidian atrial primordia and the vertebrate otic placode. This work thus illustrates the power of comparative analyses of independently duplicated genes to infer ancestral gene subfunctions, modules that can segregate independently from each other to evolving gene duplicates.
Results
Functional motif variation and gene structure of chordate Pax2/5/8 paralogs
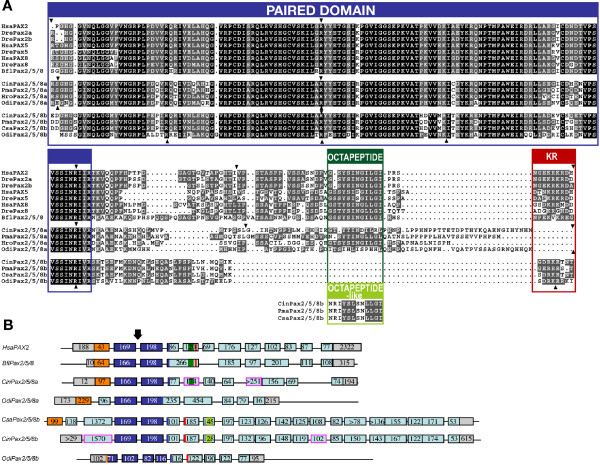
Pax2/5/8 proteins are transcription factors, whose sequence is poorly conserved across Chordata outside of two functional motifs: the paired domain, which interacts with the DNA of target genes (dark blue in Figure 1), and an octapeptide motif (dark green in Figure 1), which is conserved with other Pax proteins [57], functions in repression of Pax transactivation [58], and interacts with other transcriptional cofactors [59]. Sequence alignment of various chordate Pax2/5/8 proteins (Figure 1) reveals that while the sequence of the DNA-interacting paired domain is highly conserved across all chordate Pax2/5/8 proteins analyzed, the octapeptide motif is less conserved. In urochordates, for instance, the octapeptide is present in the ascidian Halocynthia roretzi [48], and, contrary to a previous report [60], our alignment reveals it is also present in Ciona intestinalis Pax2/5/8a (Figure 1A). The octapeptide sequence of the Oikopleura Pax2/5/8a gene is poorly conserved, and the octapeptide motif is absent from the expected position in all urochordate Pax2/5/8b proteins (Figure 1A). Interestingly, we have identified a new motif (light green in Figure 1B) that is conserved among all ascidian Pax2/5/8b paralogs, located further toward the carboxyl end. As this newly recognized sequence shows some similarities with the octapeptide motif, we have called it the 'octapeptide-like' motif (bottom Figure 1A). We could not identify an octapeptide-like motif in the Oikopleura Pax2/5/8b. Our alignment also reveals a lysine-arginine-rich domain (in red in Figure 1) that is conserved in vertebrate Pax2 and amphioxus Pax2/5/8, is variable among vertebrate Pax5 and Pax8 paralogs, and is present in urochordate Pax2/5/8b but not Pax2/5/8a. This protein motif marks the beginning of an amino acid range identified as important for greatly increasing vertebrate Pax2 protein's transactivation activity [58].
Figure 1.
Comparison of chordate Pax2/5/8 proteins and gene structures. (A) Alignment of the chordate Pax2/5/8 proteins showing the conserved DNA-binding paired-domain (dark blue), the octapeptide motif (dark green), the octapeptide-like motif (light green), and the lysine-arginine (KR) rich region (red). Arrowheads indicate the positions of introns. (B) Exon-intron organization deduced from the comparison of ESTs and genomic regions available in public databases (Ghost: http://ghost.zool.kyoto-u.ac.jp/indexr1.html, JGI: http://genome.jgi-psf.org/ciona4/ciona4.home.html, and NCBI: http://www.ncbi.nlm.nih.gov). Numbers indicate the length of the exons (boxes) in base pairs. The position of the conserved domains shown in (A) is indicated with the same code of colors. Exons containing the putative beginning of the coding sequence are labeled in orange. Exons with low degree of sequence conservation and which are hardly alignable among different organisms are labeled in light blue. Exon regions containing 5' and 3'UTR are labeled in grey. Analysis of EST sequences suggested the presence of multiple splice variants, revealing exons (in pink) that were absent from other EST sequences for the same gene; these alternates include the exon harboring the poorly conserved octapeptide motif from Ciona intestinalis. The arrow indicates the totally conserved position of the intron within the paired domain. For a phylogenetic analysis of the chordate Pax2/5/8 proteins, see Additional file 1. Bfl: Branchiostoma floridae; Cin: Ciona intestinalis; Csa: Ciona savignyi; Dre: Danio rerio; Hro: Halocynthia roretzi; Hsa: Homo sapiens; Odi: Oikopleura dioica; Pma: Phallusia mammillata.
Comparison of gene structures revealed that, while most chordate Pax2/5/8 genes have 8 to 11 exons, ascidian Pax2/5/8b paralogs have 20 exons, which code for a protein of about 1300 amino acids, three times longer than the approximately 400 amino acid-long Pax2/5/8 proteins from other chordates. This difference in size is due mainly to an exon more than 1200 nucleotides long located upstream of the paired domain. Our analysis of publicly available EST sequences confirmed the presence of this large exon in gene models predicted for Pax2/5/8b in Ciona intestinalis (EMSBL ID ENSCINT00000012344) and in Ciona savignyi (ENSCSAVG00000001640) (data not shown). This large exon is absent from O. dioica Pax2/5/8 paralogs and may have evolved in the ascidian lineage after the divergence of urochordates.
In conclusion, various Pax2/5/8 paralogs appear to have lost ancestral features and evolved new structural motifs or new exons, as would be predicted by the DDC model applied to Pax2/5/8 paralogs evolving after independent gene duplication events. The identification of these structural features focuses attention on regions to test for function to learn the roles each motif may have played in the origin of lineage-specific morphologies.
Developmental expression of the Oikopleura paralogs Pax2/5/8a and Pax2/5/8b
Tailbud stages
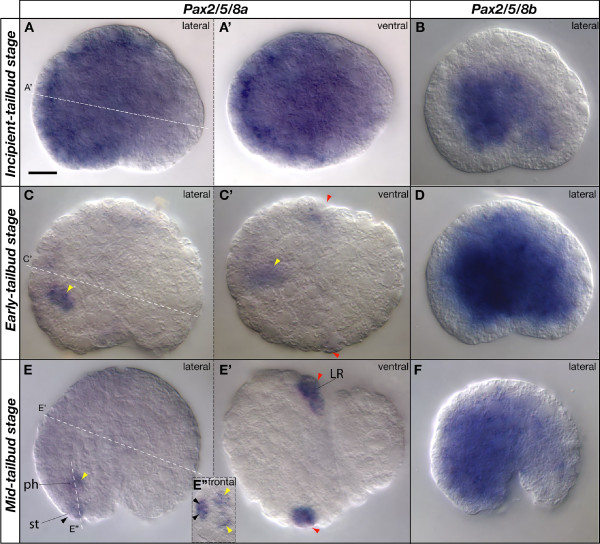
At the end of gastrulation, Pax2/5/8a and Pax2/5/8b were both broadly expressed in the Oikopleura embryo. Pax2/5/8a was expressed mainly in the ectoderm of the trunk (Figure 2A), while Pax2/5/8b was expressed primarily in the interior of the trunk (Figure 2B). In early tailbud stages, Pax2/5/8b expression continued in its broad internal expression domain (Figure 2D), but Pax2/5/8a expression became restricted to two domains: a few medial cells at the boundary of the anterior brain and the pharynx (Figure 2C,C' yellow arrowheads), and a bilateral pair of ectodermal cells in the posterior trunk (Figure 2C,C' red arrowheads). By mid- and late-tailbud stage, the signal of the Pax2/5/8a expression domains strengthened (Figure 2E,E' yellow and red arrowheads), and a new domain appeared in the rostral-most ectoderm of the trunk (Figure 2E, E" black arrowhead), probably labeling the first two cells fated to invaginate into the stomodeum and form the oral epithelium (see below; Figure 3A).
Figure 2.
Expression of Pax2/5/8 paralogs at tailbud stages in Oikopleura dioica. Whole mount in situ hybridization of Pax2/5/8a (A, C, E) and Pax2/5/8b (B, D, F) at incipient-tailbud stage (A, B), early-tailbud stage (C, D), and mid-tailbud stage (E, F) in lateral views (anterior to the left and dorsal to the top). Specific aspects of the expression domains are shown in ventral (A', C' and E') and frontal views (E") in optical sections in the plane of the dashed white lines. Colored arrowheads label expression domains (black, stomodeum, st; yellow, anterior pharynx, ph; red, placodal precursor of the Langerhans receptors, LR). Scale bar = 10 μm.
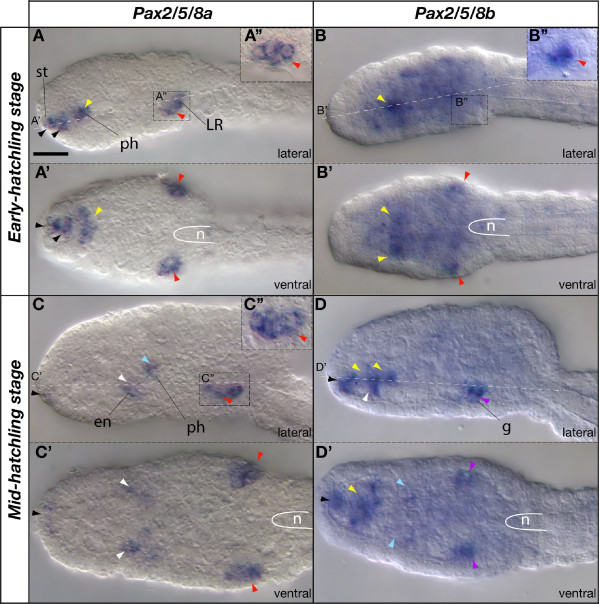
Figure 3.
Expression of Pax2/5/8 paralogs in Oikopleura dioica hatchlings. Whole mount in situ hybridization of Pax2/5/8a (A, C) and Pax2/5/8b (B, D) in early-hatchling stage (A, B) and mid-hatchling stage (C, D). Lateral (A, B, C, D) and ventral (A', B', C' and D') views for each stage. Anterior is to the left and dorsal to the top. Insets (A", B", and C") show details of the regions on the surface of the embryo where the Langerhans receptors eventually form. Colored arrowheads label expression domains (black, stomodeum, st; yellow, anterior pharynx, ph; blue, posterior pharynx, ph; white, endostyle, en; purple, presumptive cell precursors of the gills, g; red, placodal precursor of the Langerhans receptors). The position of the anterior tip of notochord (n) is demarcated (white line). Scale bar = 10 μm.
Early hatchling and mid-hatchling stages
At the early hatchling stage, Pax2/5/8a expression maintained its late-tailbud stage pattern. The rostral-most expression domain in the stomodeal region expanded internally from the surface to form a group of contiguous internal cells (Figure 3A, black arrowheads), which were separated from the internal expression domain in presumptive pharynx/endostyle precursor cells (Figure 3A, yellow arrowhead). The bilateral Pax2/5/8a expression domain in the posterior part of the trunk included at least four ectodermal cells in the area where the Langerhans mechanoreceptors eventually develop (Figure 3A, red arrowheads), at a level slightly anterior to the tip of the notochord and adjacent to the homolog of the vertebrate hindbrain [38]. During early hatchling stages, the internal broad expression domain of Pax2/5/8b that was present at tailbud stages began to fade at the same time that two separate expression domains became distinct, one in the ectoderm at about the same bilateral position as the Pax2/5/8a ectodermal domain (Figure 3A', B', red arrowheads) and the other within the pharynx (Figure 3B, yellow arrowhead). Although Pax2/5/8a and Pax2/5/8b were both expressed in the ectoderm at the eventual position of the Langerhans receptors, close inspection revealed differences in the number and morphology of the Pax2/5/8a and Pax2/5/8b positive cells (Figure 3A", B"). By mid-hatchling stages, the bilateral Pax2/5/8a expression domain expanded to at least six ectodermal cells (Figure 3C"), while the brief flash of Pax2/5/8b expression in the ectoderm became undetectable (Figure 3C', D', purple arrowheads). Simultaneously, the distal portion of each gill pouch began to express Pax2/5/8b. It is not known if ectodermal cells contribute to the formation of the gill pouches at these early stages when the ectoderm is still forming its epithelial character.
Expression of the two Oikopleura Pax2/5/8 paralogs during mid-hatchling stages appeared to be complementary in the pharynx in time and space: while Pax2/5/8a expression was diminishing in the stomodeum and the rostral pharynx (Figure 3C, C'), new Pax2/5/8b expression domains appeared (Figure 3D, D', black and yellow arrowheads). The proximal endoderm of the gill pouches began to express Pax2/5/8a while the distal portion of the gill pouches expressed Pax2/5/8b (Figure 3C, C', D', blue arrowheads). Presumptive dorsal precursor cells of the endostyle first began to express Pax2/5/8a, while presumptive ventral endostyle cells expressed Pax2/5/8b (Figure 4C, C', D, white arrowheads).
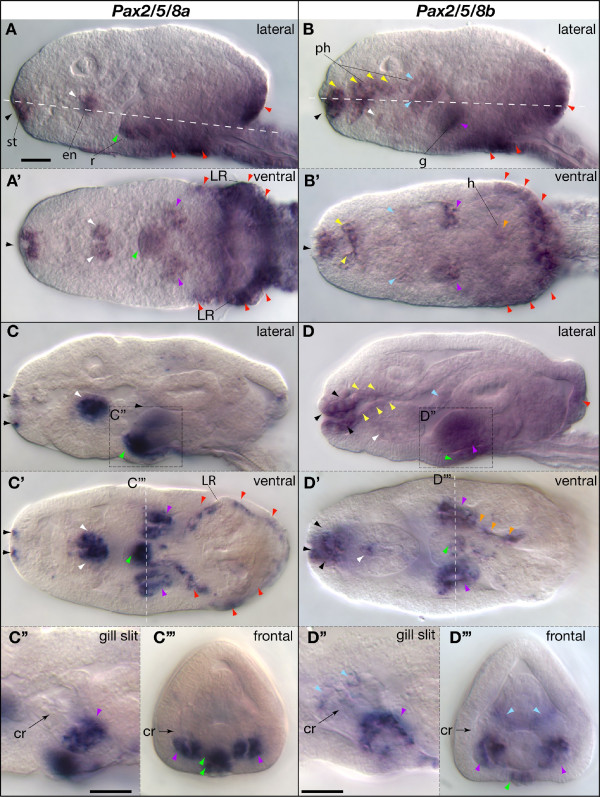
Figure 4.
Expression of Oikopleura dioica Pax2/5/8 paralogs during organogenesis. Whole mount in situ hybridization of Pax2/5/8a (A, C) and Pax2/5/8b (B, D) in late hatchling stages during the initial (A, B) and advanced state (C, D) of the expansion of internal organ cavities during organogenesis. Lateral aspect (A, B, C and D) and integrated ventral view (A', B', C' and D') for each stage are shown. Anterior is to the left and dorsal to the top. Details of the Pax2/5/8 expression domains in the gills (dashed squares and lines in C and D) are shown in lateral (C" and D") and frontal (C"', D"') views. The position of the ciliary rings (cr) is labeled with an arrow. Colored arrowheads label some of the domains (black, stomodeum, st; yellow, anterior pharynx, ph; blue, posterior pharynx; white, endostyle, en; purple, gills, g; orange, medial wall of the heart, h; green, anus and rectum, r; red, base of the Langerhans receptors, LR, and posterior part of the trunk). Scale bar = 10 μm.
Late hatchling stages
By the late hatchling stage, organs are distinct and internal cavities have started to expand. At this stage, the gills and mouth have opened to the outside, and the heart and the ciliary rings of the gills have both begun to beat. The direct development of larvacean urochordates permits the tracing of gene expression domains in organ rudiments from hatchlings until fully mature adults. In contrast, in ascidian urochordates, many organs begin to develop only at metamorphosis, just as many chordate-specific characters disappear.
Pax2/5/8a expression in late hatchling stages overlaps Pax2/5/8b in some domains: in the distal part of the gills from the ciliary rings (cr) to the external gill opening (Figure 4, purple arrowheads), and, transiently, in the posterior trunk epidermis (Figure 4A, B, C, red arrowheads). Other domains are unique to each gene, and in several regions expression of the paralogs appears complementary. For example, while the lips of the mouth express Pax2/5/8a (Figure 4A, C, black arrowheads), the pharynx just internal to the lips strongly expresses Pax2/5/8b (Figure 4B, D, black arrowheads); while dorsal cells of the endostyle express Pax2/5/8a (Figure 4A, C, white arrowheads), ventral cells of the endostyle express Pax2/5/8b (Figure 4B, D, white arrowheads); and while the most posterior section of the rectum expresses Pax2/5/8a (Figure 4A, C, green arrowheads), the anus expresses Pax2/5/8b (Figure 4D, green arrowhead). Except for the distal part of the gills, the posterior pharynx exclusively expresses Pax2/5/8b, including the gill endoderm where the two gill pouches meet medially (Figure 4B, D, blue arrowheads). And, strikingly, only one layer of the two-ply heart, the pericardium, expresses Pax2/5/8b (Figure 4B', D', orange arrowheads); the muscular myocardium does not.
A subtle metamorphic event called 'tailshift', the rapid reorientation of the tail from a posterior to a ventral position with respect to the trunk, signals the beginning of the juvenile stage in Oikopleura. Just prior to tailshift, the two Pax2/5/8 paralogs continue to show mostly non-overlapping expression patterns, now in clearly differentiated organs. Pax2/5/8a (Figure 5A) is still expressed around the mouth (including ciliated sense organs in the upper lip), in the dorsal, iodine-binding corridor cells of the endostyle, in the gill endoderm, in the rectum, and in a row of cells at the posterior margin of the oikoplastic epithelium (a specialized secretory tissue that covers much of the trunk). Expression of Pax2/5/8b (Figure 5B) is also a continuation of domains identified earlier, including the pharynx and gills, the ventral endostyle, the anus, and the pericardium. New domains, however, now appear in dorsal, ventral and lateral fields of the oikoplastic epithelium, the tissue that will soon secrete the first filter-feeding 'house' the animal inhabits [61]. Figure 5C is a schematic diagram summarizing the expression domains of both Oikopleura Pax2/5/8 paralogs in late hatchlings. Table 1 summarizes by tissue and developmental stage all Pax2/5/8 expression domains.
Figure 5.
Expression of larvacean Pax2/5/8 paralogs at pre-tailshift stage, when organogenesis nears completion. (A) Pax2/5/8a. (B) Pax2/5/8b. (C) Schematic representation summarizing the non-overlapping Pax2/5/8a (red) and Pax2/5/8b (blue) expression domains. Arrows indicate perforations where epidermal fusions occur and Pax2/5/8 paralogs are expressed. Abbreviations: a, anus; ab, anterior brain; cr, ciliary ring; en, endostyle; es, esophagus; h, heart; lr, Langerhans receptor; m, mouth; ph, pharynx; r, rectum; s, stomach. Scale bar = 10 μm.
Table 1.
Summary of Pax2/5/8 expression domains during Oikopleura development
| Tailbud | Early hatchling | Mid-hatchling | Late hatchling | |
| Stomodeum | a | a | a/b | a/b |
| Pharynx | a | a/b | a/b | b |
| Langerhans receptors | a | a+b | a | a |
| Endostyle | a/b | a/b | ||
| Gills | b | a/b | ||
| Anus | a/b | |||
| Posterior trunk epidermis | a+b | |||
| Heart | b |
a, Pax2/5/8a; b, Pax2/5/8b; a/b, expression of paralogs does not spatially overlap or only partly overlaps; a+b, both paralogs spatially overlap.
Discussion
These investigations of the structure and expression of Pax2/5/8 gene duplicates in Oikopleura, when analyzed in a comparative context with shared and independent Pax2/5/8 gene duplications in other chordate lineages, illuminate a variety of problems in the evolution of chordate developmental mechanisms and the principles that govern change in duplicated gene function over time. The work resolves alternative hypotheses for the homologies of the ascidian atrial siphon, illuminates evolution of the thyroid, identifies candidates for ancestral gene subfunctions, and defines the origin of functional innovations in this gene family.
Pax2/5/8 and the evolution and development of the chordate heart
The shared expression of cardiac developmental genes (for example, Nkx-2.5, dHand, and Mesp1) supports the homology of bilaterian hearts from chordates to flies [62-69]. The evolutionary relationship of the single-chambered heart of non-vertebrate chordates to the individual chambers of the innovative multi-chamber vertebrate heart, however, is still unclear (reviewed in [70]). Our work reveals unprecedented Pax2/5/8 expression in the developing heart of a chordate, and may provide a late ontogenetic marker for assessing homology of tissue layers among simple, urochordate hearts.
In contrast to the single-layered heart of cephalochordates, urochordates have a two-layered heart. The simple heart of Oikopleura dioica consists of two epithelial layers lying just medial to the left stomach lobe. In Oikopleura species, the lateral wall of the heart contains the muscle fibers while the medial wall is a thin pericardial membrane (called 'procardium' in [71]). Peristaltic heart contractions, which periodically reverse, cause the hemolymph to course between the heart and the stomach wall [71,72]. In comparison, the ascidian heart rolls up to become tubular rather than planar, but also has a contractile layer and a non-muscular, pericardial layer (reviewed in [70]). The larvacean pericardial membrane, therefore, is the likely homolog of the ascidian pericardium.
In ascidians, heart development can be broadly divided in two phases (reviewed in [70]). In the first phase, heart cell precursors become fated during early cleavage and embryonic stages, and after a complex process of muscle cell migration from the tail to the trunk, heart development temporarily arrests. In the second phase, after metamorphosis, heart development re-initiates and after final differentiation, the heart starts to beat and becomes functional. In larvaceans, the transparency and absence of a drastic metamorphosis provides a complementary model system for the study of heart development. In O. dioica, the heart starts beating less than 24 hours post fertilization, before tailshift. In late larvacean hatchlings, when heart differentiation is at or nearing completion, the pericardium expresses Pax2/5/8b. In ascidians, however, neither Pax2/5/8 paralog has been shown to be expressed in the heart, perhaps because most published analyses of Pax2/5/8 expression in Ciona, Halocynthia and Herdmania [48,53,60,73,74] have not included late developmental stages after metamorphosis (3 to 4 days after fertilization). Therefore, whether Pax2/5/8 expression in the heart – and more specifically in the pericardium – is a shared feature among urochordates remains to be investigated.
Cephalochordates have a single-layered, muscularized blood vessel ventral to the gut that propels the hemolymph by peristalsis [75,76]. During amphioxus development, the primordium of this muscularized vessel expresses a homolog of vertebrate NK2 class genes – a group that includes several genes necessary for vertebrate cardiac development – suggesting that the amphioxus vessel is homologous to the vertebrate heart [69]. Pax2/5/8 is not reported to be expressed in the circulatory vessels of amphioxus [52], and likewise, amphioxus has no structure that appears to be morphologically comparable to the Pax2/5/8-expressing larvacean pericardium or to the pericardia of ascidians or vertebrates.
In vertebrates, mouse Pax2 and Pax5 are not expressed in the heart [77,78], and although Pax8 expression has been detected in the heart of newborn mice, its level was equal to or less than all other tissues tested except kidney [79], suggesting that it may represent background levels. No role for Pax2, Pax5, or Pax8 has been demonstrated in the heart in knockout mutations in mouse [80-82]. Consistent with these results, our survey of EST databases in NCBI http://www.ncbi.nlm.nih.gov reveals the absence of Pax2/5/8 genes in the heart transcriptomes of human, mouse, frogs and zebrafish. Thus, we can infer that Pax2/5/8 expression in the heart was either already present in the last common ancestor of olfactores (that is, urochordates + vertebrates) and lost in the vertebrate lineage, or was a neofunctionalization event within the urochordates, and perhaps within larvaceans.
Evidence of Pax2/5/8 subfunction partitioning in thyroid homologs
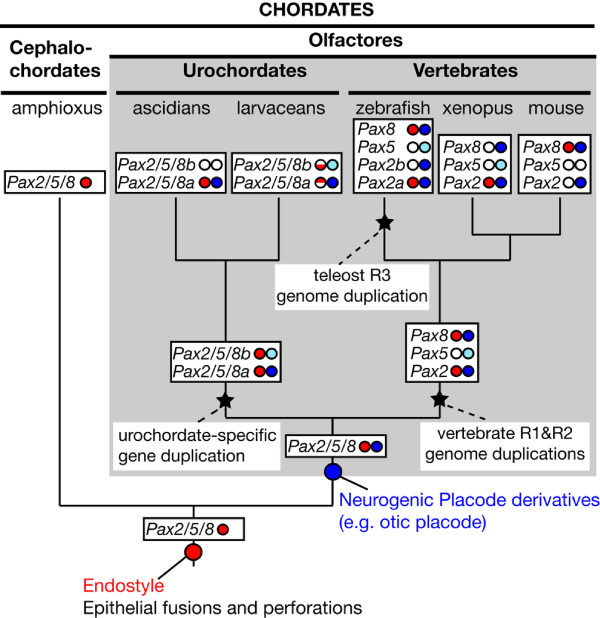
The thyroid is an endocrine gland located in the neck ventral to the pharynx in humans and other vertebrates, and it regulates energy production and growth by synthesizing tyrosine-based, iodine-containing hormones (T3 and T4). The presumed homolog of the thyroid in filter-feeding, non-vertebrate chordates is the endostyle, an organ located ventral to the pharyngeal floor that functions in iodine binding and secretion of food-trapping mucus [83]. The homology between the thyroid and the endostyle is further supported by the expression of similar molecular markers, including members of the Pax2/5/8 family ([84] and references therein). Our results show that the larvacean endostyle expresses Pax2/5/8 genes in a pattern similar to that described for amphioxus [52], ascidians [84], and vertebrates [56,85]. The last common ancestor of all three chordate subphyla, therefore, probably employed a Pax2/5/8 gene in the development of an endostyle-like organ that evolved into the endostyle of cephalochordates and urochordates and the thyroid of vertebrates (Figure 6).
Figure 6.
Hypothesis for the evolution of chordate Pax2/5/8 subfunctions. In stem chordates, Pax2/5/8 already played a role during the development of the endostyle, the homolog of the vertebrate thyroid, as well as in controlling genes for making epithelial fusions and perforations (for example, gill openings). The Pax2/5/8 subfunction related to the development of neurogenic placodes (for example, otic placode) appears to be restricted to the olfactores; like vertebrate orthologs, urochordate Pax2/5/8a genes are expressed from just after neurula stage in paired thickenings overlapping the early expression of other placode-marking genes [39]. Inferred origins for some of the functions (circles in stems) performed by the pleiotropic Pax2/5/8 gene family and inferred subfunction partitioning are schematized in the context of the chordate phylogeny. Gene duplication events (stars) in different chordate lineages permitted independent partitioning of endostyle/thyroid (red circles) and otic placode (blue circles) subfunctions among gene paralogs. Half semicircles denote dorsal and ventral expression domains. Pale blue circles denote paralogs whose expression is inferred to have become delayed, transient or spatially narrowed in development of the otic system. White circles indicate inferred subfunction losses. [43,45,48,52,56,73,84,85,118,119].
Pax2/5/8 subfunctions related to vertebrate thyroid development have likely suffered independent processes of subfunction partitioning among paralogs (Figure 6). For instance, in mouse, Pax8 is expressed during thyroid development but Pax2 is not [56,85]; in frogs, however, subfunctions of these genes are reversed, as Pax2 but not Pax8 is involved in the development of the thyroid [43]. These results suggest that ancestral vertebrate thyroid regulatory subfunctions of Pax2/5/8 were preserved by both Pax2 and Pax8 in stem amniotes, and resolved independently in the amphibian and mammalian lineages. In zebrafish, both pax8 and pax2a (previously called pax2.1) retain thyroid expression, although they are activated at different times in development [56].
Although the duplication of Pax2/5/8 paralogs in urochordates occurred before the divergence of the larvacean and ascidian lineages [38] (Additional File 1), the endostyle of Oikopleura expresses both Pax2/5/8 paralogs (Pax2/5/8a dorsally, and Pax2/5/8b ventrally), while the endostyle of ascidians does not express Pax2/5/8b, but expresses Pax2/5/8a in both the dorsal and ventral domains, the sum of the pattern for the two Oikopleura duplicates [84]. These lineage-specific differences in expression patterns of orthologous Pax2/5/8 duplicates suggest that in the last common ancestor of larvaceans and ascidians, both Pax2/5/8a and Pax2/5/8b were expressed both dorsally and ventrally, and that after lineages diverged, in larvaceans the dorsal and ventral endostyle subfunctions partitioned to different Pax2/5/8 genes, but in ascidians both subfunctions remained associated with the Pax2/5/8a paralog and both were lost by the Pax2/5/8b paralog. As vertebrate Pax8 can directly bind the promoters of thyroid follicle cell-specific genes such as thyroperoxidase [86], we raise the hypothesis that urochordate Pax2/5/8a genes, which are expressed in peroxidase-producing, dorsal cells of the endostyle [87,88], play a homologous role while urochordate Pax2/5/8b genes have lost this function.
Evidence of Pax2/5/8 subfunction partitioning in the anterior pharynx and stomodeum
Comparison between ascidians, larvaceans and cephalochordates suggests that stem chordates expressed Pax2/5/8 in the stomodeum and anterior pharynx, but that this expression domain was lost in the vertebrate lineage. Larvaceans and ascidians both have ciliated mechanosensory organs that ring the mouth and are involved in a filter-feeding, particle-rejection response [39,89,90]. Although the oral sensory cell types themselves are morphologically different in the two urochordate classes, these organs may share a common origin in a stomodeal sensory placode that has been lost in the vertebrate lineage [39,73,90-94]. Although both larvacean Pax2/5/8 paralogs are expressed in this stomodeal domain, their patterns are distinct: Pax2/5/8a expression in the rostral pharynx of early hatchlings is replaced with Pax2/5/8b by mid-hatchling stages. In mid-hatchlings, Pax2/5/8a expression is in the external ectoderm surrounding the mouth opening, including the two bristle-bearing cells of the upper lip, while Pax2/5/8b is expressed just inside the mouth, including the ciliated sensory cells of the circumoral organ. Although the two mechanosensory cell types fall into expression domains of different Pax2/5/8 paralogs, they are innervated by the same branched sensory axons emanating from a pair of rostral brain cells [39,89].
The single amphioxus Pax2/5/8 gene is expressed both externally around the developing mouth and internally in the pharynx [52]; this expression domain corresponds to the sum of expression domains occupied by different larvacean paralogs, as expected by subfunction partitioning [2] if the last stem chordate had separate regulatory elements governing the contiguous external and internal pharyngeal domains that partitioned to different larvacean paralogs after the Pax2/5/8 duplication event. Ascidian Pax2/5/8a is expressed in the invaginating stomodeum but Pax2/5/8b is expressed in the buccal cavity, a pattern comparable with the late hatchling expression domains of the Oikopleura orthologs. At least some subfunctions, therefore, appear to have partitioned between Pax2/5/8 paralogs before the larvacean and ascidian lineages diverged. The ascidian Pax2/5/8a gene, however, appears to lack expression comparable with the early, internal expression of Oikopleura Pax2/5/8a in the rostral pharynx, a difference that may derive from the developmental delay and incomplete development of endodermal organs experienced by ascidian larvae compared with the uninterrupted and complete endodermal ontogeny in larvaceans.
Pax2/5/8 expression and the Langerhans receptor, ascidian atrial siphon, and vertebrate otic placode
The expression of Pax2/5/8 genes in the Langerhans receptors of Oikopleura helps us understand apparently conflicting interpretations of the ancestral role of Pax2/5/8 in the origin of the vertebrate otic placode. Previous evidence supports the notion that larvacean Langerhans receptors are homologous to hair cell-like sensory organs in the ascidian atrium, the chamber surrounding the branchial basket [39,73,95]. Expression of Pax2/5/8a in the ascidian atrium and expression of Pax2 and Pax8 in the vertebrate hair cell-producing otic placode suggested the hypothesis that the atrium of ascidians is homologous to the otic vesicles of vertebrates [48].
An alternative to the 'placode hypothesis', however, arose from the finding that in amphioxus, the developing gill slits and mouth express Pax2/5/8. This alternative suggests that Pax2/5/8 expression in the atrial primordium of ascidians reflects an ancient gene function in the perforation, adhesion and fusion of epithelial layers, such as in the epithelia of gill openings, rather than the homology of atrial primordia and vertebrate placodes [52]. The 'epithelial fusion hypothesis' is consistent with the finding that among vertebrates, Pax2 apparently plays a role in gill slit perforation in Xenopus [96] and Pax8 is necessary for vaginal opening in mouse [97]. Furthermore, Pax2 and Pax8 regulate genes that control the composition of the extracellular matrix in epithelial fusions [42,98].
Evidence to resolve this dilemma comes from examination of gene expression and organ structure in a phylogenetic context. Our analysis of larvacean Pax2/5/8 expression teases apart epithelial fusion from placode formation and suggests that Pax2/5/8 likely functioned in both processes in ancient chordates. In Oikopleura, several Pax2/5/8 expression domains can be grouped into two overlapping categories: sites of epithelial fusion and sites of sensory cell development. At the sites of epithelial fusion, larvacean Pax2/5/8 paralogs are expressed at the junction of the gill pouch with the epidermis, the joining of the rectum to the epidermis at the anus, and the fusion of the stomodeum and pharynx at the mouth. At sites of sensory cell development, Oikopleura's Pax2/5/8 paralogs are expressed at the stomodeal placode and the putative acousticolateralis placode homolog (the Langerhans organ primordia). Therefore, in contrast to ascidians, in which hair cell-like organs and sites of perforation are conflated in the atrium [95], Oikopleura's paired mechanosensory organs are topographically separate from the gill openings.
In agreement with the perforation argument [52,99], expression of Oikopleura Pax2/5/8 as in amphioxus, in the gill openings and anus, which lack sensory cell types, supports the hypothesis of an ancient function for Pax2/5/8 in the adhesion and fusion of epithelial layers and in the promotion of epithelial perforations. On the other hand, expression of Oikopleura Pax2/5/8a at early tailbud stage in the primordia of the Langerhans organs occurs long before the fusion of the gill endoderm with the ectoderm, which happens at the late hatchling stage. This timing gap argues against the interpretation that early expression of Pax2/5/8 in the Langerhans domain is associated only with remodeling the extracellular matrix for gill perforation. Instead, early Pax2/5/8 expression defines the location of paired, thickened sensory organ primordia, in agreement with the hypothesis of Wada et al. [48] that Pax2/5/8 marks a urochordate placode. Larvacean Pax2/5/8 expression adds to a growing body of morphological and gene expression data that supports the origin of cranial placodes in early chordates, rather than in vertebrates, as had been long assumed [39,48,73,93-95], [100-105], and strengthens the case for an early origin specifically of the otic or acousticolateralis placode.
Therefore, analysis of Pax2/5/8 paralogs in Oikopleura has allowed us to reconcile the 'placode hypothesis' and 'epithelial fusion hypothesis', supporting an evolutionary scenario in which the role of Pax2/5/8 in epithelial fusions and perforations was already present in stem chordates, and the role of Pax2/5/8 related to placode development is likely also ancient, though perhaps restricted to Olfactores (Figure 6).
Evolution of Pax2/5/8 genetic pathways
Pax, Eya, Six and Dach genes form a genetic network in several biological processes, including in sensory placode development (reviewed in [81,106]). Pax2, Pax5, Pax8, Eya1, Six1, and Dach1 are co-expressed during fish otic vesicle development (see for example [107,108]), where they interact to specify otic tissue and maintain its continued ontogenesis. Larvacean Pax2/5/8 genes are expressed in presumptive sensory tissues previously reported also to express Eya and Six orthologs [39], namely around the mouth and in the mechanosensory Langerhans receptors. The two Pax2/5/8 paralogs may interact with different subsets of Eya-Six-Dach genes in different tissues. For instance, in developing sensory tissues, Pax2/5/8a expression in tailbud and early hatchling stages is similar to that of Eya and Six3/6a in the developing Langerhans receptor primordia, and to Six1/2 and Six3/6a expression around the mouth, including sensory cilia-bearing cells of the upper lip. Endodermal Pax2/5/8b expression, on the other hand, most strongly overlaps that of Six3/6a in the rostral pharynx and Eya in the gill endoderm in mid-hatchling stages. Similarly, ascidian Pax2/5/8a expression overlaps Eya and Six1/2 in the atrial primordia and Eya, Six1/2, and Six3/6 in the stomodeal domain, but Pax2/5/8b exhibits broad expression in the ectoderm and may also overlap Eya, Six1/2, and Six3/6 in a way that larvacean Pax2/5/8b does not [73]. Such differences between presumed Pax2/5/8 paralogs and orthologs is further evidence that, though the Pax-Six-Eya-Dach 'module' is conserved at the level of gene families, paralogs within each module may differ both within a developmental program and between lineages [39,99]. Not surprisingly, then, sequence analysis shows that several known protein interaction domains differ between the larvacean Pax2/5/8 paralogs and between the larvacean and ascidian orthologs (Figure 1), consistent with divergence of the molecular pathways in which these Pax proteins participate.
Pax2/5/8 gene duplications and the evolution of gene functions
A requirement for partitioning of ancestral gene functions is that the ancestral gene must have multiple independently mutable functions either in protein-coding domains or in regulatory elements that drive restricted expression: in other words, units that are by definition 'subfunctions'. Direct evidence for independently mutable Pax2/5/8 subfunctions in an extant gene comes from the Drosophila ortholog, called D-Pax2, in which mutations in separate regulatory elements affect development of either ommatidia or sensory bristles [109]. The known functions of vertebrate Pax2/5/8 genes are diverse and include the establishment of the midbrain-hindbrain border [110-113], specification and mature function of thyroid follicular cells [85], specification and morphogenesis in the pronephros [80], differentiation of interneuron subtypes in the central nervous system (see for example [114]), promotion of correct axon guidance in the optic nerves [115], and morphogenesis and sensory cell specification in the epibranchial, otic and lateral line sensory placodes (see for example [96]). It is not known, however, how many of these functions, segregated among extant genes, resulted from independently mutable ancestral subfunctions.
From the partially overlapping expression patterns of vertebrate Pax2/5/8 genes, we can infer that subfunction partitioning has occurred in this gene family. Next, comparative analysis of expression domains in the chordate phylogenetic context helps us infer when subfunctions arose. Parallel, independent partitioning of Pax2/5/8 endostyle/thyroid functions in both vertebrate and urochordate lineages suggests that the single Pax2/5/8 gene of stem olfactores had already acquired independently mutable regulation for endostyle/thyroid expression. Likewise, paralleling segregation of mammalian otic placode subfunctions to Pax2 and Pax8 [42,45], larvacean otic placode-like tissues express only one Pax2/5/8 paralog early and in a pattern overlapping orthologs of other vertebrate placode markers of the Six and Eya gene families [39] (Figure 6). It remains possible, though, that despite the similarity between vertebrate and urochordate Pax2/5/8 gene expression patterns, each lineage separately evolved independently mutable functions in the endostyle/thyroid and otic placode development and that the ancestral gene did not already bear these subfunctions.
Within the urochordate lineage, further parsing of gene functions may represent subfunctions that were present in the ancestral urochordate Pax2/5/8 gene. For example, at some sites of epithelial fusion, larvacean Pax2/5/8 paralogs exhibit complementary expression, for example just outside (Pax2/5/8a) or just inside (Pax2/5/8b) the mouth and just outside (Pax2/5/8b) or just inside (Pax2/5/8a) the anus. Therefore, though a role in epithelial fusions is probably ancient in Chordata, urochordate paralogs may have taken on separate refinements of the same function.
In addition to the partitioning of ancestral functions, we might also expect to detect apparent neofunctionalizations of gene duplicates, particularly when we compare deeply diverging lineages such as vertebrates and urochordates. The role of vertebrate Pax5 in the B-lymphoid lineage of the immune system might be one example of a vertebrate neofunctionalization [55]. Similarly, because no comparable expression can be found outside of Urochordata, a role for Pax2/5/8b in the pericardium of the heart could be a novel deployment of Pax2/5/8 exclusive to the urochordates or even to the larvacean lineage.
Though observed Pax2/5/8 expression patterns are consistent with a hypothesis of ancestral subfunction partitioning, this analysis detects only transcriptional regulatory differences and might underestimate the extent of actual subfunction partitioning. For example, mRNA expression of the paralogs may overlap in a given tissue, but the structurally different proteins the genes encode may not have redundant functions in those tissues. In addition, post-transcriptional regulation would also be missed in our analysis. Partitioned genes likely continue to evolve after their initial partitioning, and such divergence could complicate distinguishing subfunctionalization from neofunctionalization when comparing gene functions in deeply diverging lineages. Nonetheless, functional analysis of urochordate Pax2/5/8 genes could help distinguish between ancestral chordate subfunction partitioning and convergent patterns of neofunctionalization. For example, if Pax2/5/8 proteins carry out a similar function in the vertebrate thyroid as in the urochordate endostyle, this would be more concrete evidence that an ancient function was partitioned to paralogs in both chordate subphyla.
Conclusion
The present work shows how analyzing the evolution of gene families that have experienced multiple independent gene duplication events in related lineages can improve understanding of the evolution of the genetic mechanisms underlying the development of homologous structures of anatomically divergent organisms (for example the endostyle of non-vertebrate chordates and the thyroid of vertebrates), and can help to identify gene subfunctions that otherwise may be difficult to recognize because of extensive pleiotropy (for example, Pax2/5/8 subfunctions in epithelial fusions around perforations and development of placode derivatives). Analysis of the evolution of subfunctions in a phylogenetic context identifies lineage specific innovations (for example, placode derivative homologs in olfactores). The modular fashion in which gene subfunctions partition after independent gene duplication events in various lineages implies the existence of independent regulatory elements that control temporal and spatial expression for each subfunction. For instance, comparison of Pax2/5/8 expression patterns in the endostyles of amphioxus, Oikopleura, and ascidians predicts the presence of separable regulation for expression in the iodine-binding dorsal component and the supporting ventral component of the endostyle/thyroid, a hypothesis that remains to be tested. The identification of the complete set of gene orthologs and paralogs in gene families in an increasing number of completely sequenced genomes will likely reveal additional cases of independent gene duplications and subfunctionalization events, the analysis of which will improve our understanding of the evolution of gene functions and the implications in the evolution and diversity of living forms.
Methods
Biological materials
Oikopleura dioica animals were collected in the Pacific Ocean near the Oregon Institute of Marine Biology (Charleston, OR), and were cultured in the laboratory at the University of Oregon (Eugene, OR, USA) at 13°C in 10 μm-filtered seawater for several generations. The transparency of Oikopleura embryos and adults allows non-invasive study of internal anatomy at the level of individual cells and the tracing of organs from embryo to adult. For some images, we merged DIC optical sections using Adobe Photoshop software to integrate images of structures that spanned multiple focal planes.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed as described [116,117] with minor modifications. Fixed, dehydrated embryos were dechorionated manually with glass needles before re-hydration; to reduce background, Tween-20 concentration was increased from 0.1% to 0.15% in the hybridization buffer, PBT solution and post-hybridization washing buffers. Embryos were mounted in 80% glycerol for microscopy. Riboprobes for detecting the expression of Pax2/5/8a and Pax2/5/8b (Genbank accession numbers, respectively: AY870648, AY870649) genes are described in [38].
List of abbreviations
DDC: duplication-degeneration-complementation.
Authors' contributions
SB identified, cloned and characterized the expression pattern of Oikopleura Pax2/5/8b, performed part of the comparative analysis and wrote part of the manuscript. CC identified, cloned and characterized the expression pattern of Oikopleura Pax2/5/8a, performed part of the comparative analysis and wrote part of the manuscript. JHP supervised the project, performed part of the comparative analysis and wrote part of the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Phylogenetic relationships among chordate Pax2/5/8 proteins. The data provided illustrates the independent gene family expansions that permitted parallel histories of subfunction partitioning among vertebrate paralogs (Pax2, Pax5 and Pax8) and among urochordate paralogs (Pax2/5/8a and Pax2/5/8b).
Acknowledgments
Acknowledgements
We are grateful to Skipper Burley Young of the 'Charming Polly' for help in collecting larvaceans. We thank E Sanders and M Fajer for help with animal care. We thank W Cresko for helpful comments on the manuscript. This material is based on work supported by NSF Grant IOB-0719577.
Contributor Information
Susan Bassham, Email: sbassham@uoregon.edu.
Cristian Cañestro, Email: cristian@uoregon.edu.
John H Postlethwait, Email: jpostle@uoneuro.uoregon.edu.
References
- Ohno S. Evolution by Gene Duplication. New York: Springer-Verlag; 1970. [Google Scholar]
- Force A, Lynch M, Pickett FB, Amores A, Yan Y-L, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–1545. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus A. On the possibility of constructive neutral evolution. J Mol Evol. 1999;49:169–181. doi: 10.1007/PL00006540. [DOI] [PubMed] [Google Scholar]
- Chain F, Ilieva D, Evans B. Duplicate gene evolution and expression in the wake of vertebrate allopolyploidization. BMC Evol Biol. 2008;8:44. doi: 10.1186/1471-2148-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AL. The evolution of functionally novel proteins after gene duplication. Proc Biol Sci. 1994;256:119–124. doi: 10.1098/rspb.1994.0058. [DOI] [PubMed] [Google Scholar]
- Force A, Cresko WA, Pickett FB, Proulx SR, Amemiya C, Lynch M. The origin of subfunctions and modular gene regulation. Genetics. 2005;170:433–446. doi: 10.1534/genetics.104.027607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair JE, Shah P, Hedges SB. Evolutionary sequence analysis of complete eukaryote genomes. BMC Bioinformatics. 2005;6:53. doi: 10.1186/1471-2105-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwait J, Amores A, Cresko W, Singer A, Yan YL. Subfunction partitioning, the teleost radiation and the annotation of the human genome. Trends Genet. 2004;20:481–490. doi: 10.1016/j.tig.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Cañestro C, Yokoi H, Postlethwait JH. Evolutionary developmental biology and genomics. Nat Rev Genet. 2007;8:932–942. doi: 10.1038/nrg2226. [DOI] [PubMed] [Google Scholar]
- Lynch M, Force A. The origin of interspecific genomic incompatibility via gene duplication. Am Nat. 2000;156:590–605. doi: 10.1086/316992. [DOI] [PubMed] [Google Scholar]
- Bally-Cuif L, Boncinelli E. Transcription factors and head formation in vertebrates. Bioessays. 1997;19:127–135. doi: 10.1002/bies.950190207. [DOI] [PubMed] [Google Scholar]
- Meyer A, Schartl M. Gene and genome duplications in vertebrates: the one-to-four (-to-eight in fish) rule and the evolution of novel gene functions. Curr Opin Cell Biol. 1999;11:699–704. doi: 10.1016/S0955-0674(99)00039-3. [DOI] [PubMed] [Google Scholar]
- Lundin LG. Evolution of the vertebrate genome as reflected in paralogous chromosomal regions in man and the house mouse. Genomics. 1993;16:1–19. doi: 10.1006/geno.1993.1133. [DOI] [PubMed] [Google Scholar]
- Dehal P, Boore JL. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005;3:e314. doi: 10.1371/journal.pbio.0030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P. Gene duplication: past, present and future. Semin Cell Dev Biol. 1999;10:541–547. doi: 10.1006/scdb.1999.0335. [DOI] [PubMed] [Google Scholar]
- Spring J. Vertebrate evolution by interspecific hybridization – are we polyploid? FEBS Lett. 1997;400:2–8. doi: 10.1016/S0014-5793(96)01351-8. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gu X. Evolutionary patterns of gene families generated in the early stage of vertebrates. J Mol Evol. 2000;51:88–96. doi: 10.1007/s002390010069. [DOI] [PubMed] [Google Scholar]
- Larhammar D, Lundin L, Hallbook F. The human Hox-bearing chromosome regions did arise by block or chromosome (or even genome) duplications. Genome Res. 2002;12:1910–1920. doi: 10.1101/gr.445702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282:1711–1714. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, Yan Y-L, Gates M, Horne S, Amores A, Brownlie A, Donovan A, Egan E, Force A, Gong Z, Goutel C, Fritz A, Kelsh R, Knapik E, Liao E, Paw B, Ransom D, Singer A, Thomson M, Abduljabbar TS, Yelick P, Beier D, Joly J-S, Larhammar D, Talbot WS. Vertebrate genome evolution and the zebrafish gene map. Nat Genet. 1998;18:345–349. doi: 10.1038/ng0498-345. [DOI] [PubMed] [Google Scholar]
- Jaillon O, Aury JM, Brunet F, Petit JL, Stange-Thomann N, Mauceli E, Bouneau L, Fischer C, Ozouf-Costaz C, Bernot A, Nicaud S, Jaffe D, Fisher S, Lutfalla G, Dossat C, Segurens B, Dasilva C, Salanoubat M, Levy M, Boudet N, Castellano S, Anthouard V, Jubin C, Castelli V, Katinka M, Vacherie B, Biemont C, Skalli Z, Cattolico L, Poulain J, De Berardinis V, Cruaud C, Duprat S, Brottier P, Coutanceau JP, Gouzy J, Parra G, Lardier G, Chapple C, McKernan KJ, McEwan P, Bosak S, Kellis M, Volff JN, Guigo R, Zody MC, Mesirov J, Lindblad-Toh K, Birren B, Nusbaum C, Kahn D, Robinson-Rechavi M, Laudet V, Schachter V, Quetier F, Saurin W, Scarpelli C, Wincker P, Lander ES, Weissenbach J, Roest Crollius H. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature. 2004;431:946–957. doi: 10.1038/nature03025. [DOI] [PubMed] [Google Scholar]
- Taylor J, Braasch I, Frickey T, Meyer A, Peer Y Van De. Genome duplication, a trait shared by 22,000 species of ray-finned fish. Genome Res. 2003;13:382–390. doi: 10.1101/gr.640303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse K, Tanaka M, Mita K, Shima A, Postlethwait J, Mitani H. A medaka gene map: the trace of ancestral vertebrate proto-chromosomes revealed by comparative gene mapping. Genome Res. 2004;14:820–828. doi: 10.1101/gr.2004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeld SM, Holland PW. Vertebrate innovations. Proc Natl Acad Sci USA. 2000;97:4449–4452. doi: 10.1073/pnas.97.9.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley-Jones J, Robertson DL, Boot-Handford RP. On the origins of the extracellular matrix in vertebrates. Matrix Biol. 2007;26:2–11. doi: 10.1016/j.matbio.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- Bourlat SJ, Juliusdottir T, Lowe CJ, Freeman R, Aronowicz J, Kirschner M, Lander ES, Thorndyke M, Nakano H, Kohn AB, Heyland A, Moroz LL, Copley RR, Telford MJ. Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature. 2006;444:85–88. doi: 10.1038/nature05241. [DOI] [PubMed] [Google Scholar]
- Vienne A, Pontarotti P. Metaphylogeny of 82 gene families sheds a new light on chordate evolution. Int J Biol Sci. 2006;2:32–37. doi: 10.7150/ijbs.2.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Okuyama M, Satoh N, Zhang S. Molecular evolution of fibrillar collagen in chordates, with implications for the evolution of vertebrate skeletons and chordate phylogeny. Evol Dev. 2006;8:370–377. doi: 10.1111/j.1525-142X.2006.00109.x. [DOI] [PubMed] [Google Scholar]
- Oda H, Wada H, Tagawa K, Akiyama-Oda Y, Satoh N, Humphreys T, Zhang S, Tsukita S. A novel amphioxus cadherin that localizes to epithelial adherens junctions has an unusual domain organization with implications for chordate phylogeny. Evol Dev. 2002;4:426–434. doi: 10.1046/j.1525-142X.2002.02031.x. [DOI] [PubMed] [Google Scholar]
- Lynch M, Conery J. The evolutionary fate and consequences of gene duplication. Science. 2000;290:1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Troge J, Alexander J, Young J, Lundin P, Maner S, Massa H, Walker M, Chi M, Navin N, Lucito R, Healy J, Hicks J, Ye K, Reiner A, Gilliam TC, Trask B, Patterson N, Zetterberg A, Wigler M. Large-scale copy number polymorphism in the human genome. Science. 2004;305:525–528. doi: 10.1126/science.1098918. [DOI] [PubMed] [Google Scholar]
- Minguillon C, Ferrier DE, Cebrian C, Garcia-Fernandez J. Gene duplications in the prototypical cephalochordate amphioxus. Gene. 2002;287:121–128. doi: 10.1016/S0378-1119(01)00828-9. [DOI] [PubMed] [Google Scholar]
- Dalfo D, Cañestro C, Albalat R, Gonzalez-Duarte R. Characterization of a microsomal retinol dehydrogenase gene from amphioxus: retinoid metabolism before vertebrates. Chem Biol Interact. 2001;130–132:359–370. doi: 10.1016/S0009-2797(00)00261-1. [DOI] [PubMed] [Google Scholar]
- Cañestro C, Postlethwait J, Gonzalez-Duarte R, Albalat R. Is retinoic acid genetic machinery a chordate innovation? Evol Dev. 2006;8:394–406. doi: 10.1111/j.1525-142X.2006.00113.x. [DOI] [PubMed] [Google Scholar]
- Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomoso A, Davidson B, Di Gregorio A, Gelpke M, Goodstein DM, Harafuji N, Hastings KEM, Ho I, Hotta K, Huang W, Kawashima T, Lemaire P, Martinez D, Meinertzhagen IA, Necula S, Nonaka M, Putnam N, Rash S, Saiga H, Satake M, Terry A, Yamada L, Wang H-G, Awazu S, Azumi K, Boore J, Branno M, Chin-bow S, DeSantis R, Doyle S, Francino P, Keys DN, Haga S, Hayashi H, Hino K, Imai KS, Inaba K, Kano S, Kobayashi K, Kobayashi M, Lee B-I, Makabe KW, Manohar C, Matassi G, Medina M, Mochizuki Y, Mount S, Morishita T, Miura S, Nakayama A, Nishizaka S, Nomoto H, Ohta F, Oishi K, Rigoutsos I, Sano M, Sasaki A, Sasakura Y, Shoguchi E, Shin-i T, Spagnuolo A, Stainier D, Suzuki MM, Tassy O, Takatori N, Tokuoka M, Yagi K, Yoshizaki F, Wada S, Zhang C, Hyatt PD, Larimer F, Detter C, Doggett N, Glavina T, Hawkins T, Richardson P, Lucas S, Kohara Y, Levine M, Satoh N, Rokhsar DS. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- Cañestro C, Godoy L, Gonzàlez-Duarte R, Albalat R. Comparative expression analysis of Adh3 during arthropod, urochordate, cephalochordate and vertebrate development challenges its predicted housekeeping role. Evol Dev. 2003;5:157–162. doi: 10.1046/j.1525-142X.2003.03022.x. [DOI] [PubMed] [Google Scholar]
- Cañestro C, Bassham S, Postlethwait J. Development of the central nervous system in the larvacean Oikopleura dioica and the evolution of the chordate brain. Dev Biol. 2005;285:298–315. doi: 10.1016/j.ydbio.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Bassham S, Postlethwait JH. The evolutionary history of placodes: a molecular genetic investigation of the larvacean urochordate Oikopleura dioica. Development. 2005;132:4259–4272. doi: 10.1242/dev.01973. [DOI] [PubMed] [Google Scholar]
- Edvardsen RB, Seo HC, Jensen MF, Mialon A, Mikhaleva J, Bjordal M, Cartry J, Reinhardt R, Weissenbach J, Wincker P, Chourrout D. Remodelling of the homeobox gene complement in the tunicate Oikopleura dioica. Curr Biol. 2005;15:R12–13. doi: 10.1016/j.cub.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Cañestro C, Bassham S, Postlethwait JH. Seeing chordate evolution through the Ciona genome sequence. Genome Biol. 2003;4:208–211. doi: 10.1186/gb-2003-4-3-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Gruss P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development. 1996;122:3381–3391. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- Heller N, Brandli AW. Xenopus Pax2/5/8 orthologues: novel insights into Pax gene evolution and identification of Pax-8 as the earliest marker for otic and pronephric cell lineages. Dev Genet. 1999;24:208–219. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<208::AID-DVG4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Krauss S, Johansen T, Korzh V, Fjose A. Expression of the zebrafish paired box gene pax [zf-b] during early neurogenesis. Development. 1991;113:1193–1206. doi: 10.1242/dev.113.4.1193. [DOI] [PubMed] [Google Scholar]
- Pfeffer PL, Gerster T, Lun K, Brand M, Busslinger M. Characterization of three novel members of the zebrafish Pax2/5/8 family: Dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development. 1998;125:3063–3074. doi: 10.1242/dev.125.16.3063. [DOI] [PubMed] [Google Scholar]
- Kavaler J, Fu W, Duan H, Noll M, Posakony JW. An essential role for the Drosophila Pax2 homolog in the differentiation of adult sensory organs. Development. 1999;126:2261–2272. doi: 10.1242/dev.126.10.2261. [DOI] [PubMed] [Google Scholar]
- Fu W, Noll M. The Pax2 homolog sparkling is required for development of cone and pigment cells in the Drosophila eye. Genes Dev. 1997;11:2066–2078. doi: 10.1101/gad.11.16.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Saiga H, Satoh N, Holland PWH. Tripartite organization of the ancestral chordate brain and the antiquity of placodes: insights from ascidian Pax2/5/8, Hox and Otx genes. Development. 1998;125:1113–1122. doi: 10.1242/dev.125.6.1113. [DOI] [PubMed] [Google Scholar]
- O'Brien EK, Degnan BM. Expression of Pax258 in the gastropod statocyst: insights into the antiquity of metazoan geosensory organs. Evol Dev. 2003;5:572–578. doi: 10.1046/j.1525-142X.2003.03062.x. [DOI] [PubMed] [Google Scholar]
- Groger H, Callaerts P, Gehring WJ, Schmid V. Characterization and expression analysis of an ancestor-type Pax gene in the hydrozoan jellyfish Podocoryne carnea. Mech Dev. 2000;94:157–169. doi: 10.1016/S0925-4773(00)00286-0. [DOI] [PubMed] [Google Scholar]
- Kozmik Z, Daube M, Frei E, Norman B, Kos L, Dishaw LJ, Noll M, Piatigorsky J. Role of Pax genes in eye evolution: a cnidarian PaxB gene uniting Pax2 and Pax6 functions. Dev Cell. 2003;5:773–785. doi: 10.1016/S1534-5807(03)00325-3. [DOI] [PubMed] [Google Scholar]
- Kozmik Z, Holland ND, Kalousova A, Paces J, Schubert N, Holland L. Characterization of an amphioxus paired box gene, AmphiPax2/5/8 : developmental expression patterns in optic support cells, nephridium, thyroid-like structures and pharyngeal gill slits, but not in the midbrain boundary region. Development. 1999;126:1295–1304. doi: 10.1242/dev.126.6.1295. [DOI] [PubMed] [Google Scholar]
- Mazet F, Hutt JA, Millard J, Shimeld SM. Pax gene expression in the developing central nervous system of Ciona intestinalis. Gene Expr Patterns. 2003;3:743–745. doi: 10.1016/S1567-133X(03)00137-6. [DOI] [PubMed] [Google Scholar]
- Wada S, Tokuoka M, Shoguchi E, Kobayashi K, Di Gregorio A, Spagnuolo A, Branno M, Kohara Y, Rokhsar D, Levine M, Saiga H, Satoh N, Satou Y. A genomewide survey of developmentally relevant genes in Ciona intestinalis. II. Genes for homeobox transcription factors. Dev Genes Evol. 2003;213:222–234. doi: 10.1007/s00427-003-0321-0. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Urbanek P, Rolink A, Busslinger M. Essential functions of Pax5 (BSAP) in pro-B cell development: difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus. Genes Dev. 1997;11:476–491. doi: 10.1101/gad.11.4.476. [DOI] [PubMed] [Google Scholar]
- Wendl T, Lun K, Mione M, Favor J, Brand M, Wilson SW, Rohr KB. Pax2.1 is required for the development of thyroid follicles in zebrafish. Development. 2002;129:3751–3760. doi: 10.1242/dev.129.15.3751. [DOI] [PubMed] [Google Scholar]
- Burri M, Tromvoukis Y, Bopp D, Frigerio G, Noll M. Conservation of the paired domain in metazoans and its structure in three isolated human genes. Embo J. 1989;8:1183–1190. doi: 10.1002/j.1460-2075.1989.tb03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner MS, Dressler GR. Mapping of Pax-2 transcription activation domains. J Biol Chem. 1996;271:21088–21093. doi: 10.1074/jbc.271.35.21088. [DOI] [PubMed] [Google Scholar]
- Eberhard D, Jimenez G, Heavey B, Busslinger M. Transcriptional repression by Pax5 (BSAP) through interaction with corepressors of the Groucho family. Embo J. 2000;19:2292–2303. doi: 10.1093/emboj/19.10.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai KS, Satoh N, Satou Y. Region specific gene expressions in the central nervous system of the ascidian embryo. Mech Dev. 2002;119:S275–277. doi: 10.1016/S0925-4773(03)00128-X. [DOI] [PubMed] [Google Scholar]
- Thompson EM, Kallesøe T, Spada F. Diverse genes expressed in distinct regions of the trunk epithelium define a monolayer cellular template for construction of the oikopleurid house. Dev Biol. 2001;238:260–273. doi: 10.1006/dbio.2001.0414. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Venkatesh TV. Heart development in Drosophila and vertebrates: conservation of molecular mechanisms. Dev Genet. 1998;22:181–186. doi: 10.1002/(SICI)1520-6408(1998)22:3<181::AID-DVG1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Harvey RP. NK-2 homeobox genes and heart development. Dev Biol. 1996;178:203–216. doi: 10.1006/dbio.1996.0212. [DOI] [PubMed] [Google Scholar]
- Davidson B, Levine M. Evolutionary origins of the vertebrate heart: Specification of the cardiac lineage in Ciona intestinalis. Proc Natl Acad Sci USA. 2003;100:11469–11473. doi: 10.1073/pnas.1634991100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B, Shi W, Levine M. Uncoupling heart cell specification and migration in the simple chordate Ciona intestinalis. Development. 2005;132:4811–4818. doi: 10.1242/dev.02051. [DOI] [PubMed] [Google Scholar]
- Beh J, Shi W, Levine M, Davidson B, Christiaen L. FoxF is essential for FGF-induced migration of heart progenitor cells in the ascidian Ciona intestinalis. Development. 2007;134:3297–3305. doi: 10.1242/dev.010140. [DOI] [PubMed] [Google Scholar]
- Satou Y, Imai KS, Satoh N. The ascidian Mesp gene specifies heart precursor cells. Development. 2004;131:2533–2541. doi: 10.1242/dev.01145. [DOI] [PubMed] [Google Scholar]
- Imai KS, Hino K, Yagi K, Satoh N, Satou Y. Gene expression profiles of transcription factors and signaling molecules in the ascidian embryo: towards a comprehensive understanding of gene networks. Development. 2004;131:4047–4058. doi: 10.1242/dev.01270. [DOI] [PubMed] [Google Scholar]
- Holland ND, Venkatesh TV, Holland LZ, Jacobs DK, Bodmer R. AmphiNk2-tin, an amphioxus homeobox gene expressed in myocardial progenitors: insights into evolution of the vertebrate heart. Dev Biol. 2003;255:128–137. doi: 10.1016/S0012-1606(02)00050-7. [DOI] [PubMed] [Google Scholar]
- Davidson B. Ciona intestinalis as a model for cardiac development. Semin Cell Dev Biol. 2007;18:16–26. doi: 10.1016/j.semcdb.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann H. Erste Klasse der Tunicaten: Appendiculariae. In: Kükenthal W, Krumbach T, editor. Handbuch der Zoologie. Vol. 5. Berlin and Leipzig: Walter De Gruyter and Co; 1933. [Google Scholar]
- Bone Q. The Biology of Pelagic Tunicates. Oxford: Oxford University Press; 1998. [Google Scholar]
- Mazet F, Hutt JA, Milloz J, Millard J, Graham A, Shimeld SM. Molecular evidence from Ciona intestinalis for the evolutionary origin of vertebrate sensory placodes. Dev Biol. 2005;282:494–508. doi: 10.1016/j.ydbio.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Hinman VF, Degnan BM. Retinoic acid perturbs Otx gene expression in the ascidian pharynx. Dev Genes Evol. 2000;210:129–139. doi: 10.1007/s004270050019. [DOI] [PubMed] [Google Scholar]
- Rahr H. The ultrastructure of the blood vessels of Branchiostoa lanceolatum (Pallas) (Cephalochordata) Zoomorphology. 1981;97:53–74. doi: 10.1007/BF00310102. [DOI] [Google Scholar]
- Ruppert E. Microscopic Anatomy of Invertebrates. Vol. 15. New York: Wiley Liss; 1997. [Google Scholar]
- Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990;109:787–795. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- Fickenscher HR, Chalepakis G, Gruss P. Murine Pax-2 protein is a sequence-specific trans-activator with expression in the genital system. DNA Cell Biol. 1993;12:381–391. doi: 10.1089/dna.1993.12.381. [DOI] [PubMed] [Google Scholar]
- Okladnova O, Poleev A, Fantes J, Lee M, Plachov D, Horst J. The genomic organization of the murine Pax 8 gene and characterization of its basal promoter. Genomics. 1997;42:452–461. doi: 10.1006/geno.1997.4735. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16:2958–2970. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heanue TA, Reshef R, Davis RJ, Mardon G, Oliver G, Tomarev S, Lassar AB, Tabin CJ. Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophila eye formation. Genes Dev. 1999;13:3231–3243. doi: 10.1101/gad.13.24.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek P, Wang ZQ, Fetka I, Wagner EF, Busslinger M. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell. 1994;79:901–912. doi: 10.1016/0092-8674(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Muller W. Uber die hypobranchialrinne der tunicaten und deren vorhandensein bei amphioxus und den cyklostomen. Jena Z Med Naturw. 1873;7:327–332. [Google Scholar]
- Hiruta J, Mazet F, Yasui K, Zhang P, Ogasawara M. Comparative expression analysis of transcription factor genes in the endostyle of invertebrate chordates. Dev Dyn. 2005;233:1031–1037. doi: 10.1002/dvdy.20401. [DOI] [PubMed] [Google Scholar]
- Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL, Gruss P. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development. 1990;110:643–651. doi: 10.1242/dev.110.2.643. [DOI] [PubMed] [Google Scholar]
- Zannini M, Francis-Lang H, Plachov D, Di Lauro R. Pax-8, a paired domain-containing protein, binds to a sequence overlapping the recognition site of a homeodomain and activates transcription from two thyroid-specific promoters. Mol Cell Biol. 1992;12:4230–4241. doi: 10.1128/mcb.12.9.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson G, Ofverholm T, Ericson LE. Ultrastructural demonstration of iodine binding and peroxidase activity in the endostyle of Oikopleura dioica (Appendicularia) Gen Comp Endocrinol. 1985;58:319–327. doi: 10.1016/0016-6480(85)90348-X. [DOI] [PubMed] [Google Scholar]
- Cañestro C, Bassham S, Postlethwait JH. Evolution of the thyroid: Anterior-posterior regionalization of the Oikopleura endostyle revealed by Otx, Pax2/5/8, and Hox1 expression. Dev Dyn. 2008;237:1490–1499. doi: 10.1002/dvdy.21525. [DOI] [PubMed] [Google Scholar]
- Olsson R, Holmberg K, Lilliemark Y. Fine structure of the brain and brain nerves of Oikopleura dioica (Urochordata, Appendicularia) Zool Morphol. 1990;110:1–7. [Google Scholar]
- Burighel P, Lane NJ, Zaniolo G, Manni L. Neurogenic role of the neural gland in the development of the ascidian, Botryllus schlosseri (Tunicata, Urochordata) J Comp Neurol. 1998;394:230–241. doi: 10.1002/(SICI)1096-9861(19980504)394:2<230::AID-CNE7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Burighel P, Lane NJ, Fabio G, Stefano T, Zaniolo G, Carnevali MD, Manni L. Novel, secondary sensory cell organ in ascidians: in search of the ancestor of the vertebrate lateral line. J Comp Neurol. 2003;461:236–249. doi: 10.1002/cne.10666. [DOI] [PubMed] [Google Scholar]
- Manni L, Agnoletto A, Zaniolo G, Burighel P. Stomodeal and neurohypophysial placodes in Ciona intestinalis : insights into the origin of the pituitary gland. J Exp Zoolog B Mol Dev Evol. 2005;304:324–339. doi: 10.1002/jez.b.21039. [DOI] [PubMed] [Google Scholar]
- Mazet F. The evolution of sensory placodes. Scientific World Journal. 2006;6:1841–1850. doi: 10.1100/tsw.2006.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazet F, Shimeld SM. Molecular evidence from ascidians for the evolutionary origin of vertebrate cranial sensory placodes. J Exp Zoolog B Mol Dev Evol. 2005;304:340–346. doi: 10.1002/jez.b.21054. [DOI] [PubMed] [Google Scholar]
- Bone Q, Ryan KP. Cupular sense organs in Ciona (Tunicata: Ascidiacea) J Zool (Lond) 1978;186:417–429. [Google Scholar]
- Heller N, Brandli AW. Xenopus Pax-2/5/8 orthologues: novel insights into Pax gene evolution and identification of Pax-8 as the earliest marker for otic and pronephric cell lineages. Dev Genet. 1999;24:208–219. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<208::AID-DVG4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Mittag J, Winterhager E, Bauer K, Grummer R. Congenital hypothyroid female pax8-deficient mice are infertile despite thyroid hormone replacement therapy. Endocrinology. 2007;148:719–725. doi: 10.1210/en.2006-1054. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Jones FS. Developmental control of N-CAM expression by Hox and Pax gene products. Philos Trans R Soc Lond B Biol Sci. 1995;349:305–312. doi: 10.1098/rstb.1995.0118. [DOI] [PubMed] [Google Scholar]
- Kozmik Z, Holland ND, Kreslova J, Oliveri D, Schubert M, Jonasova K, Holland LZ, Pestarino M, Benes V, Candiani S. Pax-Six-Eya-Dach network during amphioxus development: Conservation in vitro but context specificity in vivo. Dev Biol. 2007;306:143–159. doi: 10.1016/j.ydbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Manni L, Caicci F, Gasparini F, Zaniolo G, Burighel P. Hair cells in ascidians and the evolution of lateral line placodes. Evol Dev. 2004;6:379–381. doi: 10.1111/j.1525-142X.2004.04046.x. [DOI] [PubMed] [Google Scholar]
- Manni L, Lane NJ, Burighel P, Zaniolo G. Are neural crest and placodes exclusive to vertebrates? Evol Dev. 2001;3:297–298. doi: 10.1046/j.1525-142X.2001.01040.x. [DOI] [PubMed] [Google Scholar]
- Manni L, Lane NJ, Joly JS, Gasparini F, Tiozzo S, Caicci F, Zaniolo G, Burighel P. Neurogenic and non-neurogenic placodes in ascidians. J Exp Zoolog B Mol Dev Evol. 2004;302:483–504. doi: 10.1002/jez.b.21013. [DOI] [PubMed] [Google Scholar]
- Kourakis MJ, Smith WC. A conserved role for FGF signaling in chordate otic/atrial placode formation. Dev Biol. 2007;312:245–257. doi: 10.1016/j.ydbio.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies R. Ceratocysties perneri A Middle Cambrian chordate with echionderm affinities. Paleontology. 1969;12:494–535. [Google Scholar]
- Jefferies R. The ancestry of the vertebrates. London: British Museum of Natural History; 1986. [Google Scholar]
- Kawakami K, Sato S, Ozaki H, Ikeda K. Six family genes – structure and function as transcription factors and their roles in development. BioEssays. 2000;22:616–626. doi: 10.1002/1521-1878(200007)22:7<616::AID-BIES4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Aghaallaei N, Bajoghli B, Czerny T. Distinct roles of Fgf8, Foxi1, Dlx3b and Pax8/2 during otic vesicle induction and maintenance in medaka. Dev Biol. 2007;307:408–420. doi: 10.1016/j.ydbio.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Kwak SJ, Vemaraju S, Moorman SJ, Zeddies D, Popper AN, Riley BB. Zebrafish pax5 regulates development of the utricular macula and vestibular function. Dev Dyn. 2006;235:3026–3038. doi: 10.1002/dvdy.20961. [DOI] [PubMed] [Google Scholar]
- Fu W, Duan H, Frei E, Noll M. shaven and sparkling are mutations in separate enhancers of the Drosophila Pax2 homolog. Development. 1998;125:2943–2950. doi: 10.1242/dev.125.15.2943. [DOI] [PubMed] [Google Scholar]
- Asano M, Gruss P. Pax-5 is expressed at the midbrain-hindbrain boundary during mouse development. Mech Dev. 1992;39:29–39. doi: 10.1016/0925-4773(92)90023-D. [DOI] [PubMed] [Google Scholar]
- Joyner AL. Engrailed, Wnt and Pax genes regulate midbrain-hindbrain development. Trends Genet. 1996;12:15–20. doi: 10.1016/0168-9525(96)81383-7. [DOI] [PubMed] [Google Scholar]
- Rowitch DH, McMahon AP. Pax-2 expression in the murine neural plate precedes and encompasses the expression domains of Wnt-1 and En-1. Mech Dev. 1995;52:3–8. doi: 10.1016/0925-4773(95)00380-J. [DOI] [PubMed] [Google Scholar]
- Ye W, Bouchard M, Stone D, Liu X, Vella F, Lee J, Nakamura H, Ang SL, Busslinger M, Rosenthal A. Distinct regulators control the expression of the mid-hindbrain organizer signal FGF8. Nat Neurosci. 2001;4:1175–1181. doi: 10.1038/nn761. [DOI] [PubMed] [Google Scholar]
- Cheng L, Arata A, Mizuguchi R, Qian Y, Karunaratne A, Gray PA, Arata S, Shirasawa S, Bouchard M, Luo P, Chen CL, Busslinger M, Goulding M, Onimaru H, Ma Q. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat Neurosci. 2004;7:510–517. doi: 10.1038/nn1221. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Scholes J, Strahle U, Brennan C, Holder N, Brand M, Wilson SW. The Pax protein Noi is required for commissural axon pathway formation in the rostral forebrain. Development. 1997;124:2397–2408. doi: 10.1242/dev.124.12.2397. [DOI] [PubMed] [Google Scholar]
- Bassham S, Postlethwait J. Brachyury (T) expression in embryos of a larvacean urochordate, Oikopleura dioica, and the ancestral role of brachyury. Dev Biol. 2000;220:322–333. doi: 10.1006/dbio.2000.9647. [DOI] [PubMed] [Google Scholar]
- Cañestro C, Postlethwait JH. Development of a chordate anterior-posterior axis without classical retinoic acid signaling. Dev Biol. 2007;305:522–538. doi: 10.1016/j.ydbio.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Heller N, Brandli AW. Xenopus Pax-2 displays multiple splice forms during embryogenesis and pronephric kidney development. Mech Dev. 1997;69:83–104. doi: 10.1016/S0925-4773(97)00158-5. [DOI] [PubMed] [Google Scholar]
- Nornes HO, Dressler GR, Knapik EW, Deutsch U, Gruss P. Spatially and temporally restricted expression of Pax2 during murine neurogenesis. Development. 1990;109:797–809. doi: 10.1242/dev.109.4.797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic relationships among chordate Pax2/5/8 proteins. The data provided illustrates the independent gene family expansions that permitted parallel histories of subfunction partitioning among vertebrate paralogs (Pax2, Pax5 and Pax8) and among urochordate paralogs (Pax2/5/8a and Pax2/5/8b).