Abstract
Prey in natural communities are usually shared by many predator species. How predators coexist while competing for the same prey is one of the fundamental questions in ecology. Here, we show that competing predator species may not only coexist on a single prey but even help each other to persist if they specialize on different life history stages of the prey. By changing the prey size distribution, a predator species may in fact increase the amount of prey available for its competitor. Surprisingly, a predator may not be able to persist at all unless its competitor is also present. The competitor thus significantly increases the range of conditions for which a particular predator can persist. This “emergent facilitation” is a long-term, population-level effect that results from asymmetric increases in the rate of prey maturation and reproduction when predation relaxes competition among prey. Emergent facilitation explains observations of correlated increases of predators on small and large conspecific prey as well as concordance in their distribution patterns. Our results suggest that emergent facilitation may promote the occurrence of complex, stable, community food webs and that persistence of these communities could critically depend on diversity within predator guilds.
Keywords: emergent facilitation, food-dependent prey development, predator coexistence, prey stage, stage-specific predation
According to the competitive exclusion principle (1), two consumers sharing the same resource cannot coexist in the absence of resource variability in time (2) or space (3) or additional limiting factors, such as intraspecific density dependence or predation (4). The contrast between this principle and the diversity of natural communities inspired Hutchinson to pose the riddle: “Why are there so many kinds of animals?” (5). In natural communities, the average number of predator species per prey species is usually larger than one (6) as many prey species are preyed on by more than a single predator species during their lifetime (7). Depending on their feeding strategies and physical limitations, predators tend to specialize on different developmental stages of the same prey (8, 9). Such specialization can be viewed as a form of niche partitioning, which in theory could allow for the stable coexistence of consumers (10). However, different prey life-history stages are linked through growth and reproduction. Even if a predator exploits one stage only, it may nonetheless decrease the abundance of other stages and thus outcompete predator species specializing on these stages. Indeed, in an age-structured model, two predators specializing on different prey stages have been shown to coexist but the range of conditions that allows for persistence of a particular predator species is always reduced by the presence of its competitor (11). Furthermore, the coexistence of an egg and a larval parasitoid species on the same insect host has been shown to be impossible unless the larval parasitoid can take over hosts already parasitized by egg parasitoids (12), in which case both parasitoids are engaged in an intraguild predation interaction.
Here, we show that a specialist predator of juvenile prey and a specialist predator of adult prey cannot only coexist but can even promote each other's persistence when ontogenetic development of prey is food- and thus density-dependent. Despite the fact that they only interact through exploitative competition for the same prey species, the predator that forages on the prey stage suffering most from intraspecific competition enables its competitor to persist even under conditions that would otherwise lead to the extinction of the latter. Consequently, extinction of the facilitating predator immediately results in the disappearance of its competitor as well, emphasizing the importance of diversity within predator guilds for community persistence. This “emergent facilitation” is a long-term, population-level effect that results from the change in prey-stage distribution brought about by the size-selective foraging of the facilitating predator.
Results
We extended a stage-structured biomass model for consumer-resource interactions (13) so as to include two specialist predators that prey exclusively on juvenile and adult consumers, respectively (see Materials and Methods and Tables 2–4). Consumer biomass dynamics is modeled by an extended version of the Yodzis and Innes approach (14) that accounts for consumer size structure (13). In equilibrium, this model is completely identical to a fully size-structured model and consistently translates individual-level assumptions on size-dependent ingestion, growth, reproduction, and maintenance to the population level (15). For predators, we do not distinguish life-history stages and hence assume that their biomass dynamics is governed by bio-energetic laws (14) in addition to losses through background mortality. Model parameterization is based on published scaling laws of ingestion, maintenance, and mortality with body mass (14, 16, 17).
Table 2.
Model equations
| Dynamic equations | Description |
|---|---|
| dR/dt = δ(Rmax − R) − Ω(R, J, A) | Resource biomass dynamics |
| dJ/dt = νA(IA)A + νJ(IJ)J − γ(νJ(IJ), μJ)J − μJ(J, PJ)J | Biomass dynamics of juvenile consumers |
| dA/dt = γ(νJ(IJ), μJ)J − μA(A, PA)A | Biomass dynamics of adult consumers |
| dPJ/dt = (νPJ(J ) − dPJ)PJ | Biomass dynamics of predators on juveniles |
| dPA/dt = (νPA(A) − dPA)PA | Biomass dynamics of predators on adults |
Table 3.
Model functions
| Functions | Expression |
Description | ||
|---|---|---|---|---|
| Model I | Model II | Model III | ||
| IJ(R) | MCR/(R + 1) | MC | MCR/(R + 1) | Resource intake rate by juvenile consumers |
| IA(R) | MC | MCR/(R + 1) | qMCR/(R + 1) | Resource intake rate by adult consumers |
| Ω(R, J, A) | IJ(R)J | IA(R)A | IJ(R)J + IA(R)A | Total resource foraging rate by all consumers |
| νJ(IJ) | σIJ(R) − TC | Net-energy production of juvenile consumers | ||
| νA(IA) | σIA(R) − TC | Net-energy production of adult consumers | ||
| γ(νJ, μJ) | (νJ(IJ) − μJ)/(1 − z(1−μJ/νJ(IJ))) | Maturation rate of juvenile consumers | ||
| μJ(J, PJ) | dC + MPJPJ/(J + 1) | Mortality rate of juvenile consumers | ||
| μA(A, PA) | dC + MPAPA/(A + 1) | Mortality rate of adult consumers | ||
| νPJ(J) | σMPJJ/(J + 1) − TPJ | Net-energy production of predators on juveniles | ||
| νPA(A) | σMPAA/(A + 1) − TPA | Net-energy production of predators on adults | ||
All functions, except total resource foraging rate by all consumers, represent mass-specific rates.
Table 4.
Model parameters
| Parameter | Value |
Description | ||
|---|---|---|---|---|
| Consumer | Predator on juveniles | Predator on adults | ||
| M | 0.1 | 0.05 | 0.03 | Mass-specific maximum ingestion rate |
| T | 0.01 | 0.005 | 0.003 | Mass-specific maintenance rate |
| d | 0.001 | 0.0005 | 0.0003 | Mass-specific background mortality rate |
| σ | 0.3 | Conversion efficiency | ||
| q | 0.65 or 1.5 | Juvenile-adult consumer ingestion ratio | ||
| z | 0.15 | Newborn-adult consumer size ratio | ||
| δ | 0.1 | Resource turn-over rate | ||
| Rmax | 2.0 | Resource maximum biomass density | ||
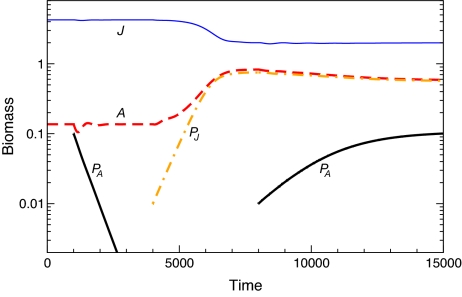
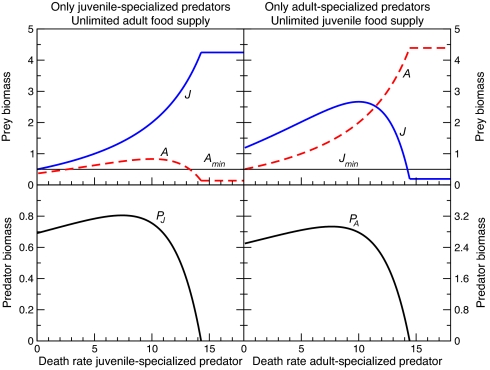
Three variants of the model are considered that differ in the extent to which juvenile and adult consumers or prey are limited by competition for resources and the mechanism giving rise to this asymmetry either through niche segregation or through interstage differences in foraging capacity. When adults have access to unlimited food and only juveniles compete for limiting resources (model I), the population is regulated through scramble competition among juveniles. Juvenile maturation is strongly food-limited and therefore slow. Juveniles then dominate the consumer population in the absence of predators (Fig. 1, t = 0; Fig. 2, Left Image, high mortality values) whereas adult densities are too low to allow invasion by adult-specialized predators (Fig. 1, t = 1,000). The high juvenile density, however, permits invasion of juvenile-specialized predators (Fig. 1, t = 4,000). These predators decrease juvenile biomass and release the scramble competition among them. This competitive release leads to an increase in the mass-specific maturation rate, which more than compensates for the decrease in juvenile biomass density such that total recruitment to the adult stage goes up (13). After establishment of juvenile-specialized predators, adult biomass increases sufficiently to allow subsequent invasion of adult-specialized predators (Fig. 1, t = 8,000). If juvenile-specialized predators were driven to extinction, however, the adult-specialized predator would die out in its wake. For persistence, adult-specialized predators thus depend crucially on the positive effect of juvenile-specialized predators on adult consumer biomass. This “emergent facilitation” is not related to any direct interaction between the two predators or any change in prey behavior but results from the change in competition among juvenile consumers and the ensuing increase in juvenile maturation rate. The juvenile-specialized predator induces the largest increase in adult biomass when it experiences intermediate levels of mortality (Fig. 2). When close to extinction itself, its impact on the consumer population is minimal and, hence, adult consumer biomass does not increase above the minimum density required to cover maintenance of adult-specialized predators. For low mortality values, the juvenile-specialized predator can continue to survive and hence impose such low densities of juvenile-consumer biomass that adult biomass is also low.
Fig. 1.
Invasion of an adult-specialized predator is only successful after establishment of a juvenile-specialized predator. Juvenile consumers compete for limiting resource (J; thin, solid trace), whereas adults have unlimited food supply (A; thick, dashed trace) (model I). Invasion of adult-specialized predators (PA; thick, solid trace; initial biomass 0.1 g, death rate dPA = 1) into consumer-resource equilibrium at t = 1,000 is unsuccessful. Invasion of juvenile-specialized predators (PJ; thick, dashed-dotted trace; initial biomass 0.01 g, death rate dPJ = 10) at t = 4,000 is successful and changes the consumer stage-distribution such that adult consumer biomass significantly increases. This allows for a subsequent, successful invasion and persistence of adult-specialized predators (initial biomass 0.01 g at t = 8,000).
Fig. 2.
Biomass levels in single predator-consumer-resource communities at equilibrium as a function of predator death rate. (Lower Left) Communities with juvenile-specialized predators (PJ, thick solid trace), (Upper) adult consumers having unlimited food supply (A, thick dashed trace), and (Upper) juveniles competing for limiting resource (J, thick solid trace) (model I). (Upper Left) Horizontal thin trace indicates the adult consumer density (Amin) that is needed to cover maintenance requirements of the adult-specialized predator. (Lower Right) Communities with adult-specialized predators (PA, thick solid trace), juvenile consumers having unlimited food supply, and adults competing for limiting resource (model II). (Upper) Horizontal thin trace indicates the juvenile consumer density (Jmin) needed to cover maintenance requirements of the juvenile-specialized predator. Note that all predator death rates are expressed as multiples of their body size-dependent background mortality (see Materials and Methods).
An analogous facilitation of the juvenile-specialized predator by the adult-specialized predator occurs when juveniles have access to unlimited food and adults compete for limiting resources (model II). In this case, the population is regulated through scramble competition for resources among adults. Whereas juveniles mature rapidly, adults have a low, strongly food-limited fecundity and dominate the population in the absence of predators (Fig. 2, Right Image, high mortality values). Low juvenile densities prevent invasion by juvenile-specialized predators unless adult-specialized predators invade first. The latter reduces adult biomass density and releases the scramble competition among them such that adult fecundity increases. This increase more than offsets the decrease in adult biomass and leads to higher total population reproduction (13). The ensuing increase in juvenile biomass allows juvenile-specialized predators to persist where they could not on their own. The increase in juvenile biomass induced by the adult-specialized predator is largest for intermediate values of its mortality (Fig. 2). However, almost always whenever adult-specialized predators can persist, their invasion results in juvenile biomass densities that are sufficient for the establishment of the juvenile-specialized predator as well.
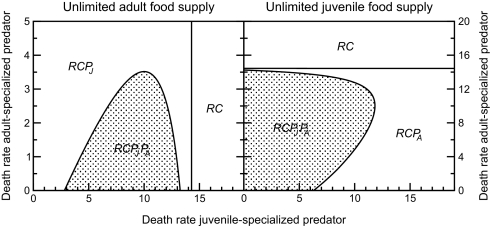
Predators specializing on prey developmental stages that experience little competition may hence be able to persist only in the presence of another predator species that attacks and reduces the food limitation in the prey life stage, which suffers most from scramble competition. Coexistence of two predator species is then possible for considerable ranges of their background mortalities for which one of them could not survive on its own (Fig. 3). When adult consumers have unlimited food (model I), adult-specialized predators cannot persist on their own even if they do not experience any background mortality at all. However, they do persist in the face of more than three times their background mortality when the juvenile-specialized predator is present and relaxes the intrastage competition among juvenile consumers (Fig. 3, Left Image). Facilitation is even stronger when juvenile consumers have unlimited food (model II) and competition is most intense among adults. The juvenile-specialized predator can in this case persist in the face of >10 times its background mortality as long as the adult-specialized predator is present and relaxes the competition among adult consumers (Fig. 3, Right Image). Without it, the juvenile-specialized predator cannot sustain at zero mortality levels as it encounters too-little food to cover its maintenance requirements.
Fig. 3.
Equilibrium community composition depending on death rates of juvenile- and adult-specialized predators when juvenile and adult consumers occupy different feeding niches. (Left) Adult consumers have unlimited food supply, whereas juveniles compete for limiting resource (model I). (Right) Juvenile consumers have unlimited food supply, whereas adults compete for limiting resource (model II). RC, consumer-resource equilibrium; RCPJ, juvenile specialized predator-consumer-resource equilibrium; RCPA, adult specialized predator-consumer-resource equilibrium; RCPJPA, all species equilibrium.
The model with two consumer stages is the simplest community model exhibiting emergent facilitation. It represents an extreme case in which the two consumer stages exploit separate resource niches with very dissimilar profitabilities such that the intensity of within-stage competition they experience is very different. Emergent facilitation, however, also occurs when the asymmetry in competition experienced by juveniles and adults results from other mechanisms, for example, interstage differences in foraging capacity. When both juveniles and adults compete for the same limiting resource but adults are better at exploiting it (model III, q = 1.5), juveniles suffer more than adults from both the inter- and intrastage competition for food. Similar to the situation in which adults have their own unlimited food resources, in this case juveniles also dominate the population in the absence of predators, and juvenile-specialized predators facilitate persistence of adult-specialized predators (results not shown, but largely similar to those shown in Fig. 3). In contrast, if juveniles are superior resource competitors (model III, q = 0.65), adults suffer more from inter- and intrastage competition for food and dominate the population when predators are absent. In this case, juvenile-specialized predators cannot persist without the presence of a predator that forages on adults and releases competition among them, analogous to the situation in which juveniles exploit their own unlimited food resources.
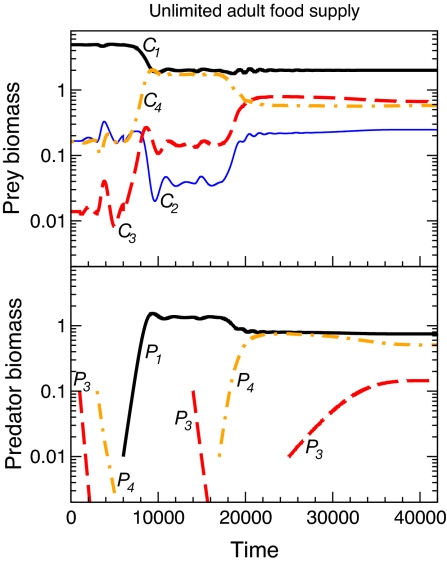
The change in prey-size distribution that gives rise to emergent facilitation also occurs in more general settings. Significant increases in the biomass density of large, adult prey because of size-selective predation on small, juvenile prey have been shown to occur as well in a model with a continuous prey-size distribution in which prey grow throughout their entire life (18). Furthermore, in a model with two juvenile and two adult prey stages (see supporting information (SI) Text and Figs. S1–S3), emergent facilitation also occurs if the juvenile- or adult-specialized predators feed only on one of the two juvenile or adult prey stages, respectively. This shows that the occurrence of emergent facilitation is not sensitive to the simplifying assumption that the two stage-specific predators specialize on nonreproductive and reproductive prey individuals. With these four prey stages, a higher-order type of emergent facilitation is also possible in which a stage-specific predator, that itself can persist only in the presence of a second stage-specific predator, facilitates the persistence of a third stage-specific predator (Fig. 4). Emergent facilitation even occurs if the niche segregation between stage-specific predators is incomplete. Body-size differences between predators will preclude them feeding equally on both prey stages even if they had access to them. Standard relations between prey preference and predator-prey body-size ratio (16) predict that juvenile- and adult-specialized predators might feed on adult and juvenile prey, respectively, at a rate that is ≈30% of their feeding rate on the prey stage in which they specialize. Analysis of a model with such niche overlap shows that in that case both predators can persist on their own at background mortality levels (data not shown). However, emergent facilitation still occurs to a similar extent but at higher levels of predator mortality. Finally, adding environmental stochasticity in resource productivity to our deterministic model does not qualitatively change model predictions either and quantitatively even tends to increase the range of parameter values over which emergent facilitation occurs (see SI Text and Figs. S4 and S5). We therefore conclude that emergent facilitation is a robust phenomenon, occurring in a wide range of settings.
Fig. 4.
Coexistence of 3 stage-specific predators on a 4-stage prey species. (Lower Graph) Specialist predators on large-adult consumers (P4; thick, dash-dotted trace) can only invade when specialist predators on small-juvenile consumers (P1; thick, solid trace) are present, while both predator species are required by specialist predators on small-adult consumers (P3; thick, dashed trace) to persist. (Upper Graph) Biomass density of small-juvenile (C1; thick, solid trace), large-juvenile (C2; thin, solid trace), small-adult (C3; thick, dashed trace) and large-adult consumers (C4; thick, dash-dotted trace). Parameter values: dP1 = 10, dP3 = 1, and dP4 = 2, expressed as multiples of their default, background mortality (see SI Text).
Discussion
In addition to coexistence of predators that compete for the same prey because of variability in time (2) or space (3) or the partitioning of available resources (10), emergent facilitation is a mechanism that not only allows for coexistence, but can promote it under conditions in which one of the competitors cannot persist on its own. In other words, the range of conditions that allows for persistence of a particular predator species increases when its competitor is present. In contrast to other coexistence mechanisms, its occurrence crucially depends on intraspecific size variability and the plasticity in prey-life history resulting from food- or density-dependent development and maturation. The direct, negative effect of predation is a decrease in total consumer biomass. However, as an indirect, positive effect, predators relax intraspecific competition among prey. If both ontogenetic development and reproduction depend on food availability, predation may lead to asymmetric increases in maturation and reproduction rate when prey competition in certain life stages is stronger than in others (13). In response, this asymmetry changes the prey-stage distribution to such an extent that, in particular size ranges, it offsets the negative effect of predation and, in fact, leads to an increase in stage-specific biomass. For predators specializing on that particular size range, this increase in food availability might be crucial for their persistence.
Emergent facilitation is related to other indirect community effects, such as trophic cascades and apparent competition, and superficially resembles the indirect mutualism occurring between two predators foraging on two competing prey species (19). However, emergent facilitation only involves a single prey population and occurs even in the absence of any competition between the prey stages. More importantly, neither of the two prey stages can grow in isolation. In fact, densities of juvenile and adult prey increase through reproduction and maturation, respectively. Density increases in one stage thus result from biomass production in the other. Emergent facilitation in essence operates within a single prey stage such that a reduction in stage biomass density translates into an increased biomass production and thus to increased recruitment to the next stage (13). These aspects make emergent facilitation fundamentally different from the previously reported types of indirect mutualism.
Food- or density-dependent growth has been suggested to characterize the life cycle of most animal species (20), but it is less clear under which conditions it results in the overcompensation in stage-specific biomass that gives rise to emergent facilitation. We reviewed the literature for three types of experimental evidence that can reveal the likely occurrence of emergent facilitation (Table 1). First, biomass increases in a particular population stage, in response to increased mortality imposed on another stage, have been shown to occur in laboratory populations of blowflies (21), soil mites (22), and fish (A. Schröder, L.P., and A.M.D.R., unpublished data). Second, increases in stage-specific prey density, because of variation in predation pressure, have been shown to occur in cladocerans in both laboratory (24) and field enclosures (25). Similarly, in whole-lake systems, the invasion of piscivorous fish with a preference for small prey fish has led to increases in density of large-sized prey (26, 27). Last, positive correlations between predator species with contrasting prey preferences have been found in artificial enclosures after the introduction of planktivorous fish (28, 29), in a range of lakes with different densities of invertebrate and fish predators foraging on small and large Daphnia individuals (30), respectively, and in the nested distribution pattern of large- and small-bodied predator communities of the same prey fish population (31).
Table 1.
Experimental and empirical data providing support for the occurrence of emergent facilitation or the mechanisms giving rise to it
| Prey species | System type | Description | Refs. |
|---|---|---|---|
| Mortality-induced increases in stage-specific prey density | |||
| Blowflies | Laboratory | In case of strong adult competition destroying 90% of emerging adults doubles density of eggs, larvae, and pupae | |
| Blowflies | Laboratory | In case of strong juvenile competition destroying 50% of young larvae doubles adult density | 21 |
| Soil mites | Laboratory | Harvesting eggs increases adult density if adults are superior competitors for food | 22 |
| Poecilliid fish | Laboratory | Size-selective harvesting of large individuals increases densities of small and large juveniles | A. Schröder, L.P., and A.M.D.R., unpublished data |
| Predator-induced increases in stage-specific prey density | |||
| Daphnia pulex | Laboratory | In populations regulated through food-dependent adult fecundity the proportion of small juveniles (<0.8 mm) increased from 27% in controls to 37% when coexisting with the positively size-selective predator Notonecta, while total population density did not change | 24 |
| Bosmina longirostris | Enclosures | Increasing fish predation from low to medium did not change total Bosmina density, but increased densities of small individuals, while decreasing densities of large individuals | 25 |
| Yellow perch | Whole lake | Establishment of walleye, a gape-limited predator on juveniles, in Canadarago Lake significantly increased density of yellow perch >200 mm | 26 |
| Artic charr | Whole lake | Thinning of prey population led to stable recovery of predator on small prey with concomitant increases in densities of both small- and large-sized prey | 27 |
| Positive associations between predators with contrasting prey size preferences (see also SI Text) | |||
| Microcrustaceans | Enclosures | Planktivorous fish significantly increased abundance of juvenile microcrustaceans and predatory macro-invertebrates that forage on them | 28, 29 |
| Daphnia | Field survey | Positive correlation between densities of invertebrate and fish predators on small and large Daphnia individuals, respectively, in a range of lakes with different predation risk densities | 30 |
| Brook stickleback | Field survey | Distribution of large, positively size-selective predators is nested within the distribution of small, negatively size-selective predators | 31 |
These studies suggest that emergent facilitation might be expected to occur in aquatic systems between vertebrate and invertebrate predators of zooplankton. Fish are visual hunters and typically select zooplankton of large body sizes (8). In contrast, gape limitations constrain invertebrate predators of zooplankton, such as larval midges, to eat only smaller sized individuals (7, 9). Furthermore, emergent facilitation may also occur between predators foraging on opposing ends of the same prey-fish size distribution such as has been found for piscivorous fish and marine mammals (32). Prey species like amphibians and many insects, which pass through a discrete habitat shift during ontogeny, will naturally be exposed to a different suite of predators in the various habitats. This opens up the possibility of emergent facilitation between predators even across ecosystem boundaries (33). In host-parasitoid systems, larval hosts may regularly overexploit their plant resource and suffer from intense-density dependence unless densities are suppressed by specialist parasitoids (34). Emergent facilitation could occur in systems with such strong, host-density dependence especially because most insect hosts are attacked by a range of different parasitoid species, each typically specializing on particular developmental stages of the host (35).
Facilitation has been deemed an important, but missing, part of ecological theory (36). Positive interactions between predator species, also referred to as emergent multiple-predator effects (37), have been shown to occur because of conflicting prey responses to multiple predators (37, 38). Experimental studies of these multipredator effects have been limited to single generations of either prey or predator and do not reveal their community effects. In contrast, emergent facilitation is a long-term, population-level positive effect, which involves only exploitative foraging by predators and occurs even in the absence of any prey behavioral response. Through emergent facilitation, diversity among predator species at higher trophic levels becomes crucial for community persistence. Our results reveal how the loss of a particular predator species may induce a cascade of secondary extinctions within its guild. Despite its positive effect on persistence and coexistence of different predators, in more complex food webs emergent facilitation may potentially also have negative effects on species coexistence. In particular, if the facilitated predator also forages on additional prey species, the latter is likely to suffer from the increased predator density induced by emergent facilitation. The overall, net effect of emergent facilitation in more complex communities thus remains unknown and a topic for future research.
Size-selective predators may not only facilitate other predator species but have been shown to also promote their own persistence. Predators foraging selectively either on small or on large prey individuals may exhibit an emergent Allee effect (18, 39). In this case, predators cannot invade a community from which they are absent, whereas an established predator population may under the same conditions nonetheless be able to persist. Emergent Allee effects arise in much the same way as emergent facilitation: Size-selective predation leads to asymmetric increases in prey maturation and reproduction because of food-dependent growth in prey body size and differences in competition in different prey life stages. In case of an emergent Allee effect, these changes increase the density of prey that the predator actually forages on and thus lead to a positive feedback of predators on their own persistence (18, 39). Together, emergent facilitation and emergent Allee effects exemplify how negative, top-down impacts of size-selective predation may indirectly turn into positive, bottom-up effects as a consequence of their interplay with the food- or density-dependent plasticity in prey life history. These indirect, positive effects make that predator species crucially depend on each other for their persistence. More generally, this interdependence suggests that the network of feeding interactions in a community is, in fact, an emergent property of the system, which to a large extent arises through self-organization. Part and parcel of this self-organized character of the food web is an inherent fragility whereby the loss of a facilitating predator species may lead to subsequent extinction of some of its guild members, making the community collapse like a house of cards.
Materials and Methods
Model Formulation.
All model equations, functions, and parameters are summarized in Tables 2–4. In the absence of consumers resource R follows semichemostat dynamics. Food intake by juvenile and adult consumers, J and A, and both stage-specific predators, PJ and PA, on juvenile and adult consumers, respectively, follows a type II functional response of their food biomass density. All species use gross biomass production to first cover maintenance requirements. Juvenile and adult consumers invest their net biomass production entirely in growth and reproduction, respectively. Juvenile biomass increases through reproduction and growth in body size. Maturation decreases juvenile and increases adult biomass. The maturation function depends on juvenile net biomass production, juvenile mortality, and on the ratio of consumer body size at birth and at maturation. This function is the unique feature of the model and therefore under equilibrium conditions the model is completely identical to a fully size-structured model (15). The model thus consistently translates individual-level assumptions on size-dependent ingestion, growth, reproduction, and maintenance to the population level. Juvenile and adult consumer biomass decreases through background and stage-specific predation mortality. Stage-specific predators only experience background mortality. Different model variants represent whether only juveniles (model I; see Table 3) or only adults (model II) forage on limited resources with the other stage having unlimited food, or both stages compete but with unequal ingestion capacity (model III).
Model Parameterization.
Time is expressed in days, whereas all biomass densities are expressed in gram per unit volume with the latter an arbitrary scaling factor of all densities. Values for mass-specific maintenance and background mortality rates follow standard quarter-power scaling laws of adult body size with proportionality constants, 0.01 (14, 16) and 0.001 (17), respectively. Maximum ingestion rates are assumed to be 10 times larger than maintenance rate (14). Without loss of generality, adult consumer body size is set to 1 g whereas body size of juvenile-specialized and adult-specialized predators equals 20 g and 100 g, respectively, reflecting a predator-prey body-size ratio of 2 orders of magnitude (16). Death rates of stage-specific predators are varied in the analysis and presented in all graphs as multiples of their background mortality. Ingested biomass is assimilated with constant efficiency (16). By doubling and halving parameter values, we verified that values different from the default do not qualitatively change model predictions but only have quantitative effects. Results shown in Figs. 2 and 3 were obtained by numerically solving the right-hand side of the model ordinary differential equations (ODE) for equilibrium solutions and assessing their stability by using numerical bifurcation software (23). Equilibria were stable for all parameter combinations considered.
Supplementary Material
Acknowledgments.
This research was supported by grants from the Swedish Research Council and the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning to L.P. T.S. was supported by the Lake Ecosystem Response to Environmental Change (LEREC) project.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803834105/DCSupplemental.
References
- 1.Gause GF. The Struggle for Existence. Baltimore: Williams and Wilkins; 1934. [Google Scholar]
- 2.Armstrong RA, McGehee R. Competitive exclusion. Am Nat. 1980;115:151–170. [Google Scholar]
- 3.Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000;31:343–366. [Google Scholar]
- 4.Levin SA. Community equilibria and stability, and an extension of the competitive exclusion principle. Am Nat. 1970;104:413–423. [Google Scholar]
- 5.Hutchinson GE. Homage to Santa Rosalia or why are there so many kinds of animals? Am Nat. 1959;93:145–159. [Google Scholar]
- 6.Cohen JE. Food Webs and Niche Space. Princeton: Princeton Univ Press; 1978. [PubMed] [Google Scholar]
- 7.Zaret TM. Predation and Freshwater Communities. New Haven, CT: Yale Univ Press; 1980. [Google Scholar]
- 8.Brooks JL, Dodson SI. Predation, body size, and competition of plankton. Science. 1965;150:28–35. doi: 10.1126/science.150.3692.28. [DOI] [PubMed] [Google Scholar]
- 9.Campbell CE. Prey selectivities of threespine sticklebacks (Gasterosteus aculeatus) and phantom midge larvae (Chaoborus spp) in Newfoundland lakes. Freshw Biol. 1991;25:155–167. [Google Scholar]
- 10.Schoener TW. Resource partitioning in ecological communities. Science. 1974;185:27–39. doi: 10.1126/science.185.4145.27. [DOI] [PubMed] [Google Scholar]
- 11.Murdoch WW, Briggs CJ, Nisbet RM. Consumer-Resource Dynamics. Princeton: Princeton Univ Press; 2003. [Google Scholar]
- 12.Briggs CJ. Competition among parasitoid species on a stage-structured host and its effect on host suppression. Am Nat. 1993;141:372–397. [Google Scholar]
- 13.De Roos AM, et al. Food-dependent growth leads to overcompensation in stage-specific biomass when mortality increases: The influence of maturation versus reproduction regulation. Am Nat. 2007;170:E59–E76. doi: 10.1086/520119. [DOI] [PubMed] [Google Scholar]
- 14.Yodzis P, Innes S. Body size and consumer resource dynamics. Am Nat. 1992;139:1151–1175. [Google Scholar]
- 15.De Roos AM, et al. Simplifying a physiologically structured population model to a stage-structured biomass model. Theor Popul Biol. 2008;73:47–62. doi: 10.1016/j.tpb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Peters RH. The Ecological Implications of Body Size. Cambridge: Cambridge Univ Press; 1983. [Google Scholar]
- 17.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- 18.De Roos AM, Persson L. In: Dynamic Food Webs: Multispecies Assemblages, Ecosystem Development, and Environmental Change. De Ruiter PC, Wolters V, Moore JC, editors. San Diego: Academic; 2005. pp. 89–100. [Google Scholar]
- 19.Wootton JT. The nature and consequences of indirect effects in ecological communities. Annu Rev Ecol Syst. 1994;25:443–466. [Google Scholar]
- 20.Werner EE. In: Size-structured Populations: Ecology and Evolution. Ebenman B, Persson L, editors. Heidelberg: Springer-Verlag; 1988. pp. 60–81. [Google Scholar]
- 21.Nicholson AJ. The self-adjustment of populations to change. Cold Spring Harbor Symp Quant Biol. 1957;22:153–173. [Google Scholar]
- 22.Cameron TC, Benton TG. Stage-structured harvesting and its effects: An empirical investigation using soil mites. J Anim Ecol. 2004;73:996–1006. [Google Scholar]
- 23.Kuznetsov YA, Levitin VV, Skovoroda AR. Report AM-R9611. Amsterdam: Centre for Mathematics and Computer Science; 1996. Continuation of stationary solutions to evolution problems in Content. [Google Scholar]
- 24.Murdoch WW, Scott MA. Stability and extinction of laboratory populations of zooplankton preyed on by the backswimmer. Notonecta Ecology. 1984;65:1231–1248. [Google Scholar]
- 25.Vonderbrink RH, Vanni MJ. Demographic and life-history response of the cladoceran Bosmina longirostris to variation in predator abundance. Oecologia. 1993;95:70–80. doi: 10.1007/BF00649509. [DOI] [PubMed] [Google Scholar]
- 26.Olson MH, Green DM, Rudstam LG. Changes in yellow perch (Perca flavescens) growth associated with the establishment of a walleye (Stizostedion vitreum) population in Canadarago Lake, New York (USA) Ecol Freshw Fish. 2001;10:11–20. [Google Scholar]
- 27.Persson L, et al. Culling prey promotes predator recovery - Alternative states in a whole-lake experiment. Science. 2007;316:1743–1746. doi: 10.1126/science.1141412. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen DL, Hillman TJ, Smith FJ. Effects of hydrological variation and planktivorous competition on macroinvertebrate community structure in experimental billabongs. Freshw Biol. 1999;42:427–444. [Google Scholar]
- 29.Nielsen DL, Hillman TJ, Smith FJ, Shiel RJ. The influence of a planktivorous fish on zooplankton assemblages in experimental billabongs. Hydrobiologia. 2000;434:1–9. [Google Scholar]
- 30.Leibold M, Tessier AJ. Contrasting patterns of body size for Daphnia species that segregate by habitat. Oecologia. 1991;86:342–348. doi: 10.1007/BF00317599. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerman MS. Predator communities associated with brook stickleback (Culaea inconstans) prey: Patterns in body size. Can J Fish Aquat Sci. 2006;63:297–309. [Google Scholar]
- 32.Duplisea DE. Running the gauntlet: The predation environment of small fish in the northern Gulf of St Lawrence, Canada. ICES J Mar Sci. 2005;62:412–416. [Google Scholar]
- 33.Vonesh JR, Osenberg CW. Multi-predator effects across life-history stages: Nonadditivity of egg- and larval-stage predation in an African treefrog. Ecol Lett. 2003;6:503–508. [Google Scholar]
- 34.Van der Meijden E, Van der Veen-Van Wijk CAM. In: Metapopulation Biology. Hanski I, Gilpin M E, editors. New York: Academic; 1997. pp. 387–405. [Google Scholar]
- 35.Waage JK, Hassell MP. Parasitoids as biological control agents - A fundamental approach. Parasitology. 1982;84:241–268. [Google Scholar]
- 36.Bruno JF, Stachowicz JJ, Bertness MD. Inclusion of facilitation into ecological theory. Trends Ecol Evol. 2003;18:119–125. [Google Scholar]
- 37.Sih A, Englund G, Wooster D. Emergent impacts of multiple predators on prey. Trends Ecol Evol. 1998;13:350–355. doi: 10.1016/s0169-5347(98)01437-2. [DOI] [PubMed] [Google Scholar]
- 38.Relyea RA. How prey respond to combined predators: A review and an empirical test. Ecology. 2003;84:1827–1839. [Google Scholar]
- 39.De Roos AM, Persson L. Size-dependent life-history traits promote catastrophic collapses of top predators. Proc Natl Acad Sci USA. 2002;99:12907–12912. doi: 10.1073/pnas.192174199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.