Abstract
To gain insight into the mechanisms that control the generation or maintenance of the characteristic bipolar morphology of cochlear spiral ganglion neurons, we have taken advantage of our recently developed procedure for culture of dissociated newborn mouse spiral ganglion. In these cultures, inclusion of the cytokine leukemia inhibitory factor (LIF) in the medium increases neuronal survival and the number of bipolar neurons. Here we tested effects of two other LIF-type cytokines (ciliary neurotrophic factor, CNTF; and human recombinant oncostatin M, hOSM) and of bone morphogenetic protein 4 (BMP4) on survival, morphology and neurite lengths of neurons in cultures of dissociated spiral ganglion. Like LIF, CNTF and hOSM increased neuronal survival and the number of surviving bipolar neurons. BMP4 also increased neuronal survival, but unlike LIF, CNTF and hOSM, increased the number of monopolar neurons and neurons with no neurites. In addition, population histograms demonstrate that the population lengths of the longer and shorter neurites of bipolar neurons were shorter in BMP4 containing cultures than in control or LIF cultures. When LIF and BMP4 were simultaneously added to the cultures, the BMP4 effects predominated. These experiments demonstrate that exposure to different environmental conditions can result in different morphologies in the surviving population of spiral ganglion neurons in culture.
Keywords: cochlea, neuron, neurite outgrowth, regeneration, cytokine, BMP4
Sound stimulation of auditory hair cells is transduced into electrical signals that are carried to the brain via the bipolar spiral ganglion neurons. When the hair cells degenerate due to overstimulation, antibiotics or other drug toxicity, there is an initial loss of a large proportion of the disconnected spiral ganglion neurons followed by a lengthier time scale of further degeneration. In the surviving neurons, peripheral processes retract back towards the spiral ganglion, potentially as far as to the cell soma (Spoendlin, 1975; 1984; Lawner et al., 1997), leaving the patient or the research animal hearing impaired or deaf. To partially overcome the deficit in damaged cochleas, cochlear implant prostheses have been designed to grossly mimic sound stimulation by generating electric current fields at different sites along the tonotopically organized cochlear spiral. These fields stimulate the cell soma or central fibers of neurons whose central fibers are still connected to the brain stem (Rubenstein and Miller, 1999; Shepherd et al., 2004; Cartee et al., 2006) and function well enough to permit adequate speech interpretation by implant users. Nonetheless, overlapping electric current fields are thought to impact the spatial resolution of prosthesis. It has been suggested that interventions aimed at reducing the distance between the implant and the neuron by inducing regrowth of peripheral nerve fibers toward the prosthesis would reduce power consumption, confine the spread of the electric field and thereby improve spatial selectivity (Roehm and Hansen, 2005).
Protection of neuronal survival in vivo is the first requirement for inducing regrowth of nerve fibers. Application of experimental agents directly to the cochlea, such as GDNF, BDNF, CNTF, NT3, or exposure to electrical stimulation has been reported to increase neuronal survival in damaged cochleas (Ernfors et al., 1996; Staecker et al., 1996; Miller et al., 1997; Mitchell et al., 1997; Ylikoski et al., 1998; Kanzaki et al., 2002; Shinohara et al., 2002; Gillespie and Shepherd, 2005; McGuinness and Shepherd, 2005; Shepherd et al., 2005). Despite this documented neuronal protection, there has to date been only limited documentation of regrowth of nerve fibers in vivo (Bohne and Harding, 1992; Lawner et al., 1997; Miller et al., 1997; Wise et al., 2005). One reason for this general lack of success may be because nearly none of the intrinsic biochemical mechanisms spiral ganglion neurons can use to develop or regenerate bipolar morphology are known. In particular, conditions that are unfavorable for neurite outgrowth have rarely been examined.
We recently developed a procedure for culture of dissociated newborn mouse spiral ganglion in order to examine mechanisms that spiral ganglion neurons can use to develop or regenerate bipolar morphology and to study conditions that are unfavorable for neurite growth (Whitlon et al., 2006). Using this method, we confirmed the results of Marzella et al (1997; 1999) in the rat, demonstrating that the cytokine, leukemia inhibitory factor (LIF) increased survival of plated spiral ganglion neurons. We extended this observation to show that LIF not only helps to maintain 100% survival of plated neurons in our system, but that this survival is associated with a preferential increase in the absolute number of bipolar neurons in the culture as compared with control. LIF is a member of the IL6 family of pleotrophic cytokines that initiate signaling mechanisms through the gp130 cell surface protein. Here we further the analysis to two other cytokines, CNTF and hOSM, that also signal through a gp130-LIFRβ heterodimer. In addition, we compare the effects of BMP4, a growth factor that is present in the cochlea during development (Oh et al., 1996; Takemura, et al., 1996; Mosli et al., 1998; Cole et a l., 2000; Lie et al., 2005; Pujades et al., 2006) that, in some systems has been shown to have opposite effects to those of LIF (Adachi et al., 2005; Bonaguidi et al., 2005).
EXPERIMENTAL PROCEDURES
Animals
Newborn and postnatal day 1 mice, CD-1 strain (Charles River Laboratories, Wilmington MA, USA) were used. The care and use of the animals in this study were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and was approved by the Animal Care and Use Committee of Northwestern University. Every effort was made to minimize the suffering of research animals and to limit their numbers.
Cell Culture
Cultures of dissociated spiral ganglion in 96-well plates were prepared as previously described in detail (Whitlon et al, 2006). Control cultures contained BDNF and NT3 (Promega, Madison, WI; final concentrations, 8ng/ml each) and 10% heat inactivated Fetal Bovine Serum (FBS). Experimental cultures additionally included CNTF (EMD Biosciences, La Jolla CA; final: 50–100ng/ml), LIF (Sigma Aldrich, St. Louis MO; final: 40–100ng/ml), hOSM (EMD Biosciences; final: 40–100ng/ml), IL6 (40–100ng/ml) and/or BMP4 (R&D Systems, Minneapolis, MN; final: 25–50ng/ml). Cell cultures were maintained for 4 hours or 42 hours in 4% CO2/air at 33°C.
Immunocytochemistry
Neurons were identified by immunolabeling with a mouse monoclonal antibody (TuJ1, Covance, Berkeley, CA) directed against the neuronal form of tubulin – βIII tubulin as described (Whitlon et al., 2006). The stained 96 well culture plate was examined either by a Zeiss Axiovert microscope or inverted to observe with an upright Zeiss Axioscope.
Neuronal Survival
Neuronal survival was counted as reported previously (Whitlon et al., 2006). Large clumps of cells that clearly failed to dissociate were not counted, and this approach did not interfere with inter-well reproducibility of counts. Otherwise, every labeled cell with a nucleus was counted as a neuron, without regard to morphology or number of processes. To account for a slightly different fraction of the spiral ganglion plated in each well in different experiments (0.143 – 0.22 ganglion per well), numbers of neurons labeled per well were normalized to βIII tubulin + cells/cochlea as described (Whitlon et al., 2006). A study of survival vs. cell concentration demonstrated that density effects on survival were constant through this range of cell concentrations (data not shown).
Neuronal Morphology
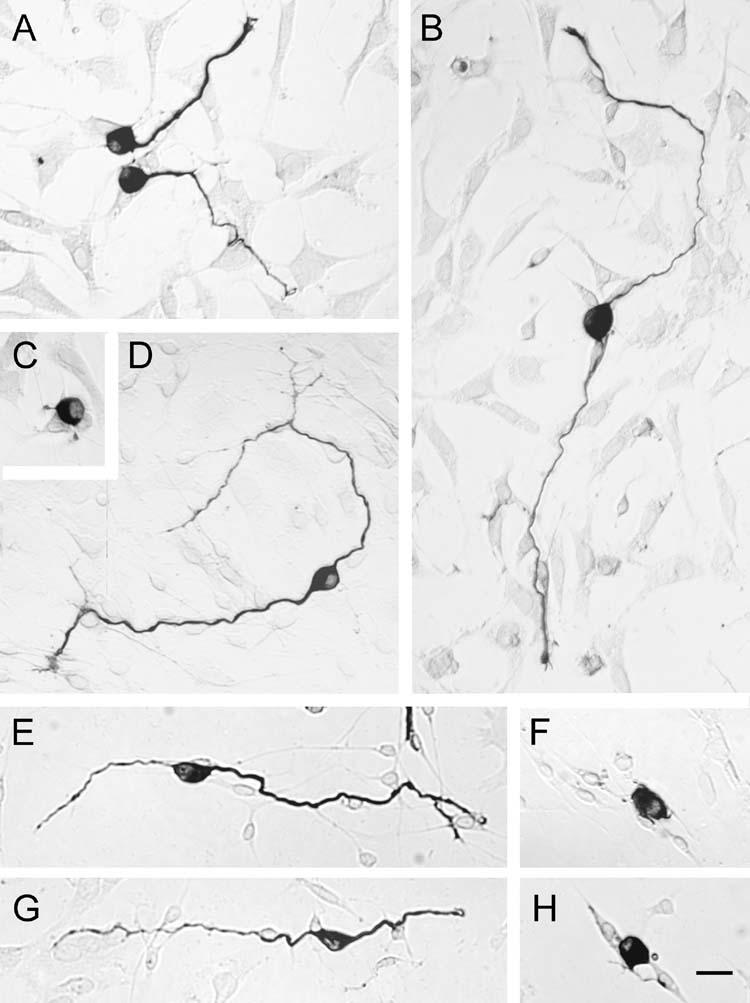
Neuronal morphology was assessed by sampling each well, following a predetermined pattern through the well. When morphology could be clearly assessed, it was scored as monopolar, bipolar, multipolar, pseudomonopolar or no processes. A process had to be longer than the diameter of the neuronal soma to be counted. Bipolar neurons were scored solely based on the number of appropriate length neurites, with no accounting for location of the processes around the cell soma i.e.: two neurites on the same side of the cell body or two on opposite sides were both counted as bipolar. A pseudomonopolar cell was defined as having one process that divided into fairly equal lengths within one cell diameter length from the neuron. Cells that could not be clearly observed were not counted. Uncounted neurons in clumps did not significantly affect results, as demonstrated by consistent reproducibility between wells within each experiment and in results across experiments. The percentages of different morphologies were calculated from the total number of neurons that were scored unambiguously in each well. Percentages were then multiplied by the surviving βIII tubulin+ cells /cochlea calculated for each well to get estimates of the absolute numbers of different morphologies in each well. Pseudomonopolar and multipolar neurons made up less than 2% of the total number of neurons. Figure 1 gives examples of monopolar (Fig. 1A) and bipolar (Fig. 1B,D,E,G) morphologies and neurons with no neurites (Fig. 1C,F,H).
Fig. 1.
Examples of neurons of different morphology immunolabeled with mouse anti-βIII tubulin (TuJ1). A) monopolar neurons; B,D,E,G) Bipolar neurons; C,F,H) neurons with no neurites. Scale bar = 20 μm.
Neurite Length
Neurons were sampled as above for morphology, with only the fibers greater than 25 m (approximate largest diameter of a neuron) measured. Nerve fibers were classified as F1 (longer fiber of a bipolar neuron), F2 (shorter fiber of a bipolar neuron) or Fm (fiber from a monopolar neuron). Each neuron was digitally photographed with a 20X objective and the fibers in the resulting picture were measured on the computer monitor with the aid of the software MetaVue. Cumulative frequency histograms, bin size 50 m starting at bin 25–75 m, were constructed for each condition in each of 4 (Figure 4) or 3 (Figure 6) experiments. Each histogram was normalized, with 100% set equal to the total number of sampled neurons. For each condition, bins were then averaged across experiments and the averages were plotted against the Bin Center value. Points ±SEM in the graph were drawn as connected curves for clarity. Table I lists the numbers of nerve fibers measured for Figures 4 and 6.
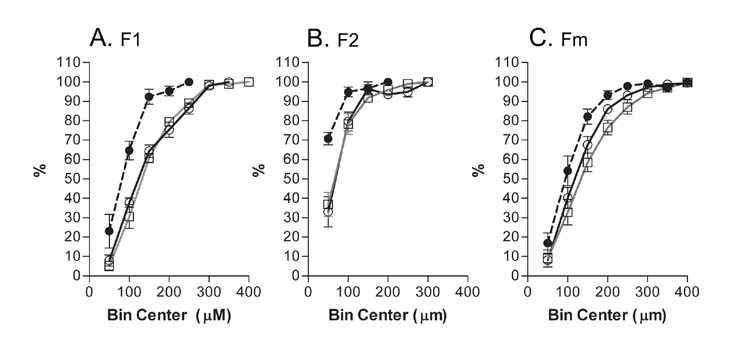
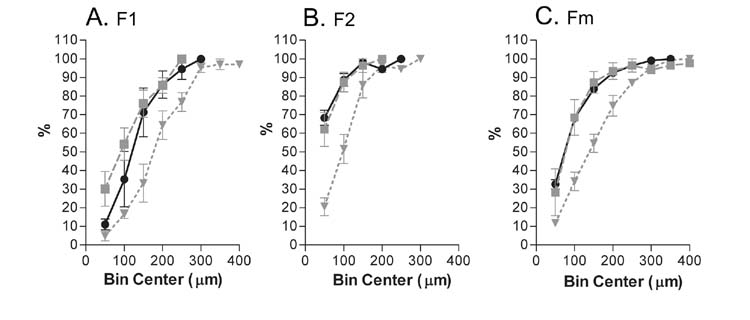
Fig. 4.
Normalized, cumulative histograms (bin size 50 m) compare the lengths of neurites after 42 hours in control cultures containing BDNF, NT3 and serum (open circles) with cultures that additionally contain LIF (open triangles) or BMP4 (closed squares). A) Comparison of the longer neurites of bipolar neurons, F1. B) Comparison of the shorter neurites of bipolar neurons F2. C) Comparison of the neurites from monopolar neurons. In all conditions, the populations of nerve fibers in BMP4 cultures are shorter than those in Control or LIF cultures. Numbers represent the averages ± SEM of normalized, cumulative histograms from 4 experiments, 2–4 wells analyzed per condition in each experiment. Points on the graph are connected for clarity. Refer to Table I for the numbers of neurites counted.
Table I.
Numbers of nerve fibers measured for A) Figure 4 and B) Figure 5. F1 – longer fiber of a bipolar neuron; F2-shorter fiber of a bipolar neuron; Fm-fiber from a monopolar neuron.
| A. | F1 | F2 | Fm |
|---|---|---|---|
| Control | 70 | 70 | 312 |
| LIF | 89 | 89 | 193 |
| BMP4 | 35 | 35 | 216 |
| Total, 4 experiments; 1109 nerve fibers counted | |||
| B. | F1 | F2 | Fm |
| LIF | 68 | 68 | 98 |
| BMP4 | 41 | 41 | 282 |
| LIF+BMP4 | 54 | 54 | 153 |
| Total, 3 experiments; 859 nerve fibers counted. | |||
Digital Imaging
Photographs were taken in color on a Zeiss Axioscope under light or partial Nomarski optics with a Nikon Digital Camera (DXM1200) at the highest possible resolution (11.6 Megp). Photographs were edited with the computer programs Photoshop and MetaVue. Editing was limited to sizing, orientation, minor adjustments to contrast and lighting, and conversion to grayscale.
Statistics
The computer programs Excel or GraphPad Prism were used to calculate, normalize, average or plot cumulative frequency histograms (for neurite lengths) and to perform and graph repeated measures ANOVA’s with HSD test by Tukey (for survival and morphology calculations). All p values < 0.05 were considered statistically significant.
RESULTS
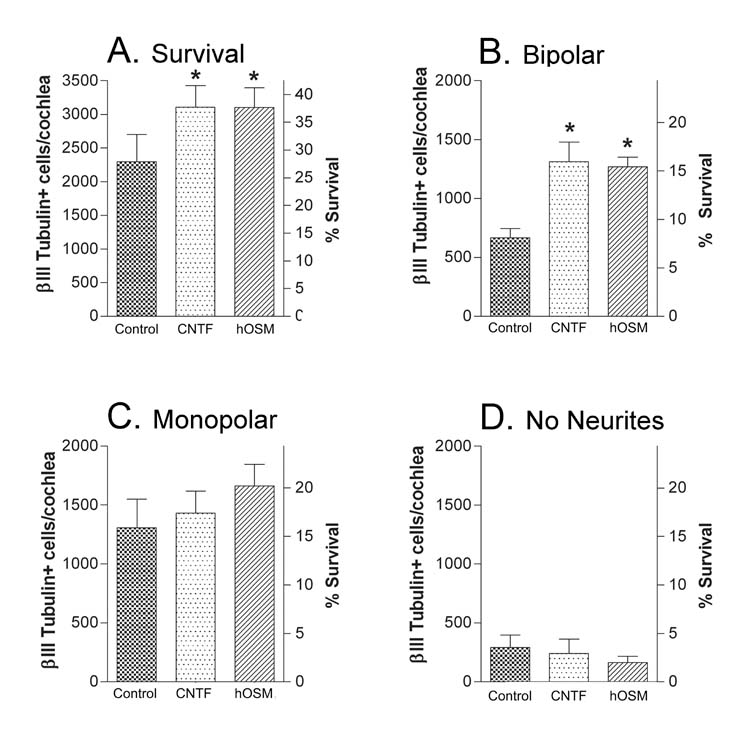
We previously demonstrated that neuronal survival 42 hour after plating could be increased when LIF was added to the control conditions of BDNF+NT3+serum. This additional survival in LIF cultures was associated with an increase in the absolute number of bipolar neurons over that control cultures (Whitlon et al., 2006). Signaling by LIF is known to be initiated at the cell surface through the LIFRβ–gp130 receptor. To determine if other cytokines that are known to function through the LIFRβ-gp130 receptor had similar effects on survival and morphology, control cultures were additionally exposed to the cytokine CNTF or the cytokine hOSM. Figure 2A shows that both CNTF and hOSM containing cultures demonstrated increased survival of neurons over control, as seen previously with LIF. In addition, the increased survival with CNTF or hOSM was associated with an increase in the absolute number of bipolar neurons (Fig. 2B) and showed smaller effects on the number of monopolar neurons or those with no neurites (Fig. 2C,D). We also examined the effects of IL6, a cytokine that functions through the gp130-gp130 homodimer. Data from 11 experiments that compared control survival (βIII Tubulin + cells/cochlea; 1926±212) to that of cultures that additionally included LIF (2475±263; p<.001) or the cytokine IL6 (2034±229; p> .05) demonstrated increased survival with LIF but no significant difference in survival with the inclusion of IL6.
Fig. 2.
Effect of CNTF and hOSM on neuronal survival and morphology after 42 hours in culture. Control cultures contained BDNF, NT3 and serum. Cytokine cultures additionally contained either CNTF or hOSM. A) Inclusion of CNTF or hOSM increases survival and B) the number of bipolar neurons in the culture over that in control cultures. C) The differences in the numbers of monopolar neurons or D) those with no neurites are not statistically significant. Numbers represent the averages ± SEM of the means from 3 experiments, 2–3 wells per condition per experiment. Neurons in each well are normalized to surviving βIII tubulin +cells/cochlea as described in the methods (left Y axis). On the right Y axis, survival is related to the total number of spiral ganglion neurons in the newborn cochlea (8240, Whitlon et al., 2006, see methods). The p values compare each cytokine culture to the control culture. *p<0.05.
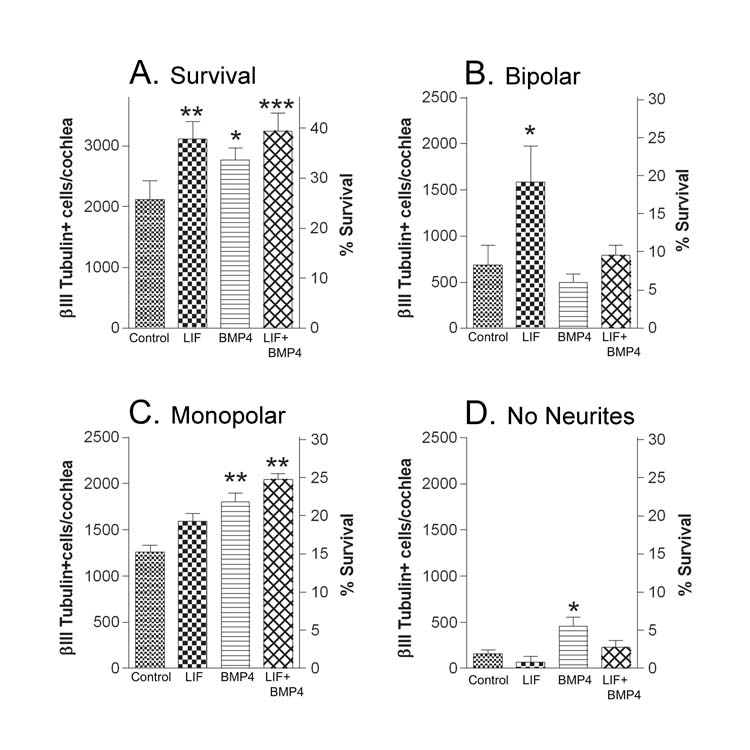
To examine the effects of BMP4 on survival and morphology of spiral ganglion neurons, we first determined in 6 separate experiments that the 4 hour survival (plating efficiency) in control cultures was unaltered by inclusion of BMP4 or LIF. For these cultures, the plating efficiency of LIF and BMP4 cultures were normalized to that of control (set at 100%). The average percent plating efficiency of all 6 experiments of LIF cultures was 103±3% of control and of BMP4 cultures was 101±5% of the control. Control, LIF and BMP4 cultures were then plated and maintained for 42 hours. Figure 3 again demonstrates that cultures respond to LIF in a manner similar to CNTF and hOSM – that is: improved survival at 42 hours (Fig. 3A), an increase in the number of bipolar neurons over that in control cultures (Fig. 3B), and with smaller or no effect on monopolar neurons or neurons with no neurites (Figs. 3C, 3D). When control cultures are additionally exposed to BMP4, survival is also maintained for 42 hours (Fig. 3A). However, in this case, the increase in survival is associated with an increase in monopolar neurons (Fig. 3C) and those with no neurites (Fig. 3D), with little or no increase in the number of bipolar neurons (Fig 3B).
Fig. 3.
Effect of LIF and BMP4 on neuronal survival and morphology after 42 hours in culture. Control cultures contained BDNF, NT3 and serum. Compare these with cultures that additionally contained LIF, BMP4 or LIF+BMP4. A). Inclusion of either LIF, BMP4 or LIF+BMP4 increased survival over that in control cultures. B) LIF cultures had more bipolar neurons than control cultures. C) There were more monopolar neurons in cultures containing BMP4 or LIF+BMP4 than in control; and D) BMP4 increased the numbers of neurons with no neurites of control levels. Numbers represent the averages ± SEM of the means from 3 experiments, 2–4 wells per condition per experiment. Neurons in each well are normalized to surviving βIII Tubulin +cells/cochlea as described in the methods (left axis) and their survival is related to the total number of spiral ganglion neurons in the newborn cochlea on the right Y axis. The p values compare the LIF, BMP4 or LIF+BMP4 conditions to the control cultures. *p<0.05; **p<0.01; ***p<0.001.
To determine whether BMP4 exerted effects on the lengths of neurites, 1109 neurites were measured across 4 experiments under control conditions and with the addition of BMP4 or LIF. The longer (F1) and shorter (F2) neurites of bipolar neurons were separately measured in addition to the single process emanating from monopolar neurons (Fm). Table I records the total number of neurites measured for each condition. The population histograms in Figure 4 demonstrate that the populations of either process of the bipolar neurons in BMP4 cultures were shorter than in control or LIF cultures. The lengths of monopolar neurites were also shorter in the BMP4 cultures than in the LIF cultures. The effect was smaller when the BMP4 and control cultures were compared.
We also compared spiral ganglion neurons in control medium containing LIF or BMP4 or with both LIF and BMP4 (Fig. 3). Figure 3A demonstrates that like cultures with LIF or BMP4, LIF+BMP4 increased survival over that of control. The number of bipolar neurons is increased over control in the presence of LIF but not when BMP4 is additionally present (Fig. 3B). Monopolar neurons are increased significantly over control levels only when BMP4 is present. (Fig. 3C). The number of neurons with no neurites was significantly elevated over control in cultures maintained with BMP4, but not with BMP4+LIF (Fig. 3D). In Figure 6, a total of 859 nerve fibers were measured over 3 separate experiments under LIF, BMP4 and LIF+BMP4 conditions. Table I shows the number of each type of nerve fiber measured. The averaged population histograms in Figure 6 demonstrate that whenever BMP4 was present in the medium, populations of neurites were shorter than those in cultures containing LIF without BMP4.
DISCUSSION
In this study, we have exposed spiral ganglion cultures to neurotrophins, LIF-type cytokines and BMP4 and examined the resulting neuronal survival, morphology and neurite lengths. Under all conditions, monopolar neurons, bipolar neurons and neurons with no neurites comprise over 95% of the neurons in the culture. However, the relative contributions of the different morphologies differs between control, LIF and BMP4 conditions. Specifically, as compared to control, inclusion of LIF type cytokines increases neuronal survival and results in an increase in the number of bipolar neurons; while inclusion of BMP4 increases neuronal survival and results in an increase in the number of monopolar neurons and neurons with no neurites. In addition, bipolar neurons in BMP4 cultures have shorter neurites than those in control or LIF cultures.
We have tested three different cytokines-LIF,CNTF and hOSM – that in other cell types signal through the LIFRβ-gp130 heterodimeric receptor (Turnley and Bartlett, 2000). LIF and hOSM use the LIFRβ-gp130 receptor directly; CNTF requires the help of an additional binding protein, CNTFRα (Marz et al., 1999; Turnley and Bartlett, 2000). All three cytokines showed similar effects on cell survival and morphology, indicating that signaling initiated through the LIFRβ-gp130 receptor increases neuronal survival and exerts a preferential effect on the maintenance of bipolar neurons. That CNTF functions at all in this culture system suggests that the CNTFRα (membrane bound or soluble) is present in the cultures (Marz et al., 1999). Interestingly, IL6, does not demonstrate similar effects to those of LIF, CNTF or hOSM. This may be due to a lack of the IL6 binding protein in the cultures (Marz et al., 1999), or a lack of effect of the IL6 type gp130 homodimeric signaling (Turnley and Bartlett, 2000) on survival and morphology of spiral ganglion neurons.
Inclusion of BMP4 in control cultures not only increases neuronal survival and alters the cellular morphology of the neuronal population, but also results in shorter neurites emanating from bipolar neurons. Even in the presence of LIF, BMP4 effects predominate. Two main possibilities present themselves: either BMP4 and LIF affect different populations of neurons, with BMP4 inhibiting the survival (and hence contribution to the bipolar pool) of LIF responsive neurons; or both support survival of the same population of neurons, with BMP4 acting as an inhibitor of LIF stimulated neurite outgrowth. With the current system, it is not possible to return a growing neuron to the time of plating and look at it a second time under different culture conditions. Thus, there is presently no direct way to determine if LIF and BMP4 exert morphologic effects on the same or different neurons. Whatever the mechanism, the result is that while maintaining survival numbers, the characteristics of the in vitro population shift between predominately bipolar and predominately monopolar and denuded neurons, depending on the factors included in the medium.
Most neurons in adult spiral ganglia in vivo are bipolar. Yet these spiral ganglion cultures return a mix of neuronal morphologies. The aim in cell culture is to induce neurons to regrow their neurites in an observable and quantifiable way. However, this entails the use of a reconstructed environment that by definition has to be different from that from which the neurons were removed, as well as different from that encountered during the initial developmental period. In the broader framework of trying to understand the mechanistic controls on bipolar morphology and neurite growth, the initial mixture of different morphologies we observe in our cultures becomes an advantage to exploit experimentally. For example: Can monopolar neurons be encouraged to regrow the missing axon? Such a mixture may also have some relevance to the in vivo situation in damaged cochleas, when hair cells degenerate and the disconnected peripheral neurites retract toward the cell soma. In particular, if the central axon remains connected to the brain stem, as cochlear implant function would indicate, then during peripheral neurite retraction the neuron can potentially arrive at a state similar to the monopolar neurons in our cultures. The use of bipolar morphology and neurite length as endpoints in our morphologic assay will allow us to examine possible biochemical and other interventions that can shift predominately monopolar populations to bipolar populations of spiral ganglion neurons, a conversion that may have implications in the whole animal.
In other tissues, both LIF and BMP4 exert effects on renewal and differentiation of neural stem/progenitor cells (Bonaguidi et al., 2005; Bauer and Patterson, 2006; Chen and Panchision, 2006). However, preliminary studies in our laboratory failed to observe BrDU labeled neurons under any of our culture conditions (D.S. Whitlon, unpublished), suggesting that the surviving neurons were not derived from cell division. Further, although it can be assumed from prior studies that neurotrophins act directly on the neurons, the present studies do not address the identity of the initial target cells for BMP4 or LIF. This a relevant question given the mixed composition of the dissociated cultures. In other tissues, both LIF type cytokines and BMPs exert effects on multiple cell types (Murphy, 1997; Dowsing et al., 1999; Turnley and Bartlett, 2000). Although it is possible that these factors directly bind neurons, it is also conceivable that survival or neurite growth effects of LIF and/or BMP4 are exerted through initial effects on non-neural cell types.
One of the many questions yet to be answered about the regrowth of spiral ganglion neurites is whether the two bipolar neurites are equivalent or different in vitro. In vivo, both processes of spiral ganglion cells are morphologically axons, and in fact there is very little to differentiate them in situ other than their position and a small narrowing at the initiation of the peripheral neurite (Brown et al., 1988; Goycoolea et al., 1990). Interestingly, we find that bipolar neurons in culture are not, in general, symmetrical with respect to neurite size. One neurite is usually longer than the other. Under control conditions, 50% of the longer neurites of bipolar neurons are longer than 150 μm, while only 15% of the shorter neurites of bipolar neurons are longer than 150 μm. It might seem reasonable that this difference in length represents differences in neurite identity and that the neurite emanating from the monopolar neurons, since similar in size, are similar in identity to the longer neurites of bipolar neurons. While this may ultimately be proven true, there is evidence in other neuronal cell types that the microtubules defining neurites are nucleated at the centrosome (Ahmad and Baas, 1995), which in cerebellar granule cells alternates between different sides of the cell soma to extend growth of different axons (Zmuda and Rivas, 1998). Therefore, without specific markers for peripheral or central fibers, it cannot yet be known whether the sizes of the neurites are statistical results of the position of the centrosome or are actually manifestations of differential neurite identities.
In the present work, we use LIF-type cytokines and BMP4 as triggers to our final endpoints –changes in survival, morphology or neurite length. The purpose is to initiate cellular mechanisms that promote or fail to promote neurite growth and to make them visible enough to measure and analyze. Although this work does not address any issues surrounding the possible use of LIF-type cytokines or BMP4 as therapeutic agents in vivo, these factors are known to be endogenous to the cochlea during development or after tissue damage (Oh et al., 1996; Takemura et al., 1996; Malgrange et al., 1998; Morsli et al., 1998; Cole et al., 2000; Cho et al., 2004; Li et al., 2005; Pujades et al., 2006). Whether there are times in development or damage that BMP4 or LIF-like mechanisms can come into play in regulating neuronal morphology, neuronal development or neurite growth are questions for further study.
Fig. 5.
Normalized, cumulative histograms (bin size 50 m) compare the lengths of neurites after 42 hours in LIF (closed triangles), BMP4 (closed squares) and LIF+BMP4 (closed circles) cultures. All cultures contain BDNF, NT3 and serum. BMP4 inclusion inhibits the lengths of neurites even in the presence of LIF. A) Comparison of the longer neurites of bipolar neurons, F1. B) Comparison of the shorter neurites of bipolar neurons F2. C) Comparison of the neurites from monopolar neurons. In all conditions, the populations of nerve fibers in LIF+BMP4 cultures are similar in length distributions to those in BMP4 cultures and shorter than those LIF cultures. Numbers represent the averages ± SEM of normalized, cumulative histograms from 3 experiments, 2–3 wells analyzed per condition in each experiment. Points in the graph are connected for clarity. Refer to Table I for the numbers of neurites counted.
Acknowledgments
The authors appreciate the advice of Dr. Adriana Ferreira on neuronal cell cultures. We thank Dr. Ferreira, Dr. James Bartles and Dr. David Tieu for their insightful comments on the manuscript. This work was supported by NIH grant #DC00653.
List of Abbreviations
- BDNF
Brain derived neurotrophic factor
- BMP4
Bone morphogenetic protein 4
- CNTF
Ciliary neurotrophic factor
- CNTFRα
Ciliary neurotrophic factor receptor alpha
- FBS
Heat inactivated fetal bovine serum
- F1
Longer neurite of bipolar neuron
- F2
Shorter neurite of bipolar neuron
- Fm
Neurite of monopolar neuron
- hOSM
Human recombinant oncostatin M
- IL6
Interleukin 6
- LIF
Leukemia Inhibitory Factor
- LIFRβ
Leukemia Inhibitory Factor Receptor Beta
- NT3
Neurotrophic factor 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi T, Takanaga H, Kunimoto M, Asou H. Influence of LIF and BMP-2 on differentiation and development of glial cells in primary cultures of embryonic rat cerebral hemisphere. J Neurosci Res. 2005;79:608–615. doi: 10.1002/jnr.20373. [DOI] [PubMed] [Google Scholar]
- Ahmad FJ, Baas PW. Microtubules released from the neuronal centrosome are transported into the axon. J Cell Sci. 1995;108:2761–2769. doi: 10.1242/jcs.108.8.2761. [DOI] [PubMed] [Google Scholar]
- Bauer S, Patterson PH. Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci. 2006;26:12089–12099. doi: 10.1523/JNEUROSCI.3047-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne BA, Harding GW. Neural regeneration in the noise-damaged chinchilla cochlea. Laryngoscope. 1992;102:693–703. doi: 10.1288/00005537-199206000-00017. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, McGuire T, Hu M, Kan L, Samanta J, Kessler JA. LIF and BMP signaling generate separate and discrete types of GFAP expressing cells. Development. 2005;132:5503–5514. doi: 10.1242/dev.02166. [DOI] [PubMed] [Google Scholar]
- Brown MC, Berglund AM, Kiang NYS, Ryugo DK. Central trajectories of type II spiral ganglion neurons. J Comp Neurol. 1988;278:581–590. doi: 10.1002/cne.902780409. [DOI] [PubMed] [Google Scholar]
- Cartee LA, Miller CA, van den Honert C. Spiral ganglion cell site of excitation I: Comparison of scala tympani and intrameatal electrode responses. Hear Res. 2006;215:10–21. doi: 10.1016/j.heares.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Chen H, Panchision D. BMP pleotrophism in neural stem cells and their derivatives -alternative pathways, convergent signals. Stem Cells. 2006 doi: 10.1634/stemcells.2006-0339. [DOI] [PubMed] [Google Scholar]
- Cho Y, Gong T-W, Kanicki A, Altschuler RA, Lomax MI. Noise overstimulation induces immediate early genes in the rat cochlea. Mol Brain Res. 2004;130:134–148. doi: 10.1016/j.molbrainres.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Cole L, Le Roux I, Nunes F, Laufer E, Lewis J, Wu D. Sensory organ generation in the chicken inner ear: contributions of bone morphogenetic protein 4, serrate 1 and lunatic fringe. J Comp Neurol. 2000;424:509–520. doi: 10.1002/1096-9861(20000828)424:3<509::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Dowing BJ, Morrison MA, Nicola NA, Satarkey GP, Bucci T, Kilpatrick TJ. Leukemia inhibitory factor is an autocrine survival factor for Schwann cells. J Neurochem. 1999;73:96–104. doi: 10.1046/j.1471-4159.1999.0730096.x. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Duan ML, El Shamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotropin-3. Nature Med. 1996;2:463–467. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Shepherd RK. Clinical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur J Neurosci. 2005;22:2123–2133. doi: 10.1111/j.1460-9568.2005.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goycoolea M, Stypulkowski P, Muchow D. Ultrastructural studies of the peripheral extensions (dendrites) of type I spiral ganglion cells in the cat. Laryngoscope. 1990;100:19–24. doi: 10.1288/00005537-199002001-00002. [DOI] [PubMed] [Google Scholar]
- Kanzaki S, Stover T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454:350–360. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- Lawner BE, Harding GW, Bohne BA. Time course of nerve-fiber regeneration in the noise-damaged mammalian cochlea. Int J Dev Neurosci. 1997;15:601–617. doi: 10.1016/s0736-5748(96)00115-3. [DOI] [PubMed] [Google Scholar]
- Li H, Corrales CE, Wang Z, Zhao Y, Wang Y, Liu H, Heller S. BMP4 signaling is involved in the generation of inner ear sensory epithelia. BMC Dev Biol. 2005;5 doi: 10.1186/1471-213X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgrange B, Rogister B, Lefebvre P, Mazy-Servais C, Welcher AA, Bonnet C, Hsu R-Y, Rigo J-M, Van de Water TR, Moonen G. Expression of growth factors and their receptors in the postnatal rat cochlea. Neurochem Res. 1998;23:1133–1138. doi: 10.1023/a:1020724506337. [DOI] [PubMed] [Google Scholar]
- Marz P, Otten U, Rose-John S. Neural activities of IL-6-type cytokines often depend on soluble cytokine receptors. Eur J Neurosci. 1999;11:2995–3004. doi: 10.1046/j.1460-9568.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- Marzella PL, Clark GM, Shepherd RK, Bartlett PF, Kilpatrick TJ. LIF potentiates the NT-3-mediated survival of spiral ganglia neurons in vitro. Neuroreport. 1997;8:1641–1644. doi: 10.1097/00001756-199705060-00017. [DOI] [PubMed] [Google Scholar]
- Marzella PL, Gillespie LN, Clark GM, Bartlett PF, Kilpatrick TJ. The neurotrophins act synergistically with LIF and members of the TGF-β superfamily to promote the survival of spiral ganglia neurons in vitro. Hear Res. 1999;138:73–80. doi: 10.1016/s0378-5955(99)00152-5. [DOI] [PubMed] [Google Scholar]
- McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol Neurotol. 2005;26:1064–1072. doi: 10.1097/01.mao.0000185063.20081.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O'Keefe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Miller JM, Finger PA, Heller JW, Raphael Y, Altschuler RA. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in deafened guinea pig. Hear Res. 1997;105:30–43. doi: 10.1016/s0378-5955(96)00202-x. [DOI] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Dutton R, Koblar S, Cheema S, Bartlett P. Cytokines which signal through the LIF receptor and their actions in the nervous system. Prog Neurobiol. 1997;52:355–378. doi: 10.1016/s0301-0082(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Oh SH, Johnson R, Wu DK. Differential expression of bone morphogenetic proteins in the developing vestibular and auditory sensory organs. J Neurosci. 1996;16:6463–6467. doi: 10.1523/JNEUROSCI.16-20-06463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujades C, Kamaid A, Alsina B, Giraldez F. BMP-signaling regulates the generation of hair cells. Dev Biol. 2006;292:55–67. doi: 10.1016/j.ydbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Roehm PC, Hansen MR. Strategies to preserve or regenerate spiral ganglion neurons. Cur Opin Otolaryngol. 2005;13:294–300. doi: 10.1097/01.moo.0000180919.68812.b9. [DOI] [PubMed] [Google Scholar]
- Rubenstein J, Miller CA. How do cochlear prostheses work? Curr Opin Neurobiol. 1999;9:399–404. doi: 10.1016/S0959-4388(99)80060-9. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Roberts LA, Paolini AG. Long-term sensorineural hearing loss induces functional change in the rat auditory nerve. Eur J Neurosci. 2004;20:3131–3140. doi: 10.1111/j.1460-9568.2004.03809.x. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, Kaksonen R, Lindstrom B, Altschuler RA, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. PNAS. 2002;99:1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H. Retrograde degeneration of the cochlear nerve. Acta Otolaryngol. 1975;79:266–275. doi: 10.3109/00016487509124683. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Factors inducing retrograde degeneration of the cochlear nerve. Ann Otol Rhinol Laryngol. 1984;93(suppl):76–81. doi: 10.1177/00034894840930s415. [DOI] [PubMed] [Google Scholar]
- Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7:889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- Takemura T, Sakagami M, Takebayashi K, Umemoto M, Nakase T, Takaoka K, Kubo T, Kitamura Y, Nomura S. Localization of bone morphogenetic protein-4 messenger RNA in developing mouse cochlea. Hear Res. 1996;95:26–32. doi: 10.1016/0378-5955(95)00233-2. [DOI] [PubMed] [Google Scholar]
- Turnley AM, Bartlett PF. Cytokines that signal through the leukemia inhibitory factor receptor-β complex in the nervous system. J Neurochem. 2000;74:889–899. doi: 10.1046/j.1471-4159.2000.0740889.x. [DOI] [PubMed] [Google Scholar]
- Whitlon DS, Ketels KV, Coulson MT, Williams T, Grover M, Edpao W, Richter CP. Survival and morphology of auditory neurons in dissociated cultures of newborn mouse spiral ganglion. Neuroscience. 2006;138:653–662. doi: 10.1016/j.neuroscience.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O'Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Virkala P, Suvanto P, Liang XQ, Magal E, Altschuler RA, Miller J, Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived neurotrophic factor from degeneration after noise trauma. Hear Res. 1998;124:17–26. doi: 10.1016/s0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Zmuda J, Rivas RJ. The Golgi apparatus and the centrosome are localized to the sites of newly emerging axons in cerebellar granule neurons in vitro. Cell Motil Cytoskel. 1998;41:18–38. doi: 10.1002/(SICI)1097-0169(1998)41:1<18::AID-CM2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]