Abstract
Hepatitis C affects about 3% of the world population, yet its current treatment options are limited to interferon-ribavirin drug regimens which achieve a 50-70% cure rate depending on the hepatitis C virus (HCV) genotype. Besides extensive screening for HCV-specific compounds, some well-established medicinal drugs have recently demonstrated anti-HCV effect in HCV replicon cells. One of these drugs is arbidol (ARB), a Russian-made broad spectrum antiviral agent, which we have previously shown to inhibit acute and chronic HCV infection. Here we show that ARB inhibits the cell entry of HCV pseudoparticles of genotypes 1a, 1b and 2a in a dose-dependent fashion. ARB also displayed a dose-dependent inhibition of HCV membrane fusion, as assayed by using HCV pseudoparticles (HCVpp) and fluorescent liposomes. ARB inhibition of HCVpp fusion was found more effective on genotype 1a than on genotypes 1b and 2a. In vitro biochemical studies revealed ARB association with membrane-like environments such as detergents, and with lipid membranes. This association was particularly prominent at acidic pH which is optimal for HCV-mediated fusion. Our results suggest that the affinity of ARB for lipid membranes could account for its anti-HCV actions, together with a differential level of interaction with key motifs in HCV glycoproteins of different genotypes.
The hepatitis C virus (HCV) infects an estimated 3% or 170 million of the world's population, and hepatitis C is now the most frequent indication for liver transplantation. Current treatment options are limited to pegylated recombinant interferon alpha (IFN-α) in combination with ribavirin. However viremia eradication is variably achieved depending on the genotype, with only 50% of virus eradication in genotype 1-infected patients. This is clearly a problem in North America, Europe and Japan, where genotype 1 is the most prevalent genotype. HCV therefore appears resistant to IFN antiviral therapy, and this is likely to be due to some factors of viral origin (1).
Historically, the development of new anti-HCV drugs has been hampered due to the lack of cell culture and small animal models that are required for pre-clinical evaluations of antiviral compounds. The generation of an HCV replicon system (2) has afforded massive anti-viral drug screening efforts (3). Distinct from specific anti-HCV compounds that target key viral functions, are a group of broad-spectrum medicinal drugs that were originally designed for other treatments or targeted toward other viruses. Using the replicon system, these compounds have been shown to possess potent antiviral activity toward HCV (4, 5). The advantage of this group of antivirals is that they have already met the pharmacological criteria for medicinal drugs and are already approved for clinical use in some countries.
One of these compounds, the Russian-made arbidol [ARB; 1H-Indole-3-carboxylic acid, 6-bromo-4-((dimethylamino)methyl)-5-hydroxy-1-methyl-2-((phenylthio)methyl)-, ethyl ester, monohydrochloride; CAS Registry Number 131707-23-8] (Fig. 1) was originally described as an anti-influenza drug with purported immunostimulant properties (6). ARB has been licensed for several years in Russia for use as prophylaxis and treatment for influenza A and B infections. It allegedly exerts its effect by activation of macrophage phagocytic activity, and may also stimulate aspects of cellular and humoral immunity. ARB inhibits influenza virus-induced membrane fusion, and may have the capacity to induce serum interferon (7). More recent studies extended its inhibitory activity to other human viruses such as the respiratory syncytial virus, parainfluenza virus 3, rhinovirus 14 (8) and hepatitis B virus (9). Bird viruses such as the avian coronavirus and the H5/N1 influenza A virus were shown to be sensitive to ARB as well (10, 11). We recently demonstrated that ARB inhibits both acute and chronic HCV infection in vitro, and HCV replication using the HCV replicon system (12). In the current study we further characterized ARB's mechanism of action. We present a detailed biochemical characterization of ARB interactions with membranes, and examin how ARB can inhibit HCV entry by using HCV pseudoparticles (HCVpp). We found that ARB inhibited the infection of cells with HCVpp of different genotypes, through the inhibition of HCVpp-mediated membrane fusion. This inhibition was particularly prominent at acidic pH that is optimal for ARB association with membranes.
Figure 1. Structures of arbidol (A), indole (B) and tryptophan (C).
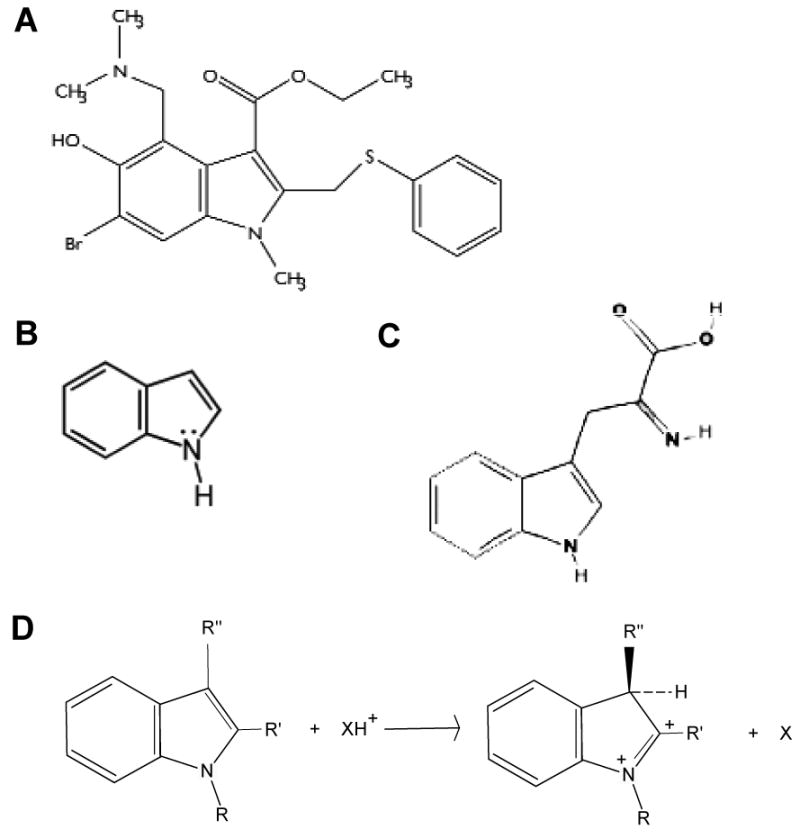
(D), scheme of the protonation of substituted indole, and its effect on the 3-position [from (22)].
Experimental procedures
Chemicals
Sodium dodecyl sulfate (SDS), n-dodecyl-β-D-maltoside (DM), L-α-palmitoyl-lysophosphatidylcholine (α-lysoPC), phosphatidylcholine from egg yolk (PC, 99% pure), cholesterol (chol, 99% pure) and Triton X-100 were purchased from Sigma. Phosphatidylserine (PS) from porcine brain was from Avanti Polar Lipids (Alabaster, AL). Octadecyl rhodamine B chloride (R18) was from Molecular Probes. Arbidol [ARB, 1H-Indole-3-carboxylic acid, 6-bromo-4-((dimethylamino)methyl)-5-hydroxy-1-methyl-2-((phenylthio)methyl)-, ethyl ester, monohydrochloride; Fig.1A] was a kind gift from A.M. Schuster and I.A. Leneva. ARB was first dissolved in absolute ethanol, and then completed to final concentration (100 mM) in sterile twice-distilled water. The final concentration of ethanol in contact with membranes varied between 0.011 and 1.1 ‰.
Vector constructs, preparation of pseudoparticles and infection assay
The CMV-Gag-Pol murine leukemia virus (MLV) packaging construct, encoding the MLV gag and pol genes, and the MLV-GFP plasmid, encoding a MLV-based transfer vector containing a CMV-GFP internal transcriptional unit, were described previously (13). The phCMV-HA (14) and NA expression vectors encode the hemagglutinin and neuraminidase of fowl plague virus, respectively. The phCMV-E1E2-HCV (13) encodes both HCV E1 and E2 glycoproteins of genotype 1a, strain H77 (AF009606), of genotype 1b (AY734975 clone UKN1b12.6, and AF333324), or of genotype 2a, isolate JFH-1 (AB047639). The phCMV-RD expression vector encodes the feline endogenous oncoretrovirus RD114 glycoprotein (15). Pseudoparticles bearing either the E1 and E2 envelope glycoproteins of HCV (HCVpp), or the hemagglutinin of the influenza virus (HApp), or the RD114 envelope protein (RD114) were then generated and purified as previously described (13, 16). Infection of Huh7 cells by concentrated pseudoparticles was performed in the absence or presence of increasing concentrations of ARB (between 0.1 and 6 μg/ml), added to cell culture medium as the ethanol/water stock solution. ARB was added to cells at increasing concentrations but under similar volumes, either before or after pseudoparticle binding. After that step, cell culture medium was removed and replaced by fresh medium, and infection was assessed 72h later by FACS analysis. Values obtained with HCVpp on untreated cells were taken as 100%.
Fluorescence assays
PC:chol (70:30 molar ratio) or PS large unilamellar vesicles (100 nm) were prepared as described (16). R18-labeled PS liposomes were added to unlabeled PS vesicles in PBS, in a 4-to-1 unlabeled/labeled ratio. After a 2 min equilibration at 37°C, CaCl2 was added to a final 2 mM Ca2+ concentration to initiate membrane fusion. Similar experiment was performed in the presence of arbidol. ARB spectral properties were investigated by spectrophotometry and spectrofluorimetry, in phosphate-buffered saline (PBS) pH 7.4, in the absence or presence of detergent micelles or liposomes. Spectral properties of indole derivatives such as tryptophan or ARB (see Fig.1) depend on their microenvironment, which in turn influences the maximum position (λmax) and quantum yield of their fluorescence. Fluorescence was recorded on an SLM Aminco 8000 fluorimeter, with excitation slits set to 8 nm, and emission slits to 4 nm. The excitation polarizer was oriented at 90° relative to the vertical, and 0° for emission, to improve the spectral resolution of fluorescence, and minimize scattering effects (17). Spectra were measured using a 10×2-mm quartz cuvette. Emission spectra were collected in the 300-450 nm region with an increment of 1 nm, and corrected for vesicle light scattering (17, 18). The increase in quantum yield is given by F/F0, where F and F0 denote the fluorescence at 340 nm after and before addition of detergent or liposomes, respectively. The measure of fusion between pseudoparticles and liposomes was based upon a lipid mixing assay, as described in details elsewhere (16). ARB was preincubated for 2 min either with pseudoparticles or liposomes, before lipid mixing was initiated by decreasing the pH to 5.0.
Results
Arbidol blocks cell infection by HCVpp
We sought to determine whether ARB had any effect on HCV entry, namely whether ARB could affect the infection of cultured cells by HCVpp. The results of these experiments are presented in Figure 2, where HApp and RD114pp were used as controls. When ARB was present during the course of HCVpp infection (Fig.2A), a decrease in infectivity was observed for all HCV genotypes tested, and the inhibition effect was proportional to ARB concentration. Maximal reduction (50% inhibition) in infectivity was observed at 6 μg/ml ARB (11.3 μM). When HCVpp were preincubated with ARB before infection, with subsequent continuous ARB presence during infection, a dose-dependent inhibition of infection was again observed for all genotypes tested (Fig.2B), with a maximum of 50% inhibition at 6 μg/ml ARB. The most pronounced inhibition was seen when Huh-7 cells were preincubated with ARB for 3h and then left in the presence of ARB onwards. Under these conditions (Fig.2C), the 50% inhibition was achieved at 1 μg/ml ARB (1.88 μM). Infectivity was further reduced at 6 μg/ml ARB, with up to 70% inhibition for genotypes 1a and 1b, and even 80% for genotype 2a. The HApp behaved in a very similar way, showing a dose-dependent decrease in their infectivity (Fig.2 A,B,C), with a maximal ARB effect at 6 μg/ml (from 40 to 90% inhibition). Note that a drastic reduction in infectivity (90% inhibition) could also be observed when cells were preincubated overnight with ARB, with no toxicity observed (data not shown).
Figure 2. Arbidol effect on HCVpp infectivity.

Huh-7 cells were seeded at 4.104 cells per well in 24-well plates the day before infection (day -1). At day 0, HCVpp (as a 100 × concentrated fraction; see Experimental Procedures) were added to cells, and incubated for 3h at 37°C, in the absence or presence of arbidol (see Experimental Procedures for other details). Panel A, ARB was added at indicated concentrations during the course of infection. Panel B, ARB was first preincubated at indicated concentrations with concentrated pseudoparticles for 3h, and infection was then performed in the presence of the same concentration of ARB. Panel C and D, ARB was preincubated at indicated concentrations with Huh-7 cells for 3h before infection, and HCVpp infection was performed in the presence (panel C) or absence (panel D) of ARB. HCV pseudoparticles tested were harboring the E1 and E2 glycoproteins of genotypes 1a (AF009606), 1b (AY734975 for 1b-I, and AF333324 for 1b-II), and 2a (AB047639). HApp and RD114pp are shown as positive and negative controls, respectively. Infectivity is expressed as the percentage of control, i.e. pseudoparticle infection of Huh-7 cells not treated with ARB, taken as 100%. Results are the mean ± standard deviation of 4 separate experiments.
Conversely cell infection by RD114pp was unaffected by the presence of ARB, whatever drug concentration tested and whatever infection conditions designed. This suggests that ARB might achieve a certain level of specificity toward some viral glycoproteins in particular, namely HCV and influenza glycoproteins in our study.
Interestingly, when cells were preincubated with ARB for 3h, but the drug was removed from cells immediately before HCVpp infection, no significant effect on HCVpp infectivity was observed (Fig.2D), although we noted up to a 20% reduction of infectivity of HCVpp-2a and HApp at the highest concentrations of ARB (6 μg/ml).
Taken together, these data indicate that ARB exerts its inhibitory effect on infection when it is in physical contact with either the pseudoparticles or cell membranes or both, and prominently at acidic pH. The absence of infection inhibition observed after ARB withdrawal from the incubation medium before infection suggests that its transient accumulation at the cell surface and/or in intracellular compartments is not enough to exert sustained HCV inhibitory action. The effect of ARB on HCVpp-mediated fusion and the role of pH were next directly tested in our in vitro lipid mixing assay (16).
Arbidol inhibits HCVpp-mediated lipid mixing
The effect of arbidol on HCVpp membrane fusion activity is presented in Figure 3. After decreasing the pH to 5.0, a rapid and significant dequenching was observed as a measure of lipid mixing between HCVpp and R18-labeled liposomes, for genotypes 1a [Fig.3A; see also (16)], 1b (Fig.3B) and 2a (Fig.3C). In the presence of ARB, HCVpp fusion ability was inhibited. This inhibition was directly proportional to ARB concentration, with some variations among HCV genotypes, though. Indeed the most prominent inhibition of fusion by ARB was observed for genotype 1a, where fusion was completely abolished at 1 μg/ml ARB (1.88 μM). Conversely a similar block in fusion was achieved at only 6 μg/ml ARB (11.3 μM) for genotype 1b. For genotype 2a, a measurable fusion was still observed at this concentration. This observation was reproducible (n = 4), but the reason for that difference in fusion behaviour between genotypes is presently unclear. ARB concentrations higher than 6 μg/ml were not tested to avoid toxicity to the cells (12). Note also that immunoblot analyses of the glycoproteins revealed that they were present in each viral preparation (data not shown, and (16). We also controlled the global input of the different pseudoparticles by estimating the amount of MLV core protein in each viral preparation on immunoblots. This amount of core was similar for all pseudoparticle preparations assayed in fusion (data not shown, and (16). We suggest that ARB might differentially interact with HCV glycoproteins of different genotypes (see Discussion). Note that the fusion mediated by RD114pp could not be investigated in our assay, since the RD114 glycoprotein is activated for fusion in a pH-independent but receptor-dependent manner (data not shown).
Figure 3. Arbidol inhibits HCVpp-mediated lipid mixing.
Lipid mixing curves of HCVpp genotypes 1a (A), 1b (B), and 2a (C), in the absence or presence of ARB, with R18-labeled liposomes (representative of 4 separate experiments). Pseudoparticles (40 μl) were added to R18-labeled PC:chol liposomes (15 μM final lipid concentration), in PBS pH 7.4 at 37°C, with or without indicated concentrations of ARB. After a 2-min equilibration, lipid mixing was initiated by decreasing the pH to 5.0 (time 0), and recorded as R18 fluorescence dequenching as a function of time. Panel D, influence of ARB on Ca2+-induced fusion of PS vesicles. Liposomes consisting of PS:R18 (96:4) were mixed with non-labeled PS vesicles (molar ratio 1:4) in 5 mM Hepes/100 mM NaCl pH 7.4 at 37°C, at a final lipid concentration of 100 μM. Fusion was induced by rapid injection of Ca2+ into the medium (2 mM final), and the kinetics of lipid dilution was continuously monitored. ARB was incubated with labeled and non-labeled PS vesicles for 1 min in buffer at pH 7.4 or at pH 5.0 (by adding diluted HCl), then Ca2+ was added at 2 mM final to initiate fusion.
The inhibition of fusion by ARB was comparable when ARB was preincubated with HCVpp or with liposomes (data not shown). ARB-induced inhibition is also not dependent upon the lipid composition of liposomes, since similar HCV fusion inhibition was observed with plain PC (not shown) or with PC:chol liposomes (Fig. 3).
We next asked whether ARB could inhibit fusion in other membrane systems as well. We therefore investigated ARB effect toward the Ca-induced fusion of PS liposomes (19). Kinetics of PS vesicles lipid mixing by Ca2+ were comparable at neutral and acidic pH (Fig.3D), as previously reported (20). In the presence of 6 μg/ml ARB, inhibition of lipid mixing was observed at both pH, but with a more pronounced effect at acidic than neutral pH (Fig.3D). ARB spectral properties were not affected by Ca2+ (data not shown), which rules out the direct interaction between ARB and Ca2+ that would artifactually lead to fusion inhibition.
Altogether these results suggest that ARB is a membrane fusion inhibitor, exerting its effect on viral (protein-induced) fusion and on a protein-free membrane fusion systems. Thus, ARB might associate with membranes to exert its inhibitory effect. However this is probably not its only mode of action, since : (i) HCVpp of different genotypes have differential sensitivity to the antiviral effects of ARB, and (ii) in contrast to HA and HCV E1/E2, RD114 envelope protein is not sensitive to ARB. These observations suggest that ARB might differentially interact with key motifs in HCV fusion protein(s), sequences that might differ among HCV genotypes. Moreover ARB acts predominantly at low pH. In our in vitro fusion assays, two hypotheses might explain such a behaviour: ARB could block fusion either by counteracting the acidification process similarly to chloroquine (21) and/or through its protonated form at low pH [see Fig.1D for the protonated form of a substituted indole; (22)]. Since the Ca-induced PS liposome fusion system is not pH-dependent, but is markedly inhibited by ARB at low pH, the first hypothesis of ARB blocking endosomal acidification as an explanation for ARB-induced fusion inhibition was deemed unlikely. Nonetheless, since ARB is a weak base (Fig.1), we tested this hypothesis directly. To this end, increasing concentrations of ARB (1, 10 and 50 μg/ml) were added to the suspension of HCVpp and liposomes in PBS pH 7.4. After a 2-min equilibration, an exactly measured volume of diluted HCl required to decrease the pH to 5.0 was added in each tube, and pH recorded with the pH meter. Within the tested range of ARB concentrations from 1 to 50 μg/ml, we could detect only slight pH variations of no more than 0.1 pH unit (data not shown). Such a narrow pH range alteration could be critical for influenza virus-induced fusion (HApp) but is unlikely to affect the broad-spectrum (pH 5.0-6.3) fusogenic properties of HCV (16, 23, 24). Therefore our results essentially ruled out the direct buffering role of ARB as an explanation for its fusion inhibition. The behaviour of ARB at low pH will be examined in detail in the next paragraphs.
Arbidol spectral properties in solution
ARB is an indole derivative, and shares this structure with tryptophan (Trp) (Fig.1). The preference of indole derivatives for membrane interfaces (and specifically of Trp in membrane proteins) is now well documented (25, 26). This interfacial preference is related to the flat rigid structure of these molecules, and to their aromaticity which allows them to establish cation-π interactions with lipid headgroups, i.e. interactions between the indole ring through its π-electron cloud and the nitrogen of the lipid headgroup (25, 27). We therefore reasoned that ARB might behave in a similar way, i.e. that it might enter in similar interactions between its indole group (Fig.1B) and the N(CH3)3+ group of phosphatidylcholine. In order to investigate ARB behaviour toward membranes, we first analyzed its spectral properties in solution. Its light absorption properties were studied by spectrophotometry over a 240-700 nm wavelength range. The ARB absorbance peaked at 255 nm (λexc) and 315 nm, both at pH 7.4 (Fig.4, dotted line) and at pH 5.0 (data not shown). The former λexc was then used as the excitation wavelength to study ARB fluorescence properties, since we reasoned that a shorter excitation wavelength would improve spectral separation of emission and scattering [see also (17)]. In order to achieve an adequate signal-to-noise ratio, we have analyzed the average of 5 emission spectra (Fig.4, solid lines). In PBS at pH 7.4, ARB displays a λmax at 360 nm (thin solid line); this λmax is slightly blue-shifted to 357 nm at pH 5.0 (thick solid line). For our further investigations on ARB behaviour toward membranes or membrane-like environments, λexc= 255 nm and λem= 350 nm were then used, unless otherwise indicated.
Figure 4. Fluorescence properties of arbidol.
All measurements were recorded at 37°C, with excitation (exc) and emission (em) slits set to 8 and 4 nm respectively. Relative orientations of exc. and em. polarizers were 90° and 0°, respectively. ARB absorbance (dotted curve) was recorded at pH 7.4, and fluorescence emission spectra were recorded at pH 7.4 (thin solid line) or pH 5.0 (thick solid line). Fluorescence was monitored using a 113 μM (60 μg/ml) ARB solution, with λexc = 255 nm.
Arbidol interacts with detergent micelles
Three detergents were chosen as membrane-like environments to study the interaction of ARB with micelles: sodium dodecyl sulfate (SDS, negatively-charged), dodecylmaltoside (DM, neutral and of glucidic nature), and α-palmitoyl-lysophosphatidylcholine (zwitterionic and of lipidic nature). At detergent concentrations well above the critical micellar concentration [cmc, (28)], and at pH 7.4, the addition of ARB to micelles led to a dramatic increase in the quantum yield of fluorescence for each detergent tested (Fig. 5A; Table 1), in the order DM > α-lysoPC > SDS. This effect was even more pronounced at pH 5.0, where the increase in quantum yield was concomitant to a moderate blue shift of the maximum emission wavelength for SDS and α-lysoPC (Fig. 5B; Table 1). These two phenomena are indicative of an association of ARB to micelles, accompanied by a partial, and most likely, superficial burial of the ARB molecule into the micelles. This interaction is more pronounced at acidic pH, where the ARB molecule is more protonated than at neutral pH. This suggests that the protonated form of ARB is probably more effective at inhibiting membrane fusion than the actual ARB molecule.
Figure 5. Arbidol interaction with detergent micelles.
Fluorescence conditions were similar as those in Fig.4. ARB (18.8 μM, 10 μg/ml) was added to PBS buffer containing either 100 mM SDS, or 100 μM α-lysoPC, or 100 mM DM, or no detergent, (A) at pH 7.4 or (B) at pH 5.0.
Table 1.
Fluorescence characteristics of arbidol in buffer, or in the presence of detergent micelles, or of PC:chol liposomes a.
| Medium | λmax (nm) | F/F0 (340 nm) b |
|---|---|---|
| PBS at pH 7.4 | 360 | / |
| PBS at pH 5.0 | 357 | / |
|
| ||
| pH 7.4 | ||
|
| ||
| SDS 100 mM | 351 | 11.88 |
| α-lysoPC 100 μM | 352 | 14.14 |
| DM 100 mM | 354 | 16.99 |
| PC:chol 12.5 μM | 351 | 0.99 |
| PC:chol 25.0 μM | 344 | 1.18 |
| PC:chol 62.5 μM | 342 | 1.36 |
| PC:chol 100 μM | 338 | 1.54 |
| pH 5.0 | ||
|
| ||
| SDS 100 mM | 348 | 6.42 |
| α-lysoPC 100 μM | 350 | 9.38 |
| DM 100 mM | 377 | 20.58 |
| PC:chol 12.5 μM | 353 | 1.28 |
| PC:chol 25.0 μM | 345 | 1.41 |
| PC:chol 62.5 μM | 338 | 1.81 |
All measurements were performed at 37°C after a 5-min pre-equilibration.
Variation in the fluorescence quantum yield is expressed as F/F0, where F and F0 denote the fluorescence intensity at 340 nm after and before addition of detergent or PC:chol liposomes, respectively.
We then monitored the binding of ARB at pH 5.0 to increasing concentrations of SDS, α-lysoPC and DM micelles from the resulting fluorescence changes at 350 nm (Fig.5). Results are shown in Fig.6. The shapes of the three binding curves are similar, showing that binding was similar in all cases. For all detergents studied, a steep increase in fluorescence was observed till the cmc value of the detergent was reached (28). From detergent concentrations above the cmc, fluorescence plateaued, which indicates that virtually all ARB molecules are bound to micelles at high detergent concentrations. Under these conditions, almost all of the observed ARB fluorescence was therefore due to ARB incorporated into or interacting with the detergent micelles. This confirms the affinity and tropism of ARB for membrane-like environments, particularly at acidic pH.
Figure 6. Binding of arbidol to SDS (A), α-lysoPC (B) and DM (C) at pH 5.0.
Fluorescence conditions were similar as those in Fig.4. Increasing concentrations of detergent were added to a cuvette containing ARB (18.8 μM) in PBS buffer acidified at pH 5.0 and equilibrated at 37°C. Emission spectra for each concentration were recorded after a 5-min equilibration. The fluorescence intensities obtained after each detergent addition, corrected using blank values (detergent alone in buffer), were plotted as a percentage of the initial value, as a function of final detergent concentration.
The influence of pH on ARB binding to detergent micelles was then studied using DM solely, since this detergent gave rise to the largest increase in fluorescence quantum yield (Fig.5; Table 1), and is neutral in charge. When ARB was added to a 5 mM solution of DM in a buffer, it appeared clearly that ARB fluorescence increased while pH was decreasing (Fig.7A). The increase in ARB fluorescence between pH 7.4 and 4.5 at 350 nm is 60%, as shown in Fig.7B. Since we know from the previous experiment that for the ARB:DM ratio used here (1:266), virtually all ARB molecules are bound to micelles, the observed fluorescence increase with pH decrease may arise from a difference in ARB ionization state at neutral or acidic pH. Depending on pH, ARB may therefore exist as a deprotonated, neutral form, or as a protonated, cationic form [see Fig.1D; (22)]. ARB's position within/on detergent micelles and its intrinsic fluorescence properties may then depend on its ionization state.
Figure 7. Binding of arbidol to DM micelles : effect of pH.
DM (final concentration 5 mM) was added to a 18.8 μM (10 μg/ml) ARB solution in PBS pH 7.4. Fluorescence conditions were similar as those in Fig.4. pH was decreased by addition of diluted HCl to the cuvette, and (A) emission spectra were recorded at pH 7.4 (a), 6.5 (b), 5.5 (c) or 4.5 (d), after a 5-min equilibration at 37°C. (B) plot of the fluorescence obtained at 350 nm from panel A as a function of pH, and expressed as a percentage of the initial value at pH 7.4.
Taken together, the data indicate that arbidol has tropism for membrane-like environments, and that this propensity to interact with hydrophobic environments is more pronounced at acidic than at neutral pH, possibly due to the ionization state of ARB.
Arbidol has tropism for lipid membranes
The interaction of ARB with liposomes was then studied at neutral and acidic pH. Results are presented in Fig.8 and Table 1. In the presence of increasing concentrations of PC:chol liposomes, λmax is progressively shifted to lower wavelengths (blue shift), with a concomitant increase in quantum yield (Table 1). These changes most likely result from a significant increase in the hydrophobicity of the ARB microenvironment upon binding to liposomes. Moreover fluorescence emission depends upon phospholipid concentration (compare curves a to c or d for each panel, Fig.8), which indicates that the ARB microenvironment becomes more hydrophobic in the presence of liposomes, and therefore points toward the interaction of ARB with lipid membranes. This trend is even more pronounced at acidic pH (Fig.8B, Table 1). Indeed larger blue shifts (up to ∼ 20 nm), were associated with greater increases in quantum yield at pH 5.0 as compared to pH 7.4, which may reflect enhanced incorporation of the ARB molecule into the hydrophobic core of lipid bilayers at low pH. This extends our previous observations that ARB inhibits fusion more prominently at acidic pH than at neutral pH in the pH-independent Ca-induced PS vesicle fusion model. In addition, for similar pH and ARB concentration of 10 μg/ml, much larger blue shifts are observed with liposomes than with any detergent micelles (Table 1). This indicates that the environment of ARB is significantly more hydrophobic in lipid bilayers than in micelles, i.e. that ARB reaches the hydrophobic core of the lipid bilayer whereas it is most likely associated shallowly to the surface of detergent micelles. This is consistent with the view that the fluorescent indole moiety of ARB is partially buried in the liposome membranes.
Figure 8. Influence of liposomes on ARB fluorescence.
(A) Neutral pH. PC:chol (70:30 molar ratio) liposomes were added at 12.5 μM (a), 25 μM (b), 62.5 μM (c) or 100 μM (d), to a 18.8 μM (10 μg/ml) ARB solution in PBS pH 7.4 (dotted curve). Emission spectra were recorded after 5 min of incubation, and corrected for blank (buffer) and liposome scattering effects. (B) Acidic pH. ARB solution (10 μg/ml) in PBS buffer pH 7.4 was acidified with HCl to pH 5.0 final reading (dotted curve), and PC:chol liposomes were subsequently added [final lipid concentration 12.5 μM (a), 25 μM (b) or 62.5 μM (c)].
Taken together, the data provide direct evidence that ARB incorporates into artificial lipid bilayers (liposomes), as well as biological membranes such as cellular and viral membranes, indicating that ARB inhibits HCV entry into cells and HCV-mediated fusion by acting directly at the membrane level.
Discussion
This study aimed at assessing the anti-HCV properties of arbidol, a broad-spectrum Russian-made oral antiviral drug. Using HCV pseudoparticles, we analysed the effects of ARB on HCV entry at the fusion step and demonstrated that ARB is able to inhibit this process in a dose-dependent manner.
HCV entry and membrane fusion are early steps in the lifecycle of the virus (29, 30). HCV first interacts through its envelope glycoproteins with a set of co-receptors at the plasma membrane level [reviewed in (31)], and eventually becomes endocytosed (32, 33). Due to a combined action of acidification in the endosome and particular lipids like cholesterol (16), viral fusion occurs over a broad spectrum of pH ranging from 6.3 to 5.0 (16, 24). This suggests that fusion could occur in early as well as in late endosomes, or at least that a cascade of reorganizations could occur in the fusion protein(s) during the progression of the virus in the endocytic pathway. In this respect, the inhibitory action of ARB on HCVpp entry and fusion could be envisaged at various levels including the viral glycoprotein, membrane, or the endosomal lumen.
Viral infectivity studies with HCVpp showed that ARB exerted its inhibitory effect only when it was present throughout the course of infection, and that an almost complete inhibition was achieved when the cells were preincubated with ARB (Fig.2). Similarly, ARB inhibition of primary infection of Huh7.5.1 cells with JFH-1 virus was complete only when cells were preincubated with ARB 24 or 48h before infection (12). This points to an action of ARB on cell membranes, and suggests that a certain level of membrane impregnation/saturation with ARB must be achieved in order to efficiently block HCV infection. HCVpp-1a-mediated in vitro fusion was completely blocked at low ARB concentration (1 μg/ml; Fig.3). Fusion was inhibited similarly when viral envelope membranes or target (liposome) membranes were preincubated with ARB, indicating again an effect on membranes, although a specific effect of ARB on HCV glycoproteins themselves cannot be excluded. From a physico-chemical point of view, ARB displays tropism for membranes or membrane-like environments such as detergent micelles, particularly prominent at low pH (Fig.1, 5-8, Table 1). This may be due to ARB's indole-derived structure (Fig.1). Moreover ARB may interact with lipids via other hydrophobic and aromatic moieties on the molecule.
Concerning detergents, ARB bound similarly at acidic pH to the micelles of all three detergents tested, binding being total at 10 μM α-lysoPC (C16:0), or almost total at 1 mM SDS and at 2 mM DM. Under these conditions most of the detergent is in a micellar form, since the cmc values for C16:0 α-lysoPC, SDS and DM are ∼ 6 μM (34), 1.2 mM and 0.18 mM (28), respectively. The enhancement of ARB fluorescence is greater upon binding to DM micelles than upon binding to other detergent micelles or lipid vesicles (Table 1). The maltose headgroup of DM is rigid, giving a high cohesion to the headgroup region of the micelles (35). These maltose rings might therefore exert a stacking effect with the indole rings of ARB, leading to the observed dramatic increase in ARB fluorescence. However we consistently observed a lack of shift toward lower wavelengths (blue shift) in ARB fluorescence with DM micelles. At low pH this could come from a shielding effect on the protonation of ARB, played by the numerous hydroxyl groups of DM.
Concerning lipid vesicles, ARB membrane association was accompanied by a blue shift and an increase in quantum yield of fluorescence for all ARB-to-lipid ratios tested, particularly prominent at low pH (Fig.8, Table 1). When comparing these data to those obtained with detergent micelles, we can infer that ARB probably embeds itself more deeply into lipid bilayers than in micelles, and is probably in a more hydrophobic microenvironment in bilayers than in micelles. This difference in fluorescence behaviour between lipid bilayers and detergent micelles most likely comes from the very low cmc of lipids in aqueous buffers, where all lipids are in the condensed membrane phase. Compared to DM micelles more specifically, the flexible choline headgroups of PC might allow a better accessibility to the hydrophobic core of the particle (vesicle or micelle) than the rigid sugar headgroup of DM, as already described for the indole derivative tryptophan octyl ester (36).
Indole derivatives have been shown to exhibit a preference for membrane interfaces (25, 26), due to the flat rigid structure of these molecules, and to their aromaticity which allows them to establish cation-π interactions with the positively-charged quaternary ammonium lipid headgroups (25, 27). The S-phenyl groups of ARB could also interact with the hydrophobic fatty acid chains of phospholipids inside the bilayer. The amino groups could bond the phosphate moieties of phospholipids, and establish a salt bridge between two adjacent phospholipid molecules as an ion-pair complex. At low pH these interactions would be favoured due to the protonation of the amino groups. Particularly protonation of the 3-position could displace the ester group out of the indole plane and place it in a better position to bond with neighbouring molecules (see Fig.1D). This could in turn lead to a better membrane association. Considering these chemically plausible interactions, ARB might have the propensity to intercalate into lipids in the membrane while adopting a consistent orientation by filling the gaps between lipid molecules. The molar ratio between ARB and lipids in our in vitro fusion assays is ca. 1:10, so a plausible mode of action could be the formation of a stable and dose-dependent “ARB cage” at the surface of membranes, and therefore to excessive stabilization of these membranes, which become resistant to fusion. This mode of action would be quite reminiscent to that observed for the tripeptide inhibiting fusion, carbobenzoxy-D-phenylalanyl-L-phenylalanylglycine Z-fFG. Z-fFG was shown to inhibit viral fusion by stabilizing bilayers in their lamellar phase, thereby inhibiting non-bilayer phase formation most likely from the surface of the bilayer, i.e. not by forming a tight complex with phospholipids (37-39). The carbobenzoxy group is important for the effectiveness of fusion inhibition (38); it is interesting to note that ARB contains a phenyl-thio-methyl group in position 2 of the indole ring, which could play a comparable role as that of the carbobenzoxy of Z-fFG in the fusion inhibition. Z-fFG inhibition of the formation of highly curved surfaces, occurring during membrane fusion, was found to be more effective at low pH where the protonated form of the tripeptide is predominant (39). This correlates well with ARB behaviour as observed in our study, where the protonated form might as well penetrate deeper into the membrane than the charged form.
This hypothesis of membrane stabilization as a mode of action of ARB in the viral fusion system finds support from the PS/Ca model system, since ARB also blocks fusion in this membrane system, and again more efficiently at low than at neutral pH (Fig.3). The PS/Ca model also brings the information that ARB can inhibit fusion at neutral pH, which could be an explanation for the ARB inhibition of fusion of pH-independent viruses such as some paramyxoviruses and coronaviruses (8).
At the virus level, ARB might block the uncoating of the membrane during the fusion process. From our infection and fusion data we cannot exclude a direct action of ARB on endosomal pH and/or on HCV glycoproteins. Based upon its chemical structure, ARB is a weak base that could undergo hydrolysis of its ester function in vivo. For these reasons, ARB could exert its effect on HCVpp infection and fusion through inhibition of the acidification in the endosomes, and/or through accumulation of hydrolysed ARB molecules. The first hypothesis was tested directly by measuring the pH of a liposome/HCVpp suspension in the conditions of our fusion assay. Even at very high ARB concentrations, we could not detect any significant variation in pH. A behaviour of ARB as an ionophore or as a blocker of proton pumps inside the endosome cannot be excluded in vivo. However if this effect were operative, it would unlikely be the predominant mechanism of action of the molecule, since ARB inhibits pH-dependent and pH-independent viruses with a similar efficacy (8), and we showed in this study that ARB could block fusion in the pH-independent Ca-induced PS vesicle fusion system. The second hypothesis of ARB hydrolysis in vivo followed by intracellular accumulation of the cleaved compound finds support from our results that ARB displayed optimal activity when administered 24-48 hours before primary HCV infection (12), or preincubated 3 hours with cells before HCVpp infection (this study).
We also reproducibly noticed that ARB inhibition of cell entry and/or fusion concerned HCVpp and HApp, but not RD114pp (Fig.2-3). This suggests that ARB might display specificity for the recognition of key motifs inside the fusion protein, leading to inhibition of the properties of this protein. Furthermore HCVpp fusion was more pronounced on genotype 1a-derived than on genotypes 1b and 2a-derived pseudoparticles (Fig.3). Since different HCV genotypes possess sequence variability, it is conceivable that ARB might differently affect fusion induced by genetically different glycoproteins. In our fusion assay, liposomes are devoid of HCV receptors; therefore subtle changes in envelope protein conformation between genotypes might affect fusion more than infectivity, since receptors could help in conformational rearrangements at the plasma membrane of cells. Since ARB is most likely able to enter into a number of chemical interactions with lipid molecules, these interactions might exist for ARB / HCV glycoprotein(s) as well. Hydrophobic interactions, formation of salt bridges, cation-π interactions govern the binding of several ligands to proteins [e.g. (27)]. This hypothesis cannot be tested experimentally at present in the absence of any 3D-structure of HCV E1 and E2. However, further studies using the recently described genotype 1a (40) and chimeric (41) infectious culture systems may provide further insight into the mechanisms accounting for the possible genotype-specific effects of ARB.
Arbidol also exerted a clear inhibitory effect on HCV replication, with loss of protein expression and decline in RNA level (12). This effect was visible after 2 weeks of treatment of cells containing an HCV-1b replicon, and no rebound effect was observed after removal of the drug (12). Since this replicon did not produce infectious particles, the antiviral effects of ARB in this system cannot involve inhibition of virus fusion and infectivity. The HCV replication complex associates with endoplasmic reticulum (ER) membranes to form membranous webs (42). The web is formed via the association of HCV non-structural proteins with ER membranes (43, 44). One can therefore speculate that ARB-induced inhibition of HCV non-structural protein interactions with organelle membranes that are required for HCV replication might also contribute to ARB's anti HCV actions.
Current interferon-based anti-HCV therapies have shown their limits, since about 40 to 50% of patients do not achieve sustained viral eradication with IFN treatment (45). A number of antiviral strategies against HCV are presently being developed, some of which are directed against viral enzymes or viral genome, or aimed at modulating the host immune response (46). Recently two groups described the anti-HCV replication activity of small molecules that perturb the intracellular lipid metabolism. Sakamoto and coworkers (47) described the activity of NA255, which prevents the de novo synthesis of sphingolipids, and thus disrupts the association of HCV nonstructural proteins on the lipid rafts. Pezacki's group studied the anti HCV effect of an antagonist of the peroxisome proliferator-activated receptor (PPAR) (48). Inhibition of PPAR led to HCV inhibition of replication through disregulation of lipid metabolism, phospholipid secretion, cholesterol catabolism and triglyceride clearance.
Thus, lipids and membranes are clearly central to the HCV lifecycle, where each step is directly related to membrane activity. Arbidol, by its tropism for membranes and its inhibitory effect on HCV entry, fusion and replication, opens promising perspectives in the search for new and efficient anti-HCV therapies.
Acknowledgments
We gratefully acknowledge Dr François Penin (IBCP, Lyon, France) for continuous support, critical reading and helpful discussions at several stages of this manuscript. E.I.P. thanks Elodie Teissier for technical assistance. Fluorescence measurements were performed on the facility “Production et Analyse physico-chimique des Protéines” (PAP) of the IFR128 BioSciences Lyon Gerland (http://www.ifr128.prd.fr/ifr128.htm).
Abbreviations
- ARB
arbidol
- chol
cholesterol
- cmc
critical micellar concentration
- DM
dodecyl maltoside
- HA
influenza hemagglutinin
- HCV
hepatitis C virus
- HCVpp
HCV pseudoparticles
- PC
phosphatidylcholine
- α-lysoPC
L-α-palmitoyl-lysophosphatidylcholine
- PS
phosphatidylserine
- R18
octadecyl rhodamine B chloride
- RD114
feline endogenous oncoretrovirus RD114
- SDS
sodium dodecyl sulfate
Footnotes
This work was supported by the ANRS (Agence Nationale de Recherches sur le SIDA et les hepatites virales) to E.I.P., by the French CNRS and INSERM, by LSHB-CT-2004-005246 (COMPUVAC) to F.L.C., by the Ligue Nationale Contre le Cancer and the Rhône-Alpes Region. Y.S.B. was partially supported by the Fulbright Visiting Scholar Program. S.J.P. is supported by NIH grants RO1 DK62187 and U19 A1066328.
References
- 1.Di Bisceglie AM, Fan X, Chambers T, Strinko J. Pharmacokinetics, pharmacodynamics, and hepatitis C viral kinetics during antiviral therapy: the null responder. J Med Virol. 2006;78:446–451. doi: 10.1002/jmv.20560. [DOI] [PubMed] [Google Scholar]
- 2.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 3.Horscroft N, Lai VC, Cheney W, Yao N, Wu JZ, Hong Z, Zhong W. Replicon cell culture system as a valuable tool in antiviral drug discovery against hepatitis C virus. Antivir Chem Chemother. 2005;16:1–12. doi: 10.1177/095632020501600101. [DOI] [PubMed] [Google Scholar]
- 4.Paeshuyse J, Kaul A, De Clercq E, Rosenwirth B, Dumont JM, Scalfaro P, Bartenschlager R, Neyts J. The non-immunosuppressive cyclosporin DEBIO-025 is a potent inhibitor of hepatitis C virus replication in vitro. Hepatology. 2006;43:761–770. doi: 10.1002/hep.21102. [DOI] [PubMed] [Google Scholar]
- 5.Duong FH, Christen V, Filipowicz M, Heim MH. S-Adenosylmethionine and betaine correct hepatitis C virus induced inhibition of interferon signaling in vitro. Hepatology. 2006;43:796–806. doi: 10.1002/hep.21116. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous. Arbidol. Drugs R&D. 1999;2:171–172. doi: 10.2165/00126839-199902030-00003. [DOI] [PubMed] [Google Scholar]
- 7.Gagarinova VM, Ignat'eva GS, Sinitskaia LV, Ivanova AM, Rodina MA, Tur'eva AV. The new chemical preparation arbidol: its prophylactic efficacy during influenza epidemics. Zh Mikrobiol Epidemiol Immunobiol. 1993:40–43. [PubMed] [Google Scholar]
- 8.Brooks MJ, Sasadeusz JJ, Tannock GA. Antiviral chemotherapeutic agents against respiratory viruses: where are we now and what's in the pipeline? Curr Opin Pulm Med. 2004;10:197–203. doi: 10.1097/00063198-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Chai H, Zhao Y, Zhao C, Gong P. Synthesis and in vitro anti-hepatitis B virus activities of some ethyl 6-bromo-5-hydroxy-1H-indole-3-carboxylates. Bioorg Med Chem. 2006;14:911–917. doi: 10.1016/j.bmc.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 10.Fediakina IT, Leneva IA, Iamnikova SS, Livov DK, Glushkov RG, Shuster AM. Sensitivity of influenza A/H5 viruses isolated from wild birds on the territory of Russia to arbidol in the cultured MDCK cells. Vopr Virusol. 2005;50:32–35. [PubMed] [Google Scholar]
- 11.Leneva IA, Fediakina IT, Gus'kova TA, Glushkov RG. Sensitivity of various influenza virus strains to arbidol. Influence of arbidol combination with different antiviral drugs on reproduction of influenza virus A. Ter Arkh. 2005;77:84–88. [PubMed] [Google Scholar]
- 12.Boriskin YS, Pécheur EI, Polyak SJ. Arbidol: a broad-spectrum antiviral that inhibits acute and chronic HCV infection. Virol J. 2006;3:56. doi: 10.1186/1743-422X-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med. 2003;197:633–642. doi: 10.1084/jem.20021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatziioannou T, Valsesia-Wittmann S, Russell SJ, Cosset FL. Incorporation of fowl plague virus hemagglutinin into murine leukemia virus particles and analysis of the infectivity of the pseudotyped retroviruses. J Virol. 1998;72:5313–5317. doi: 10.1128/jvi.72.6.5313-5317.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandrin V, Boson B, Salmon P, Gay W, Negre D, Le Grand R, Trono D, Cosset FL. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood. 2002;100:823–832. doi: 10.1182/blood-2001-11-0042. [DOI] [PubMed] [Google Scholar]
- 16.Lavillette D, Bartosch B, Nourrisson D, Verney G, Cosset FL, Penin F, Pécheur EI. Hepatitis C Virus Glycoproteins Mediate Low pH-dependent Membrane Fusion with Liposomes. J Biol Chem. 2006;281:3909–3917. doi: 10.1074/jbc.M509747200. [DOI] [PubMed] [Google Scholar]
- 17.Ladokhin AS, Jayasinghe S, White SH. How to measure and analyze tryptophan fluorescence in membranes properly, and why bother? Anal Biochem. 2000;285:235–245. doi: 10.1006/abio.2000.4773. [DOI] [PubMed] [Google Scholar]
- 18.Pécheur EI, Martin I, Ruysschaert JM, Bienvenue A, Hoekstra D. Membrane fusion induced by 11-mer anionic and cationic peptides: a structure-function study. Biochemistry. 1998;37:2361–2371. doi: 10.1021/bi972697f. [DOI] [PubMed] [Google Scholar]
- 19.Wilschut J, Papahadjopoulos D. Ca2+-induced fusion of phospholipid vesicles monitored by mixing of aqueous contents. Nature. 1979;281:690–692. doi: 10.1038/281690a0. [DOI] [PubMed] [Google Scholar]
- 20.Breisblatt W, Ohki S. Fusion in phospholipid spherical membranes. II. Effect of cholesterol, divalent ions and pH. J Membr Biol. 1976;29:127–146. doi: 10.1007/BF01868956. [DOI] [PubMed] [Google Scholar]
- 21.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinman RL, Lang J. The Protonation of Indoles. Basicity Studies. The Dependence of Acidity Functions on Indicator Structure. Journal of the American Chemical Society. 1964;86:3796–3806. [Google Scholar]
- 23.Stegmann T, Hoekstra D, Scherphof G, Wilschut J. Kinetics of pH-dependent fusion between influenza virus and liposomes. Biochemistry. 1985;24:3107–3113. doi: 10.1021/bi00334a006. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi M, Bennett MC, Bercot T, Singh IR. Functional analysis of hepatitis C virus envelope proteins, using a cell-cell fusion assay. J Virol. 2006;80:1817–1825. doi: 10.1128/JVI.80.4.1817-1825.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen FN, Jensen MO, Nielsen CH. Interfacial tryptophan residues: a role for the cation-pi effect? Biophys J. 2005;89:3985–3996. doi: 10.1529/biophysj.105.061804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yau WM, Wimley WC, Gawrisch K, White SH. The preference of tryptophan for membrane interfaces. Biochemistry. 1998;37:14713–14718. doi: 10.1021/bi980809c. [DOI] [PubMed] [Google Scholar]
- 27.Zacharias N, Dougherty DA. Cation-pi interactions in ligand recognition and catalysis. Trends Pharmacol Sci. 2002;23:281–287. doi: 10.1016/s0165-6147(02)02027-8. [DOI] [PubMed] [Google Scholar]
- 28.le Maire M, Champeil P, Moller JV. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim Biophys Acta. 2000;1508:86–111. doi: 10.1016/s0304-4157(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 29.Bartenschlager R, Lohmann V. Replication of hepatitis C virus. J Gen Virol. 2000;81:1631–1648. doi: 10.1099/0022-1317-81-7-1631. [DOI] [PubMed] [Google Scholar]
- 30.Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- 31.Bartosch B, Cosset FL. Cell entry of hepatitis C virus. Virology. 2006;348:1–12. doi: 10.1016/j.virol.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Bartosch B, Vitelli A, Granier C, Goujon C, Dubuisson J, Pascale S, Scarselli E, Cortese R, Nicosia A, Cosset FL. Cell Entry of Hepatitis C Virus Requires a Set of Co-receptors That Include the CD81 Tetraspanin and the SR-B1 Scavenger Receptor. J Biol Chem. 2003;278:41624–41630. doi: 10.1074/jbc.M305289200. [DOI] [PubMed] [Google Scholar]
- 33.Tscherne DM, Jones CT, Evans MJ, Lindenbach BD, McKeating JA, Rice CM. Time- and temperature-dependent activation of hepatitis C virus for low-pH-triggered entry. J Virol. 2006;80:1734–1741. doi: 10.1128/JVI.80.4.1734-1741.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar VV, Baumann WJ. Lanthanide-induced phosphorus-31 NMR downfield chemical shifts of lysophosphatidylcholines are sensitive to lysophospholipid critical micelle concentration. Biophys J. 1991;59:103–107. doi: 10.1016/S0006-3495(91)82202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tortech L, Jaxel C, Vincent M, Gallay J, de Foresta B. The polar headgroup of the detergent governs the accessibility to water of tryptophan octyl ester in host micelles. Biochim Biophys Acta. 2001;1514:76–86. doi: 10.1016/s0005-2736(01)00370-4. [DOI] [PubMed] [Google Scholar]
- 36.de Foresta B, Gallay J, Sopkova J, Champeil P, Vincent M. Tryptophan octyl ester in detergent micelles of dodecylmaltoside: fluorescence properties and quenching by brominated detergent analogs. Biophys J. 1999;77:3071–3084. doi: 10.1016/S0006-3495(99)77138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeagle PL, Dentino AR, Smith FT, Spooner P, Watts A. The antiviral peptide carbobenzoxy-D-phenylalanyl-L-phenylalanylglycine changes the average conformation of phospholipids in membranes. Biochemistry. 1993;32:12197–12202. doi: 10.1021/bi00096a032. [DOI] [PubMed] [Google Scholar]
- 38.Epand RM, Epand RF, Richardson CD, Yeagle PL. Structural requirements for the inhibition of membrane fusion by carbobenzoxy-D-Phe-Phe-Gly. Biochim Biophys Acta. 1993;1152:128–134. doi: 10.1016/0005-2736(93)90239-v. [DOI] [PubMed] [Google Scholar]
- 39.Dentino AR, Westerman PW, Yeagle PL. A study of carbobenzoxy-D-phenylalanine-L-phenylalanine-glycine, an inhibitor of membrane fusion, in phospholipids bilayers with multinuclear magnetic resonance. Biochim Biophys Acta. 1995;1235:213–220. doi: 10.1016/0005-2736(95)80007-3. [DOI] [PubMed] [Google Scholar]
- 40.Yi M, Villanueva RA, Thomas DL, Wakita T, Lemon SM. Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proc Natl Acad Sci U S A. 2006;103:2310–2315. doi: 10.1073/pnas.0510727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, Steinmann E, Abid K, Negro F, Dreux M, Cosset FL, Bartenschlager R. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc Natl Acad Sci U S A. 2006;103:7408–7413. doi: 10.1073/pnas.0504877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moradpour D, Gosert R, Egger D, Penin F, Blum HE, Bienz K. Membrane association of hepatitis C virus nonstructural proteins and identification of the membrane alteration that harbors the viral replication complex. Antiviral Res. 2003;60:103–109. doi: 10.1016/j.antiviral.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Appel N, Schaller T, Penin F, Bartenschlager R. From structure to function: new insights into hepatitis C virus RNA replication. J Biol Chem. 2006;281:9833–9836. doi: 10.1074/jbc.R500026200. [DOI] [PubMed] [Google Scholar]
- 44.Gretton SN, Taylor AI, McLauchlan J. Mobility of the hepatitis C virus NS4B protein on the endoplasmic reticulum membrane and membrane-associated foci. J Gen Virol. 2005;86:1415–1421. doi: 10.1099/vir.0.80768-0. [DOI] [PubMed] [Google Scholar]
- 45.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 46.De Francesco R, Migliaccio G. Challenges and successes in developing new therapies for hepatitis C. Nature. 2005;436:953–960. doi: 10.1038/nature04080. [DOI] [PubMed] [Google Scholar]
- 47.Sakamoto H, Okamoto K, Aoki M, Kato H, Katsume A, Ohta A, Tsukuda T, Shimma N, Aoki Y, Arisawa M, Kohara M, Sudoh M. Host sphingolipid biosynthesis as a target for hepatitis C virus therapy. Nat Chem Biol. 2005;1:333–337. doi: 10.1038/nchembio742. [DOI] [PubMed] [Google Scholar]
- 48.Rakic B, Sagan SM, Noestheden M, Belanger S, Nan X, Evans CL, Xie XS, Pezacki JP. Peroxisome proliferator-activated receptor alpha antagonism inhibits hepatitis C virus replication. Chem Biol. 2006;13:23–30. doi: 10.1016/j.chembiol.2005.10.006. [DOI] [PubMed] [Google Scholar]








