Abstract
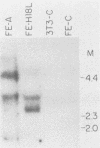
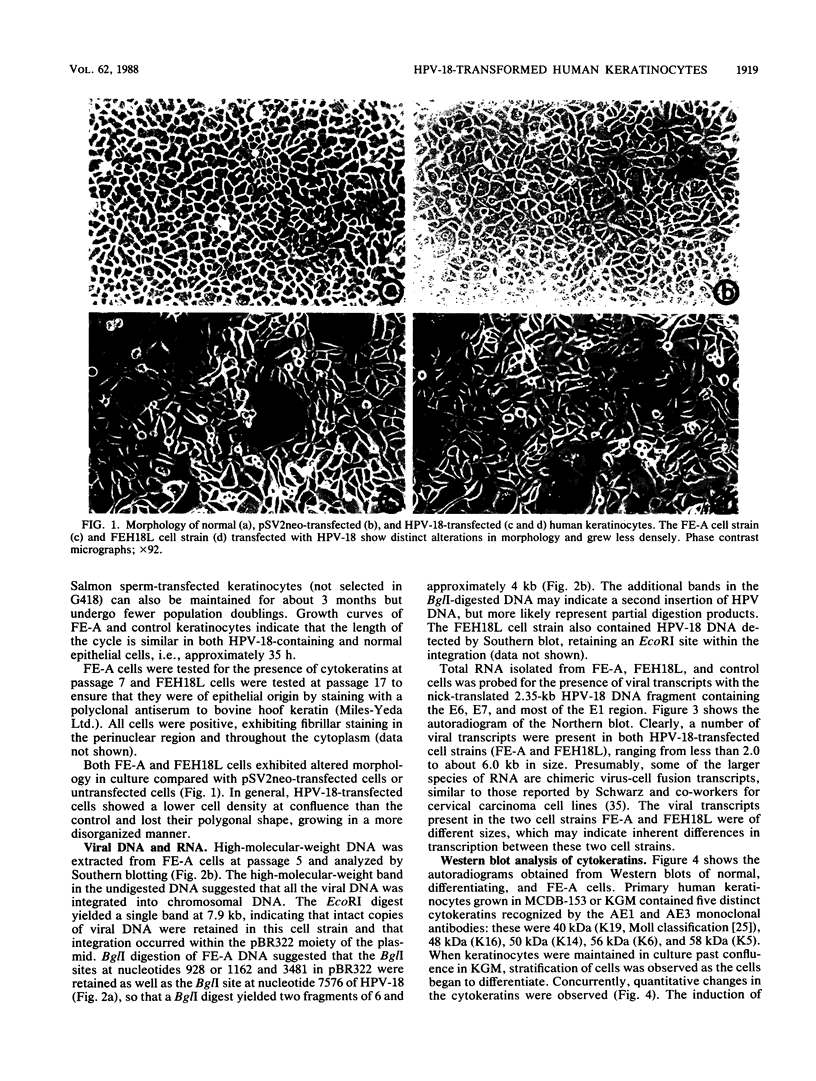
Primary human epithelial cells were cotransfected with pHPV-18 and pSV2neo, and cell strains were generated by selecting in G418. One cell strain (FE-A), which exhibits an extended life span, is currently in its 30th passage. In comparison, control cultures can only be maintained up to the seventh passage. Southern blot analysis revealed the presence of at least one intact, integrated viral genome in these cells. FE-A cells showed altered growth properties, characterized by a change in morphology, and clonal density. Differentiation markers analyzed by Western blotting (immunoblotting), such as cytokeratins and involucrin, indicated that the cells resembled a partially differentiated epithelial population. Increased expression of the 40-kilodalton cytokeratin was observed in FE-A cells, similar to that observed in simian virus 40-immortalized human keratinocytes (M. Steinberg and V. Defendi, J. Cell Physiol. 123:117-125, 1985). FE-A cells were also found to be defective in their response to terminal differentiation stimuli. Calcium and 12-O-tetradecanoyl-phorbol-13-acetate treatment induced normal epithelial cells to differentiate, whereas the human papillomavirus 18 (HPV-18)-containing keratinocytes were resistant to these signals, indicating their partially transformed nature. These cells were not able to induce tumors in nude mice over a period of up to 8 months. A second cell strain, FE-H18L, also generated by transfecting HPV-18, also exhibited an extended life span and similar alterations in morphology. Viral RNA transcribed from the early region of HPV-18 was detected in both cell strains by Northern (RNA) blot analysis. These cell strains should provide a useful model for determining the role of HPV in carcinogenesis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bobrow L. G., Makin C. A., Law S., Bodmer W. F. Expression of low molecular weight cytokeratin proteins in cervical neoplasia. J Pathol. 1986 Feb;148(2):135–140. doi: 10.1002/path.1711480203. [DOI] [PubMed] [Google Scholar]
- Boshart M., Gissmann L., Ikenberg H., Kleinheinz A., Scheurlen W., zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984 May;3(5):1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshart M., zur Hausen H. Human papillomaviruses in Buschke-Löwenstein tumors: physical state of the DNA and identification of a tandem duplication in the noncoding region of a human papillomavirus 6 subtype. J Virol. 1986 Jun;58(3):963–966. doi: 10.1128/jvi.58.3.963-966.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce S. T., Ham R. G. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983 Jul;81(1 Suppl):33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dürst M., Dzarlieva-Petrusevska R. T., Boukamp P., Fusenig N. E., Gissmann L. Molecular and cytogenetic analysis of immortalized human primary keratinocytes obtained after transfection with human papillomavirus type 16 DNA. Oncogene. 1987;1(3):251–256. [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Kleinheinz A., Hotz M., Gissmann L. The physical state of human papillomavirus type 16 DNA in benign and malignant genital tumours. J Gen Virol. 1985 Jul;66(Pt 7):1515–1522. doi: 10.1099/0022-1317-66-7-1515. [DOI] [PubMed] [Google Scholar]
- Gissmann L., Boshart M., Dürst M., Ikenberg H., Wagner D., zur Hausen H. Presence of human papillomavirus in genital tumors. J Invest Dermatol. 1984 Jul;83(1 Suppl):26s–28s. doi: 10.1111/1523-1747.ep12281143. [DOI] [PubMed] [Google Scholar]
- Gissmann L., Wolnik L., Ikenberg H., Koldovsky U., Schnürch H. G., zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983 Jan;80(2):560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissmann L., deVilliers E. M., zur Hausen H. Analysis of human genital warts (condylomata acuminata) and other genital tumors for human papillomavirus type 6 DNA. Int J Cancer. 1982 Feb 15;29(2):143–146. doi: 10.1002/ijc.2910290205. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Hronis T. S., Steinberg M. L., Defendi V., Sun T. T. Simple epithelial nature of some simian virus-40-transformed human epidermal keratinocytes. Cancer Res. 1984 Dec;44(12 Pt 1):5797–5804. [PubMed] [Google Scholar]
- Kulesz-Martin M. F., Koehler B., Hennings H., Yuspa S. H. Quantitative assay for carcinogen altered differentiation in mouse epidermal cells. Carcinogenesis. 1980;1(12):995–1006. doi: 10.1093/carcin/1.12.995. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C. Animal papillomaviruses. Microbiol Rev. 1982 Jun;46(2):191–207. doi: 10.1128/mr.46.2.191-207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner J. F., McClendon I. A., LaVeck M. A., Shamsuddin A. M., Harris C. C. Differential control by platelet factors of squamous differentiation in normal and malignant human bronchial epithelial cells. Cancer Res. 1983 Dec;43(12 Pt 1):5915–5921. [PubMed] [Google Scholar]
- Masui T., Lechner J. F., Yoakum G. H., Willey J. C., Harris C. C. Growth and differentiation of normal and transformed human bronchial epithelial cells. J Cell Physiol Suppl. 1986;4:73–81. doi: 10.1002/jcp.1041290414. [DOI] [PubMed] [Google Scholar]
- McCance D. J. Human papillomaviruses and cancer. Biochim Biophys Acta. 1986;823(3):195–205. doi: 10.1016/0304-419x(86)90002-8. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Parkinson E. K. Defective responses of transformed keratinocytes to terminal differentiation stimuli. Their role in epidermal tumour promotion by phorbol esters and by deep skin wounding. Br J Cancer. 1985 Oct;52(4):479–493. doi: 10.1038/bjc.1985.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson E. K., Emmerson A. Non-promoting hyperplasiogenic agents do not mimic the effects of phorbol, 12-myristate, 13-acetate on terminal differentiation of normal and transformed human keratinocytes. Carcinogenesis. 1984 May;5(5):687–690. doi: 10.1093/carcin/5.5.687. [DOI] [PubMed] [Google Scholar]
- Parkinson E. K., Emmerson A. The effects of tumour promoters on the multiplication and morphology of cultured human epidermal keratinocytes. Carcinogenesis. 1982;3(5):525–531. doi: 10.1093/carcin/3.5.525. [DOI] [PubMed] [Google Scholar]
- Parkinson E. K., Grabham P., Emmerson A. A subpopulation of cultured human keratinocytes which is resistant to the induction of terminal differentiation-related changes by phorbol, 12-myristate, 13-acetate: evidence for an increase in the resistant population following transformation. Carcinogenesis. 1983;4(7):857–861. doi: 10.1093/carcin/4.7.857. [DOI] [PubMed] [Google Scholar]
- Pfister H. Biology and biochemistry of papillomaviruses. Rev Physiol Biochem Pharmacol. 1984;99:111–181. doi: 10.1007/BFb0027716. [DOI] [PubMed] [Google Scholar]
- Pfister H. Human papillomaviruses and genital cancer. Adv Cancer Res. 1987;48:113–147. doi: 10.1016/s0065-230x(08)60691-0. [DOI] [PubMed] [Google Scholar]
- Pirisi L., Yasumoto S., Feller M., Doniger J., DiPaolo J. A. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987 Apr;61(4):1061–1066. doi: 10.1128/jvi.61.4.1061-1066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald J. G., Beckett M. A. Defective terminal differentiation in culture as a consistent and selectable character of malignant human keratinocytes. Cell. 1980 Nov;22(2 Pt 2):629–632. doi: 10.1016/0092-8674(80)90373-6. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Shirasawa H., Tomita Y., Kubota K., Kasai T., Sekiya S., Takamizawa H., Simizu B. Detection of human papillomavirus type 16 DNA and evidence for integration into the cell DNA in cervical dysplasia. J Gen Virol. 1986 Sep;67(Pt 9):2011–2015. doi: 10.1099/0022-1317-67-9-2011. [DOI] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staquet M. J., Viac J., Thivolet J. Keratin polypeptide modifications induced by human papilloma viruses (HPV). Arch Dermatol Res. 1981;271(1):83–90. doi: 10.1007/BF00417391. [DOI] [PubMed] [Google Scholar]
- Steinberg M. L., Defendi V. Transformation and immortalization of human keratinocytes by SV40. J Invest Dermatol. 1983 Jul;81(1 Suppl):131s–136s. doi: 10.1111/1523-1747.ep12540905. [DOI] [PubMed] [Google Scholar]
- Tseng S. C., Jarvinen M. J., Nelson W. G., Huang J. W., Woodcock-Mitchell J., Sun T. T. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982 Sep;30(2):361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- Watts S. L., Phelps W. C., Ostrow R. S., Zachow K. R., Faras A. J. Cellular transformation by human papillomavirus DNA in vitro. Science. 1984 Aug 10;225(4662):634–636. doi: 10.1126/science.6330900. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Eichner R., Sun T. T. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984 Apr;98(4):1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey J. C., Moser C. E., Jr, Lechner J. F., Harris C. C. Differential effects of 12-O-tetradecanoylphorbol-13-acetate on cultured normal and neoplastic human bronchial epithelial cells. Cancer Res. 1984 Nov;44(11):5124–5126. [PubMed] [Google Scholar]
- Wu Y. J., Rheinwald J. G. A new small (40 kd) keratin filament protein made by some cultured human squamous cell carcinomas. Cell. 1981 Sep;25(3):627–635. doi: 10.1016/0092-8674(81)90170-7. [DOI] [PubMed] [Google Scholar]
- Yasumoto S., Burkhardt A. L., Doniger J., DiPaolo J. A. Human papillomavirus type 16 DNA-induced malignant transformation of NIH 3T3 cells. J Virol. 1986 Feb;57(2):572–577. doi: 10.1128/jvi.57.2.572-577.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C., Krishnan-Hewlett I., Baker C. C., Schlegel R., Howley P. M. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am J Pathol. 1985 Jun;119(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- Yoakum G. H., Lechner J. F., Gabrielson E. W., Korba B. E., Malan-Shibley L., Willey J. C., Valerio M. G., Shamsuddin A. M., Trump B. F., Harris C. C. Transformation of human bronchial epithelial cells transfected by Harvey ras oncogene. Science. 1985 Mar 8;227(4691):1174–1179. doi: 10.1126/science.3975607. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Ben T., Hennings H., Lichti U. Divergent responses in epidermal basal cells exposed to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1982 Jun;42(6):2344–2349. [PubMed] [Google Scholar]
- Yuspa S. H., Ben T., Hennings H., Lichti U. Phorbol ester tumor promoters induce epidermal transglutaminase activity. Biochem Biophys Res Commun. 1980 Nov 28;97(2):700–708. doi: 10.1016/0006-291x(80)90321-6. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Morgan D. L. Mouse skin cells resistant to terminal differentiation associated with initiation of carcinogenesis. Nature. 1981 Sep 3;293(5827):72–74. doi: 10.1038/293072a0. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Vass W., Scolnick E. Altered growth and differentiation of cultured mouse epidermal cells infected with oncogenic retrovirus: contrasting effects of viruses and chemicals. Cancer Res. 1983 Dec;43(12 Pt 1):6021–6030. [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses and their possible role in squamous cell carcinomas. Curr Top Microbiol Immunol. 1977;78:1–30. doi: 10.1007/978-3-642-66800-5_1. [DOI] [PubMed] [Google Scholar]