Abstract
A novel fluorescent substrate (termed FRET-HA) to quantitatively assess hyaluronidase activity was developed. Hyaluronan (HA), the major substrate for hyaluronidase, was dual labeled with fluorescein amine and rhodamine B amine. The fluorescein amine fluorescence signal was significantly quenched while the rhodamine B amine signal was significantly enhanced due to fluorescence resonance energy transfer (FRET). In the presence of bovine testes hyaluronidase, cleavage of HA disrupted FRET resulting in a loss of the fluorescein amine quenching that was dependent upon both enzyme concentration and time. Increase in the fluorescein amine signal could be conveniently monitored in both non-continuous and continuous fashions. The Km value for bovine testes hyaluronidase was determined using FRET-HA in a continuous fluorescent assay. Importantly, the estimated Km value for bovine testes hyaluronidase using FRET-HA as the substrate was in excellent agreement with Km values previously reported for this enzyme using native (i.e., unlabeled) HA. Therefore, FRET-HA is a reliable substrate for quantitatively assessing the HA/ hyaluronidase molecular interaction. The simplicity, sensitivity and versatility of the FRET-HA substrate suggest that it will have utility in a variety of assay platforms and should be a new tool for assessing hyaluronidase activity.
Keywords: Hyaluronan, FRET, Hyaluronidase, Continuous Assay, Non-continuous Assay
Introduction
Hyaluronan (HA), a non-sulfated glycosaminogylcan, is composed of glucuronic acid (GlcA) and N-acetylglucosamine (GlcNAc) and has the structure (-4-GlcA- β-1,3-GlcNAc- β1-)n where n is the number of repeating disaccharide subunits. Although originally considered an inert filling material HA is now known to have a number of biological activities including wound healing [1], angiogenesis [2][3] and cancer cell metastasis [4]. In many cases, HA biological function is thought to be related to its molecular size. For example, low molecular weight HA fragments stimulate endothelial cells to form tube-like structures reminiscent of angiogenic vessels. By contrast, high molecular weight HA does not stimulate endothelial tube formation [5].
The hyaluronidases are a class of enzymes that degrade HA, and to a lesser extent chondroitin and chondroitin sulfates. These enzymes have been classified based on their mode of HA degradation and consist of hydrolases, lyases and endo-β-glucuronidases. All mammalian hyaluronidases are hydrolases [6]. Despite their description and classification > 30 years ago, the hyaluronidases have generally been a neglected group of enzymes [7]. However, interest in the hyaluronidases has resurged in recent years due to their potential role in regulating HA biological function by modulating the size distribution of this molecule.
Sensitive and reliable biochemical assays to evaluate hyaluronidase activity are essential for better understanding their physicochemical properties. A number of biochemical assays have been developed for assessing hyaluronidase activity including measurements that record the changes in HA turbidity, viscosity and the colorimetric detection of reducing ends as the HA co-polymer is hydrolyzed [8]. Solid phase assays 4 including ELISA-like assays and zymography techniques have also been developed to assess hyaluronidase activity [8]. Solid phase methods and colorimetric assays are often labor intensive and do not allow for continuous data acquisition. Although turbidity and viscosity assays can be performed continuously, they require high concentrations of reagents that are not always routinely available and/ or economical. Moreover, turbidity and viscosity methods may not be reliable for quantitative analysis [7]. Thus, new methods to ascertain hyaluronidase activity simply and quantitatively are highly desirable.
Several enzymatic assays have been developed that monitor changes in fluorescent intensity during the conversion of substrate to product [9][10][11]. Fluorescence based enzymatic assays are convenient, sensitive, and reliable and often are useful for determining parameters (e.g., Vmax and Km) from kinetic data. Based on the broad utility and robustness of fluorescence based enzymatic assays we have developed a Fluorescence Resonance Energy Transfer (FRET) HA substrate (termed FRET-HA) to monitor hyaluronidase activity.
FRET is the non-radiative transfer of energy from a donor fluorophore to an acceptor fluorophore primarily due to the dipole-dipole interactions between them. The rate of energy transfer between the acceptor and donor depends on their distance apart, the orientation between their dipole transition moments and the spectral overlap between the donor emission and acceptor absorption [12]. We conceived that HA could be intra-molecularly labeled with a fluorescent donor (fluorescein amine) and a fluorescent acceptor (rhodamine B amine). During FRET, the donor fluorescence would be quenched; hyaluronidase digestion of the HA co-polymer would disrupt FRET resulting in fluorescent enhancement of the donor molecule. Although most FRET based enzymatic assays have used peptide substrates, a FRET heparin substrate has recently been reported [13]. Thus, carbohydrate FRET substrates can also be designed to study other enzymatic reactions.
Herein, we report the preparation and fluorescent properties of the FRET-HA substrate. Moreover, we describe non-continuous and continuous assays using bovine testes hyaluronidase that show the potential utility of FRET-HA to serve as a fluorescent substrate in both assay formats. Based on our results, we propose that FRET-HA is a new substrate for conveniently measuring hyaluronidase activity. Furthermore, the versatility and sensitivity of FRET-HA suggest that it can be incorporated into a number of different experimental designs for multiple applications.
Materials and methods
Materials
Sodium hyaluronate from bacterial fermentation was from Acros Organics, Inc. Fluorescein amine, rhodamine B amine, DMSO, guanidine hydrochloride, acetaldehyde, cyclohexyl isocyanide, Sephadex G-75 and bovine testes hyaluronidase (EC 3.2.1.35, Type 1-S, 451 U/mg) were all from Sigma-Aldrich. Dulbeccos PBS was from Invitrogen Life Technologies and was adjusted to pH 6.0 with 0.1 N HCl after reconstitution in dH2O. Slide-A-Lyser dialysis cassettes (10,000 molecular weight cut-off) were purchased from Pierece Chemical Co. The HA ELISA kit was from Echelon Biosciences, Inc.
Preparation of conjugated HA
HA was covalently conjugated with fluorescein amine and rhodamine B amine using the condensation reaction as described by de Belder and Wik [14]. In this reaction, the fluorescein amine and rhodamine B amine molecules were coupled to the carboxylic acid functional groups of the glucuronic acid monosaccharides. Briefly, HA was dissolved to 1.25 mg/mL in dH2O. The HA solution was diluted 1:2 in DMSO and fluorescein amine and rhodamine B amine (both pre-dissolved as DMSO stock solutions) were simultaneously added to final concentrations of 0.42 mg/mL for each of the fluorophores. Acetaldehyde and cyclohexyl isocyanide were added to 0.04% (vol/vol) and the reaction was allowed to proceed for 16 h at 25 °C. Afterwards, the solution was diluted 1:14 in ethanol:guanidine HCl (50 µL of 3 M guanidine HCl per 900 µL of 100% ethanol) and the HA allowed to precipitate overnight at −20 °C. The precipitate was then dissolved in 1 mL of dH2O followed by extensive dialysis against dH2O. Purity of the dual labeled HA conjugate was ascertained by applying the sample to a Sephadex G-75 size exclusion column and monitoring the fluorescence of the fractions using a spectrophotometer. The concentration of HA was estimated using the HA ELISA kit and the concentrations of fluorescein amine and rhodamine B amine were determined using their molar extinction coefficients in Dulbeccos PBS, pH 6.0. The extinction coefficient for fluorescein amine at 490 nm was 49,000 M−1 cm−1 and 107,034 M−1 cm−1 for rhodamine B amine at 565 nm.
Fluorescence measurements
All fluorescence measurements were acquired using a PC-1 photon counter (ISS) with Vinci software for instrument control. A 510 nm cut-off filter was included in the emission path for all experiments. Experiments were performed in a quartz cuvette (NSG Precision Cells) with a 1-cm pathlength.
We compared the fluorescent intensities of the FRET-HA substrate with HA labeled with fluorescein amine alone (i.e., FL-HA) using equal concentrations relative to the fluorescein amine (2×10−7 M). The % quenching of the FRET-HA fluorescein amine was determined from the following equation,
Where IF is the intensity of fluorescien amine conjugated to HA without the rhodamine B amine acceptor and IFR is the intensity of the fluorescein amine donor conjugated to HA with the rhodamine B amine acceptor. Because dense substitution of macromolecules with fluorophores can result in their fluorescence autoquenching [10] (and hence over or under estimate the fluorescein amine quenching due to FRET when the above equation is used) we compared preparations of FRET-HA with FL-HA that had low and comparable densities of substitution (0.047 moles of fluorescein amine per mole of HA disaccharide for FRET-HA compared with 0.053 moles of fluorescein amine per mole of HA disaccharide for FL-HA). Sample excitation was at 490 nm and emission was monitored at 530 nm.
Similarly, we compared the intensity of the FRET-HA substrate with HA labeled with rhodamine B amine alone (i.e., R-HA) using equal concentrations relative to rhodamine B amine (2×10−7 M). The % enhancement of rhodamine B amine was calculated from the equation,
Where IFR is the intensity of the rhodamine B amine acceptor conjugated to HA with the fluorescein amine donor and IR is the intensity of rhodamine B amine conjugated to HA without the fluorescein amine donor. Again, because dense substitution of macromolecules with fluorophores can result in their fluorescence autoquenching [10] (and hence over or under estimate the rhodamine B amine enhancement due to FRET when the above equation is used) we compared preparations of FRET-HA with R-HA that had low and comparable densities of substitution (0.029 moles of rhodamine B amine per mole of HA disaccharide for FRET-HA compared with 0.036 moles of rhodamine B amine per mole of HA disaccharide for R-HA). Sample excitation was 490 nm and emission was monitored at 580 nm.
Corrections for inner filter effects
Inner filter effects can arise from high extinction coefficients at the excitation and emission wavelengths used for data acquisition. If left uncorrected, this effect can cause quenching of the donor fluorescence which can be misinterpreted as FRET. Inner filter effects can be assessed by sample dilution or the measured fluorescence values can be corrected using the equation [12],
Where Icorrected is the corrected fluorescent intensity, Iobserved is the observed fluorescent intensity, AEx is the absorption at the excitation wavelength and AEm is the absorption at the emission wavelength.
Enzymatic assays
All enzymatic assays were performed in Dulbeccos PBS, pH 6.0. Solutions of bovine testes hyaluronidase were prepared immediately before use. HA concentrations were calculated based on disaccharide molarity.
In order to determine the impact of hyaluronidase on FRET, we incubated the FRET-HA substrate (5.5 µg/mL) with different concentrations of hyaluronidase (0 to 10 U/mL) for 2 h at 37 °C. Samples were then boiled at 100 °C for 10 min to inactivate the enzyme. After cooling the solutions to room temperature the samples were transferred to a cuvette, excited at 490 nm and the fluorescent emissions collected at 530 nm. The fluorescent intensity of mock treated FRET-HA (incubated at 37 °C without hyaluronidase and then boiled) was then subtracted from the fluorescent intensities of treated samples.
To monitor the kinetics of bovine testes hyaluronidase we added 100 µL of the FRET-HA solution at different concentrations to the bottom of a cuvette. Next, 900 µL of enzyme solution was flushed into the cuvette using the instrument set-up exactly as described [15]. The final concentration of hyaluronidase was 100 U/mL and the reactions were performed at 25 °C. The change in the fluorescence emission was recorded at 530 nm (excitation = 490 nm) for 1000 s using the Vinci software.
Curve fitting
All curve fitting was performed using Sigma Plot software, version 10.0. The Sigma Plot program uses the Marquardt-Levenberg algorithm to estimate the coefficients of the independent variables.
We determined the ratio of degraded FRET-HA at time t relative to undegraded FRET-HA at t = 0 using the equation,
where It is the intensity at time t, Im is the maximum intensity and Io is the intensity at t = 0. Plots of [HA] / [HA]o versus time were fit with the first order rate equation,
where the coefficients of the independent variables were [FRET-HA]o and k.
The initial reaction velocities were determined from,
where k is the first order reaction rate (s−1) and [FRET-HA] is the HA concentration expressed in mol/L of disaccharide subunits.
The Vmax and Km values for bovine testes hyaluronidase were estimated by non-linear regression using the Michaelis-Menten equation,
where vo is the initial velocity, [FRET-HA] is the concentration of HA expressed in mol/L of disaccharide subunits and Vmax and Km are the coefficients of the independent variables.
Results
Development of a FRET-HA substrate
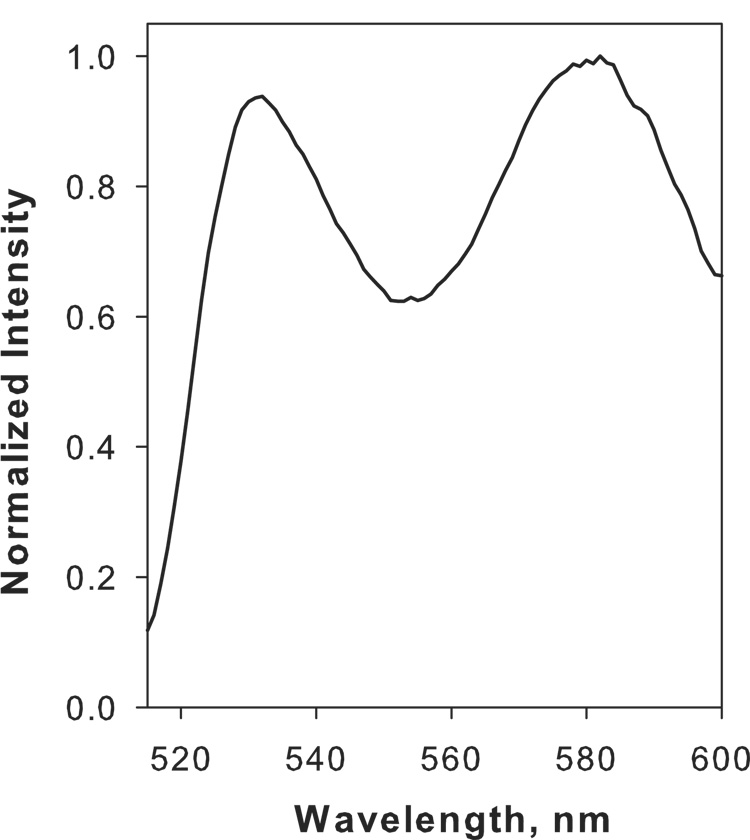
In order to develop a substrate to monitor hyaluronidase activity, we conjugated fluorescein amine and rhodamine B amine to HA using the condensation reaction. Precipitation of the dual labeled conjugate followed by dialysis effectively removed free fluorescein amine and rhodamine B amine as ascertained by the single peak from size exclusion chromatography. The density of substitution (expressed as the moles of fluorescein amine or rhodamine B amine per mole of HA disaccharide) was 0.047 for fluorescein amine and 0.029 for rhodamine B amine. The emission spectrum for the HA conjugate was biphasic with emission maxima at 530 nm and 586 nm which corresponds to fluorescein amine and rhodamine B amine, respectively (Fig. 1). Next, we compared the fluorescent intensities of HA conjugated with fluorescein amine or HA conjugated with both fluorescein amine and rhodamine B amine. As shown in Fig. 2A, the fluorescent intensity of the fluorescein amine donor in the presence of the conjugated rhodamine B amine acceptor was significantly quenched (78.73 ± 3.46%) relative to HA conjugated to fluorescein amine alone. On the other hand, the fluorescent intensity of the rhodamine B amine acceptor when conjugated to HA with the fluorescein amine donor was significantly enhanced (68.21 ± 4.35%) relative to HA conjugated to rhodamine B amine alone (Fig. 2B). These results indicated that conjugation of HA with fluorescein amine and rhodamine B amine resulted in energy migration.
Fig. 1.
Normalized fluorescence spectrum of the FRET-HA substrate in Dulbeccos PBS, pH 6.0 at 25 °C. The sample was excited at 490 nm and the emission spectrum accumulated in triplicate. Note the presence of two major peaks at 530 nm and 580 nm corresponding to the emission maximum of fluorescein amine and rhodamine B amine, respectively.
Fig. 2.
Impact of dual labeling on the fluorescent intensities of fluorescein amine and rhodamine B amine. (A) The fluorescent intensity of FRET-HA was compared with the fluorescent intensity of HA labeled with fluorescein amine alone. The rhodamine B amine acceptor significantly quenched the fluorescence of the fluorescein amine donor. Samples were excited at 490 nm and their emissions collected at 530 nm. (B) The fluorescent intensity of FRET-HA was compared with the fluorescent intensity of HA labeled with rhodamine B amine alone. The fluorescein amine donor significantly enhanced the fluorescent intensity of the rhodamine B amine acceptor. Samples were excited at 490 nm and their emissions collected at 580 nm. Data in this figure show the means ± SD from triplicate samples. Asterisks indicate significant differences (** P < 0.01) assessed by the two-tailed Students t-test.
As already discussed, FRET is sensitive to the distance between the energy donor and energy acceptor. Because we did not have control over the region-specific attachment of fluorescein amine and rhodamine B amine, we evaluated a second batch of FRET-HA in order to ascertain the consistency of different FRET-HA preparations. We found similar low levels of substitution in terms of both fluorescein amine (0.047 in batch 1 versus 0.058 in batch 2) and rhodamine B amine (0.029 in batch 1 versus 0.087 in batch 2). Importantly, the fluorescein amine fluorescent intensities were similarly quenched in both batches (78% in batch 1 versus 65% in batch 2) which allowed us to monitor hyaluronidase activity in either continuous or non-continuous assays for both preparations. Thus, our results suggest that FRET-HA can be prepared in a relatively reproducible fashion.
As briefly described in the Introduction, FRET involves the non-radiative transfer of energy due to the dipole-dipole coupling of the donor-acceptor pair. On the other hand, radiative transfer of energy (i.e., inner filter effects) can also occur. In order to ascertain the impact of inner filter effects on the fluorescent properties of FRET-HA, we serially diluted the HA conjugate. Serial dilutions of FRET-HA resulted in marked decreases of the fluorescein amine fluorescent intensities (Fig. 3). Decreases in the fluorescent intensities were linear showing that inner filter effects played little or no role in the fluorescence properties of FRET-HA. Finally, the calculated correction values for each of the FRET-HA concentrations were unity, reinforcing the notion that inner filter effects did not contribute to the observed fluorescence intensities in this concentration range. These results show that energy migration in our system is most likely due to FRET. Thus, we concluded that the dual labeled HA conjugate is a novel FRET probe.
Fig. 3.
Impact of inner filter effects on the fluorescent properties of FRET-HA. The fluorescein amine fluorescent intensities of serially diluted FRET-HA were assessed by exciting the samples at 490 nm and collecting the fluorescent emissions at 530 nm. Results are expressed as the means ± SD from triplicate samples. The error bars are smaller than the symbols for the mean values in this figure.
Sensitivity of FRET to hyaluronidase
In order to assess if the FRET-HA probe can serve as a hyaluronidase substrate, we incubated the labeled HA with different concentrations of bovine testes hyaluronidase and then measured the fluorescein amine fluorescent intensities. As shown in Fig. 4, the fluorescent intensities at 530 nm emission increased with increasing concentrations of bovine testes hyaluronidase. Importantly, an increase in the fluorescein amine fluorescent intensity could be detected with as little as 0.01 U/mL of this enzyme. These results show that the FRET-HA substrate is sensitive to hyaluronidase activity. Moreover, the non-continuous assay described herein is simple and could easily be expanded to accommodate additional analytical techniques (e.g., HPLC and other chromatographic methods).
Fig. 4.
Enzyme concentration dependent digestion of the FRET-HA substrate. FRET-HA was incubated with graded concentrations of bovine hyaluronidase in Dulbeccos PBS, pH 6.0. After heat denaturing the enzyme, samples were excited at 490 nm and the fluorescence emission collected at 530 nm. Data in this figure show the means ± SD from triplicate samples. In this figure, the error bars are smaller than the symbols for the mean values.
Measurement of hyaluronidase kinetics
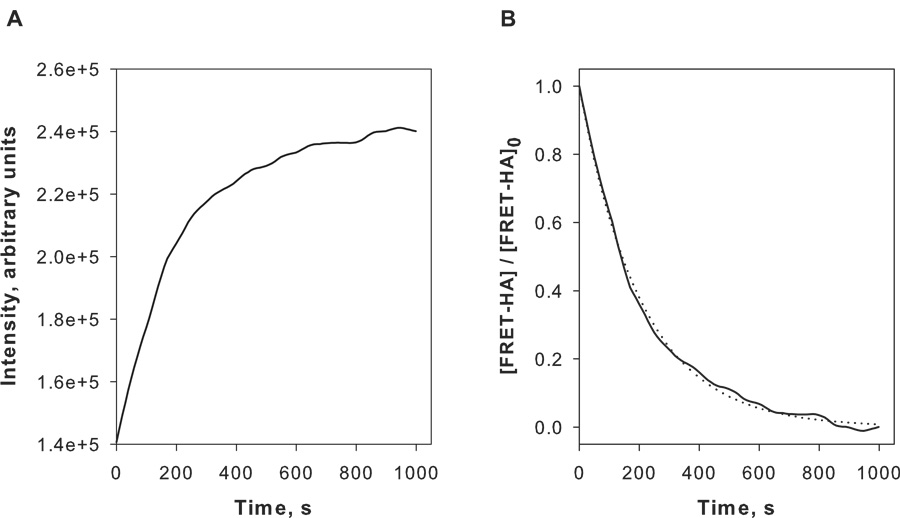
To measure the kinetics of bovine testes hyaluronidase we continuously monitored the increase in the fluorescein amine signal for 1000 s. As shown in Fig. 5A, addition of bovine testes hyaluronidase resulted in a time-dependent increase of the fluorescent intensity. Based on the Michaelis-Menten model, when [S]≪Km the enzyme kinetics are expected to behave as a first order reaction. Because the concentrations of HA that we used were significantly less than the reported Km values for bovine testes hyaluronidase (0.16–0.90 mM) [16][17], we curve-fit our kinetic data using the first order rate law. We found excellent correlation between the experimental data and predicted behavior for first order kinetics (Fig. 5B). Importantly, we were able to conduct these kinetic experiments using relatively low concentrations of HA and enzyme (concentrations in the microgram range) compared to the classic turbidity or viscometry continuous assay systems (concentrations typically in the milligram range). Thus, we conclude that the FRET-HA substrate is useful for rapidly acquiring kinetic data continuously and with relatively modest requirements for reagent concentrations. Again, as noted above for the non-continuous assay, it should be possible to implement the continuous assay into other formats (e.g., microtiter plate assays).
Fig. 5.
Time dependent degradation of FRET-HA by bovine testes hyaluronidase. (A) Progress curve of increasing fluorescein amine fluorescence intensity after addition of bovine testes hyaluronidase (100 U/mL) in Dulbeccos PBS, pH 6.0 at 25 °C. In this example, the FRET-HA substrate is at 42 µM. (B) The data shown in (A) was converted to the fractional change in the FRET-HA concentration as described in the text. Data were fit to the first order rate law using non-linear least squares regression. The experimental data is shown by the solid line and the curve-fit by the dashed line.
Substrate dependence of the initial velocity
We calculated the initial velocities of bovine testes hyaluronidase as the product of the first-order rates of reactions and the concentrations of FRET-HA substrate. Plots of the initial velocities versus time were parabolic and allowed us to estimate values for the Km and Vmax based on the Michaelis-Menten model (Fig. 6). Importantly, the estimated Km value that we obtained (0.16 mM) is in excellent agreement with the range of Km values previously estimated for bovine testes hyaluronidase (0.16–0.90 mM) using non-continuous assays that measured the concentration of reducing ends generated during the course of the enzymatic reaction. Thus, we conclude that FRET-HA is a reliable substrate for assessing the hyaluronidase kinetics.
Fig. 6.
Substrate dependence of the initial degradation velocities for bovine testes hyaluronidase. Initial velocities were plotted against the substrate concentrations and fit to the Michaelis-Menten model (Vmax = 0.05 mM/min, Km = 0.16 mM). Data in this figure shows the means ± SD from triplicate samples.
Discussion
HA was originally considered to be an inert filling material of the extracellular matrix. However, more recent investigations have shown that HA likely plays a significant role in a number of diverse biological activities. Because the size of the HA co-polymer is important for mediating many of its biological functions, the pathways that regulate HA degradation are key to understanding the biology of HA in its proper physiological context. Because the hyaluronidases may be pivotal players in regulating HA size, these enzymes have recently received increased interest.
Although a number of assays have been described to assess hyaluronidase activity, the development of rapid yet robust methods are highly desirable. To this end, we have developed a novel fluorescent HA substrate (FRET-HA) for use in either non-continuous or continuous assays. The condensation reaction for covalently labeling HA with amine containing fluorophores is easily performed in a one step reaction. Previous studies have shown that the reaction conditions used for the condensation reaction are mild and very little of the HA molecule is depolymerized [14]. In addition, removal of free fluorophores can be performed efficiently by precipitation of the HA molecule and dialysis.
Inner filter effects can limit the utility of FRET-based substrates for quantitatively evaluating enzymatic activity due to deviations of the fluorescent intensity from linearity [18]. No corrections were required for inner filter effects for the FRET-HA even up to relatively high substrate concentrations (56 µM) that typically require correction for the inner filter effect (~20 µM) [9][11]. This is most likely due to the modest levels of substitution of HA with the fluorescein amine molecules (<0.1 mole of fluorescein amine per mole of HA disaccharide). For example, even at 56 µM of HA disaccharide the concentration of fluorescein amine remained relatively low (2.6 µM).
The FRET-HA substrate relies on the detection of a change in the quenching status of the energy donor as hyaluronidase disrupts the energy migration between the donor-acceptor pair due to digestion of the HA molecule. Thus, the FRET-HA substrate takes advantage of the simplicity and high sensitivity afforded by fluorescent assays. Moreover, assays can be performed in either continuous or non-continuous formats depending on investigator needs. Non-continuous assays can be performed without the need for additional steps (e.g., colorimetric detection) thus streamlining hyaluronidase assays. Finally, continuous assays are implemented by simply mixing hyaluronidase to the FRET-HA substrate.
A number of applications for the FRET-HA substrate can be envisioned. First, the ability to continuously monitor hyaluronidase activity suggests that at high substrate concentrations kinetic analysis of progress curves can be performed [19][20][21]. As mentioned in the Introduction, different hyaluronidases degrade HA using different catalytic mechanisms (i.e., hydrolases, lyases and endo-β-glucuronidases ). Thus, the FRET-HA substrate may be a valuable new tool for testing and comparing kinetic models for the different hyaluronidase classes. Second, because of the potential importance of the hyaluronidases in pathological states (e.g., angiogenesis for tumor development), inhibitors for these enzymes obtained from screening chemical libraries may yield beneficial molecules for drug development. Therefore, the FRET-HA substrate may provide the foundation for the development of high throughput screening strategies. Techniques for measuring and analyzing FRET signals in living cells have been reported [22]. Previous investigations have indicated that exogenous HA is transported to the endosomal/ lysosomal system for degradation [23]. Thus, the FRET-HA substrate may be a new tool for investigating hyaluronidase activity in intact living cells. Finally, FRET-HA is unique from most other FRET based enzymatic substrates. The majority of FRET-substrates are fluorescently labeled peptides that have been developed to assess proteolytic activity. By contrast, the FRET-HA substrate is a carbohydrate designed to ascertain hydrolase activity. Therefore, FRET-HA may provide a template for designing other FRET-based carbohydrate substrates.
In conclusion, we have developed a fluorescent HA substrate (termed FRET-HA) based on FRET between fluorescein amine (energy donor) and rhodamine B amine (energy acceptor). Our studies show that FRET-HA is a sensitive and robust substrate for measuring hyaluronidase activity. Furthermore, the convenience and versatility of fluorescent assays suggest that FRET-HA may have broad applications in basic as well as applied hyaluronidase research.
Acknowledgements
We thank Drs. Edward W. Voss, Jr. and Peter Antich for their insightful comments and critiques and Susan Milberger for her secretarial assistance. This work was supported by a Galderma Pilot and Feasibility grant and NIH grant AR48840.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Savani RC, Wang C, Yang B, Zhang S, Kinsella MG, Wight TN, Stern R, Nance DM, Turley EA. Migration of bovine aortic smooth muscle cells after wounding injury. The role of hyaluronan and RHAMM. J. Clin. Invest. 1995;95:1158–1168. doi: 10.1172/JCI117764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deed R, Rooney P, Kumar P, Norton JD, Smith J, Freemont AJ, Kumar S. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non- angiogenic, high-molecular-weight hyaluronan. Int. J. Cancer. 1997;71:251–256. doi: 10.1002/(sici)1097-0215(19970410)71:2<251::aid-ijc21>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J. Biol. Chem. 2001;276:36770–36778. doi: 10.1074/jbc.M102273200. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Underhill CB, Chen L. Hyaluronan on the surface of tumor cells is correlated with metastatic behavior. Cancer Res. 1995;55:428–433. [PubMed] [Google Scholar]
- 5.Rahmanian M, Pertoft H, Kanda S, Christofferson R, Claesson-Welsh L, Heldin P. Hyaluronan oligosaccharides induce tube formation of a brain endothelial cell line in vitro. Exp. Cell Res. 1997;237:223–230. doi: 10.1006/excr.1997.3792. [DOI] [PubMed] [Google Scholar]
- 6.Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem. Rev. 2006;106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreil G. Hyaluronidases--a group of neglected enzymes. Protein Sci. 1995;4:1666–1669. doi: 10.1002/pro.5560040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hynes WL, Ferretti JJ. Assays for hyaluronidase activity. Methods Enzymol. 1994;235:606–616. doi: 10.1016/0076-6879(94)35174-0. [DOI] [PubMed] [Google Scholar]
- 9.Holskin BP, Bukhtiyarova M, Dunn BM, Baur P, de CJ, Pennington MW. A continuous fluorescence-based assay of human cytomegalovirus protease using a peptide substrate. Anal. Biochem. 1995;227:148–155. doi: 10.1006/abio.1995.1264. [DOI] [PubMed] [Google Scholar]
- 10.Voss EW, Jr, Workman CJ, Mummert ME. Detection of protease activity using a fluorescence-enhancement globular substrate. BioTechniques. 1996;20:286–291. doi: 10.2144/96202rr06. [DOI] [PubMed] [Google Scholar]
- 11.Grahn S, Ullmann D, Jakubke H. Design and synthesis of fluorogenic trypsin peptide substrates based on resonance energy transfer. Anal. Biochem. 1998;265:225–231. doi: 10.1006/abio.1998.2902. [DOI] [PubMed] [Google Scholar]
- 12.Lakowicz JR. Principles of fluorescence spectroscopy. New York: Plenum Press; 1983. [Google Scholar]
- 13.Enomoto K, Okamoto H, Numata Y, Takemoto H. A simple and rapid assay for heparanase activity using homogeneous time-resolved fluorescence. J Pharm. Biomed. Anal. 2006;41:912–917. doi: 10.1016/j.jpba.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 14.de Belder AN, Wik KO. Preparation and properties of fluorescein-labelled hyaluronate. Carbohydr. Res. 1975;44:251–257. doi: 10.1016/s0008-6215(00)84168-3. [DOI] [PubMed] [Google Scholar]
- 15.Voss EW, Jr, Croney JC, Jameson DM. Discrete bathochromic shifts exhibited by fluorescein ligand bound to rabbit polyclonal anti-fluorescein Fab fragments. J Protein Chem. 2002;21:231–241. doi: 10.1023/a:1019789118530. [DOI] [PubMed] [Google Scholar]
- 16.Asteriou T, Vincent JC, Tranchepain F, Deschrevel B. Inhibition of hyaluronan hydrolysis catalysed by hyaluronidase at high substrate concentration and low ionic strength. Matrix Biol. 2006;25:166–174. doi: 10.1016/j.matbio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Hofinger ES, Bernhardt G, Buschauer A. Kinetics of Hyal-1 and PH-20 hyaluronidases: comparison of minimal substrates and analysis of the transglycosylation reaction. Glycobiology. 2007;17:963–971. doi: 10.1093/glycob/cwm070. [DOI] [PubMed] [Google Scholar]
- 18.Kruger RG, Dostal P, McCafferty DG. Development of a high-performance liquid chromatography assay and revision of kinetic parameters for the Staphylococcus aureus sortase transpeptidase SrtA. Anal. Biochem. 2004;326:42–48. doi: 10.1016/j.ab.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Orsi BA, Tipton KF. Kinetic analysis of progress curves. Methods Enzymol. 1979;63:159–183. doi: 10.1016/0076-6879(79)63010-0. [DOI] [PubMed] [Google Scholar]
- 20.Liao F, Zhu XY, Wang YM, Zuo YP. The comparison of the estimation of enzyme kinetic parameters by fitting reaction curve to the integrated Michaelis-Menten rate equations of different predictor variables. J Biochem. Biophys. Methods. 2005;62:13–24. doi: 10.1016/j.jbbm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Palmier MO, Van D., Sr Rapid determination of enzyme kinetics from fluorescence: overcoming the inner filter effect. Anal. Biochem. 2007;371:43–51. doi: 10.1016/j.ab.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallrabe H, Chen Y, Periasamy A, Barroso M. Issues in confocal microscopy for quantitative FRET analysis. Microsc. Res. Tech. 2006;69:196–206. doi: 10.1002/jemt.20281. [DOI] [PubMed] [Google Scholar]
- 23.Tammi R, Rilla K, Pienimaki JP, MacCallum DK, Hogg M, Luukkonen M, Hascall VC, Tammi M. Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J. Biol. Chem. 2001;276:35111–35122. doi: 10.1074/jbc.M103481200. [DOI] [PubMed] [Google Scholar]